Abstract
A polypyrrole (PPy) film containing dodecyl sulfonate (SDS) as an organic counter ion and primary phosphate as an inorganic counter ion was electrodeposited on a stainless steel wire. The process was carried out under a constant deposition potential of 1.5 V that was applied to an aqueous solution containing pyrrole monomer, SDS and phosphate ions in varying concentrations. Films (with thicknesses 66 μm) were synthesized in the presence and absence of phosphate ions, and their surfaces were characterized by scanning electron microscopy. The surface structure of the PPy–SDS–H2PO4 − film compared to films made from PPy-SDS only or PPy-phosphate only, are more porous and less smooth. The effects of counter ions and their weight ratios, of applied voltage and time for electrodeposition were optimized. The applicability of this unbreakable coating is demonstrated by headspace solid-phase micro extraction of selected chlorobenzenes from aqueous samples. Extraction temperature and time, ionic strength, desorption conditions and stirring rates were also optimized. Under optimum conditions and by a gas chromatography–mass spectrometry for separation and detection, the relative standard deviations for the determination of chlorobenzenes in distilled water spiked at a level of 30 ng L−1 were 3–8 % (for n = 3), the limits of detection are between 0.5 and 1 ng L−1, and the calibration plots cover the 2.5 to 1,000 ng L−1 range. The method was applied to the analysis of (spiked) water samples, and relative recoveries were found to range from 95 to 103 %.

A polypyrrole film containing dodecyl sulfonate as an organic counter ion and primary phosphate as an inorganic counter ion was electrodeposited on a stainless steel wire. The applicability of this coating is demonstrated by headspace solid-phase micro extraction of selected chlorobenzenes from aqueous samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid phase micro extraction (SPME) has been successfully applied for the sampling and analysis of a wide variety of compounds, especially for the extraction of volatile and semi − volatile organic compounds from environmental, biological and food samples. This versatile and simple methodology combines sampling, extraction, preconcentration and sample introduction in one step and while does not require solvents, desorption process can directly be conducted in the injector of the chromatographic system. The extraction efficiency of this micro-oriented technique is predominantly determined by intermolecular and steric interactions between the analyte species and the extracting media [1–4]. The fragility of fused-silica substrate is a major drawback and it is therefore necessary to use metallic substrates in order to have an unbreakable SPME fiber [5, 6]. In recent years, many researchers have focused on the development of metallic fibers coated with a variety of materials including conducting polymers. PPy and its derivatives are among the most frequently investigated conductive polymers and this is due to their facile synthesis and good environmental and excellent thermal stability [7]. This polymer can be easily prepared by either an oxidative chemical or electrochemical polymerization of pyrrole [8]. PPy and its derivatives have been one of the most widely used and intensively studied classes of conducting polymers because: (1) they can be easily polymerized from organic or aqueous media at neutral pH electrochemically or chemically, (2) they are relatively stable in air and solution, and (3) pyrrole monomer and some of its derivatives are readily available. PPy and its derivatives, due to their multi functionality, are assisted by different adsorption mechanisms such as hydrophobic, acid–base, π–π interaction, polar functional groups interactions, ion exchange, hydrogen bonding, and electro activity [9–12].
If the polymer is prepared by electropolymerization, it is possible to create very small polymeric structures on conductive substrates, which makes PPy coatings highly adaptable for miniaturized analytical systems [13]. The polymer is easily prepared from both aqueous [14] and non-aqueous solutions [15] containing a suitable dopant ion. The most commonly used dopants or counter-ions are oxalate, dodecyl-sulfate, perchlorate, chloride and sulfate [16–20]. In general, the properties of the PPy films are strongly dependent on polymerization conditions such as, the type of counter-ion, the concentrations of monomer and the counter-ion, the applied voltage, the solvent and the reaction temperature [21, 22]. The doping of the polymer is generally performed simultaneously by incorporation of the doping anion into the polymer to ensure the electrical neutrality of the film. The physical properties of PPy films such as morphology, porosity, mechanical property and thermal stability are also influenced by the nature and size of counter ions. It has been shown that improved mechanical and thermal properties can be achieved for PPy coatings incorporated with sulfonated aromatic counter ions [23–25]. Also PPy layers doped with amphiphilic dopant anions like dodecylsulfate (DS) and dodecylbenzenesulfonate gave layers with short range order, whereas small BF4 −or ClO4 − anions produced amorphous porous layers, with rough surface morphology [26].
It was also found that the adhesion of the PPy film to the platinum wire surface was affected by the counter ion used. PPy was synthesized in the presence of different dopants such as chloride, perchlorate, acetate, sulfate, and dodecylsulfate ions in aqueous medium [27]. Polymer films doped with chloride, perchlorate and acetate anions showed weak adhesion on the Pt wire and were unstable at temperatures higher than 200 °C, while the fibers coated with sulfate doped PPy (PPy–S) and PPy–DS under argon atmosphere are thermally stable up to 300 °C. Effect of different types of doping ions in PPy-based SPME fibers have been followed by exploration of sodium polyphosphate (PP), as a new dopant for the electropolymerization of pyrrole on the surface of a steel fiber with the aim of achieving a polymer coating with improved qualities for SPME analysis. It was revealed that PPy/PP film is thermally more stable than others. Also, the prepared PPy/PP coating showed to be more stable and did not show any major weight loss even at 450 °C. Moreover, the PPy doped with polyphosphate could overcome the adhesion problem through incorporation of the inorganic polyphosphate into the organic polymeric structure, which led to a chemically adhered coating through Fe-P bonding to the metal surface [28].
Although a number of sorbent-based pyrrole polymers were prepared using organic and inorganic dopant ions [13–29], there are no known reports on the preparation of PPy in which contains both organic and inorganic dopant ions. In this study, for the first time, two counter ions of SDS and H2PO4 − were selected to prompt the porosity and adhesion property from one side and the improvement in the extraction efficiency from other side. These are originated form the incorporation of phosphate ions into the PPy structure through Fe-P bonding to the metal surface and the presence of organic counter ion. Therefore, the electrochemical polymerization technique was applied to synthesize the desired PPy films on the SPME stainless steel substrate at the presence of both SDS and KH2PO4 as dopant ions.
Experimental
Chemicals
Pyrrole (98 %) was obtained from Aldrich (Mississauga, Canada, https://www.sigmaaldrich.com) and distilled before use. SDS, and KH2PO4 were purchased from Aldrich and their aqueous solution was used as the electrolyte for electropolymerization of pyrrole. The Chlorobenzene compounds (CBs) including monochlorobenzene (MCB), 1,4-dichlorobenzene (14DCB), 1,2-dichlorobenzene (12DCB), 1,2,4-trichlorobenzene (124TCB), 1,2,3-trichlorobenezne (123TCB) and 1,2,3,4-tetrachlorobenezne (1234TeCB) were purchased from Merck (Darmstadt, Germany, http://www.merckgroup.com). Standard solution (1,000 mgL−1) of CBs mixture was prepared in HPLC-grade methanol (Merck) and stored in the refrigerator. The working standard solutions were prepared weekly by diluting the standard solution with methanol, and more diluted working solutions were prepared daily by diluting this solution with double distilled water (DDW). Sodium chloride was purchased from Merck.
Apparatus
A gas chromatograph model Agilent 6820 (http://www.agilent.com), with a split/splitless injection port and a flame ionization detection system, was used for optimization. The separation of analytes was carried out using a wide bore column HP − 5 MS (30 m, 0.53 mm i.d.) with 0.25 μm film thickness (Hewlett − Packard, Palo Alto, CA, USA). The carrier gas was nitrogen (99.99 %) at a flow rate of 2.5 mLmin−1. The sample introduction was performed in the splitless mode and the split valve was kept closed for 2 min. The injector and detector temperatures were set at 260 and 290 °C, respectively. For real samples analysis and the quantitative survey, a Hewlett − Packard (HP, Palo Alto, CA, USA) HP 6890 series GC equipped with a split/splittless injector and a HP 5973 mass − selective detector system were used. The MS was operated in the EI mode (70 eV). Helium (99.999 %) was used as carrier gas and its flow-rate was adjusted to 1 mL min−1. The separation of the selected CBs was performed on a 30 m × 0.25 mm HP − 5 MS column with 0.25 μm film thickness. The GC–MS interface, ion source and quadrupole temperatures were set at 270, 230 and 150 °C, respectively. For quantitative analysis, the MS was set on the selected ion monitoring (SIM) mode and two characteristics ions for each compound were monitored (Table 1). The column temperature was programmed at 40 °C for 2 min, increased to 120 °C at a rate of 10 °Cmin−1 and kept at this temperature for 1 min. The scanning electron microscopy (SEM) images were obtained by a TESCAN VEGA II XMU (Brno, Czech Republic, www.tescan.com). A homemade glass water bath connected to a refrigerated circulating water bath (Neslab, USA, www.thermoscintific.com) was used for heating the samples while they were stirred using a MR Hei − Mix S magnetic stirrer (Heidolph, Germany).
Preparation of polypyrrole– dodecyl sulfonate –H2PO4 −
Firstly, pyrrole was distilled under reduced pressure and stored in darkness before use. The electrolyte solution contained SDS, H2PO4 − and double distilled water. An electrochemical cell consisting of stainless steel working and counter electrodes was used. In this work, different amount of H2PO4 − and SDS counter ions were dissolved in 2 mL of pyrrole solution and stirred for 10 min to obtain a homogenous solution. Then, a length of 1.5 cm from the end part of plunger wire was placed in the solution as the working electrode. Each PPy coating was directly synthesized on the surface of the plunger needle inserted in a electrolyte solution (0.05 % SDS) containing 0.05 M of pyrrole monomer and different amount of H2PO4 − by applying constant potential at constant time (Fig. 1). The stainless steel plunger coated with PPy film was washed with distilled water to remove the unwanted chemicals such as monomers and the supporting electrolyte and then dried under a gentle stream of nitrogen.
Headspace solid phase micro extraction analysis
For the headspace solid phase micro extraction (HS-SPME) determination, a manual home-made SPME holder with the prepared fiber coatings (~66 μm film thickness) was used. Each fiber was conditioned prior to the extraction by its exposure to a temperature of 260 °C of the injection port for 120 min. One-factor-at-a-time method was used for optimizing the HS-SPME parameters. For HS-SPME sampling, the fiber was then inserted into the sample bottle, and situated at about 1.5 cm above the surface of the aqueous phase. In all experiments, 1.2 g NaCl was added to 4 mL of aqueous sample, and then the solution was spiked with the mixture of CBs. Then the sample vial was sealed with a PTFE-faced septum and an aluminum cap. The sample vials were heated in a circulating water bath and agitated by a magnetic stirrer operated at maximum rate. The preliminary extraction was performed by exposing the fiber coating to the headspace over the aqueous sample for 25 min at 26 °C. After performing the extraction, the fiber was withdrawn into the external needle (22 G) and then it was immediately inserted into the GC injector port for thermal desorption of analytes at 260 °C for 2 min.
Results and discussion
Synthesis of polypyrrole– dodecyl sulfonate –H2PO4 −
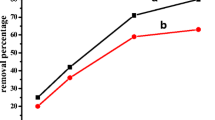
Surely, when the surface area and porosity of a polymeric structure are improved, higher extraction efficiency would be expected. An alternative is to use different counter ions including SDS and H2PO4 − to provide a multi-functional characteristics polymeric media. Evaluating the extraction efficiency was implemented for synthesizing the most appropriate PPy-based film. Firstly, the influence of the mixing ratio of counter ions was investigated while electrodeposition was carried for 10 min at a voltage of 1 V using a 0.05 M pyrrole solution. According to Fig. 2a, at constant concentration of SDS the peak areas were enhanced as the H2PO4 − content raised to 3 mg, indicating the efficient role of the H2PO4 − ions in the extraction process. The applied voltage and its duration time can influence the fiber coating thickness and morphology. If the applied voltage is high, the thick of coating due to the reduction process becomes kinetically prominent was increased. Therefore, by manipulation of the working electrode voltage and electrodeposition time, a variety of coatings with different thicknesses and pore sizes were obtained. In order to achieve the optimum value for the working electrode voltage, this parameter was studied in the range of 0.5–2.5 V while the coating prepared under the applied voltage of 1.5 V exhibited higher extraction efficiency (Fig. 2b). A duration time of 20 min was also found to be practically the most appropriate time for the coatings preparation.
The polypyrrole-based films characteristics
The surface characteristics of the PPy-based films were investigated by SEM. Figure 3a, b and c show the micrographs of PPy–H2PO4 −, PPy–SDS and PPy–SDS–H2PO4 − film coatings, respectively. Apparently, the synthesized PPy–SDS–H2PO4 − coating possesses a non-smooth and porous structure in comparison with the PPy film containing only one counter ion.
Fourier transform infrared spectroscopy (FTIR) was also employed to examine the modification process and recognition of any possible change on the PPy-based coatings (Fig. S1, Electronic Supplementary Material, ESM). For PPy, the bands at 1,547 and 1,454 cm−1 may be assigned to typical ring vibrations and the bands at 1313, 1040 and 890 cm−1 may correspond to = C–H band in plane vibration while the peaks at ∼3,000–3,600 cm−1 correspond to the N-H stretching vibrations. In addition, for PPy-SDS the absorption peaks at 2,800–3,000 cm−1 correspond to the C-H stretching vibrations for SDS while for PPy-H2PO4 −, the intense bands in the range of 1,155–1,026 cm−1 are attributed to the asymmetric and symmetric stretching vibrations of the PO3 groups originated from the presence of H2PO4 − in the film. Clearly, the spectrum of PPy–SDS–H2PO4 − exhibits two characteristic absorption peaks which are due to the presence of both H2PO4 − and SDS counter ions.
Evaluation
To evaluate the capability of the PPy–SDS–H2PO4 − fiber coating, the feasibility of HS-SPME of some important organic pollutants such as CBs from water samples was considered. The use of headspace mode is usually preferred due to limited harm to the sorbent caused by the sample matrix. Furthermore, CBs have sufficient vapor pressure to be analyzed by headspace mode. Effects of different parameters such as the extraction temperature, desorption time and temperature, the ionic strength and extraction time on the amounts of extracted CBs from water samples were investigated using the developed HS-SPME method using the PPy–SDS–H2PO4 −film.
The effect of ionic strength was studied by addition of sodium chloride in the range of 0–1.2 g to 4 mL sample. An increase in the relative peak areas was observed as the salt content increased to 1.2 g. This was due to the salting out effect produced by an increase in the ionic strength of the sample.
Magnetic stirring is used commonly in most extraction techniques to accelerate the mass transfer. Sampling agitation enhances extraction process and reduces its time because the equilibrium between the aqueous and vapor phases can be achieved more rapidly. Five different stirring rates of 0 (static case), 200, 400, 600, 800 and 1,000 rpm were selected. Clearly stirring causes an increase in the analytical signal compared to the stagnant case. The results revealed that the analytical signals increased as the stirring rate changed from 0 to 1,000 rpm. For further experiments, a stirring rate of 1,000 rpm was chosen.
Desorption was investigated after HS-SPME of the solution containing selected CBs. The injection port temperature is an important factor since the coating/gas distribution constants of the adsorbed analytes rapidly decrease when temperature increases allowing an efficient desorption of analytes. On the other hand, desorption temperature is limited by the coating thermal stability. Five desorption temperatures ranging from 200 to 280 °C were tested and the responses increased with the rising temperature up to 260 °C and then remained constant. For the desorption time, a duration of 1 to 5 min was considered and the compounds of interest were fully desorbed from the fiber coating after 2 min.
For the extraction temperature, a temperature range of 20–80 °C was examined and a temperature of 26 °C was chosen (Fig. 4a). HS-SPME is not an exhaustive process but relies on the equilibrium between the concentration of analytes in the headspace of the sample solution and those trapped by the fiber coating. Therefore, equilibrium time is a crucial parameter which could increase the extraction efficiency. The SPME equilibrium time was investigated by exposing the PPy film fiber to the headspace of sample containing the target analytes for 5 to 45 min (Fig. 4b). The mass transfer in SPME follows first order kinetics, that is, the rate of mass transfer from analyte of headspace sample to fiber coating is proportional to the difference between the concentration of analyte in the headspace sample and those trapped by the fiber coating. For all compounds studied, a rapid increase in the analytical signal was observed up to 25 min and then remained almost constant. Therefore, an extraction time of 25 min was used as the optimum quantity.
Method validation
Based on the method development observed above, the following conditions were selected for the determination of CBs in aqueous sample. Double-distilled water spiked with the selected CBs was used to evaluate the precision of the measurements, the limits of detection and the dynamic range of method. The intraday precision of the method was determined by performing three consecutive extractions from the aqueous solution. The working standard solutions of chlorobenzenes were prepared in double distilled water at the concentration levels of 2.5, 30, 80, 200, 400, 800 and 1,000 ng L−1 by dilution of the stock solutions prepared at concentration of 2,000 ng L−1. The linearity of the method was tested by preparing the calibration curve for each analyte. The tested concentration range was from 2.5 to 1,000 ng L−1. The coefficient of determination for all analytes was rather satisfactory (R2 > 0.9992). Limits of detection (LOD), based on a signal-to-noise ratio of 3:1, and were between 0.5 and 1 ng L−1, using SIM mode. The standard deviations of the extraction of the CBs, spiked at the level of 30 ng L−1, were below 8 %. To examine the applicability of the method for CBs determination in an actual aqueous sample, a Calan dam water sample was collected from the agriculture area, Malayer County, Iran, and subsequently analyzed with the use of the developed method. Figure S2 (ESM) shows the chromatogram obtained after SPME of a Calan dam water sample spiked with CBs (30 ng L−1) using SIM mode at optimized conditions. Recoveries were determined by comparison of the responses obtained from 4 mL real water sample spiked at 30 ngL−1 versus the responses achieved after analyzing 4 mL DDW spiked at the same level (95–103 %, Table 2). The developed method was compared to other relevant reports and data are shown in Table 3. As illustrated, this method possesses lower LODs and a wider linear range (LDR) but comparable precision. Additionally, the long lifetime of this novel fiber was validated by no tangible change in extraction efficiency of selected CBs after 115 times. The extraction of CBs was used to assess the prepared PPy–SDS–H2PO4 − fiber durability after repeating the sampling/desorption cycles. The result shown that the extraction capabilities of CBs after 115 cycles were almost unchanged. These results provide strong indication that the developed fiber has a rather high durability.
Conclusion
In this study, a PPy-based film with dual, SDS and H2PO4 − as organic and inorganic, counter ions was electrodeposited on a SPME stainless steel substrate. The surface structure of the PPy–SDS–H2PO4 − film in comparison with PPy–SDS and PPy–H2PO4 − was more porous and non-smooth. The applicability of this unbreakable fiber coating was examined by HS-SPME of some selected CBs from aqueous samples. Relative recoveries along with other analytical data confirm that the PPy film containing both counter ions is an appropriate candidate as a SPME fiber for extracting ultra traces of aromatic-based pollutants. In addition, the synthesis of the PPy coating can be carried out conveniently in a rather inexpensive, easy, simple and rapid fashion. The chemical structure of PPy–SDS–H2PO4 − contributes to hydrophobic and π-π interaction between analytes and the polymeric coating, making it a sensitive probe for extracting ultra traces of organic compounds.
References
Belardi RG, Pawliszyn J (1989) The application of chemically modified fused silica fibres in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Pollut Res J Can 24:179–191
Zhang Z, Pawliszyn J (1995) Quantitative extraction using internally cooled solid phase micro extraction device with simultaneous heating of the sample. Anal Chem 67:34–43
Pawliszyn J (1999) Applications of Solid-Phase Micro extraction. Royal Society of Chemistry, UK
Qin Z, Bragg L, Ouyang G, Niri VH, Pawliszyn J (2009) Solid-phase micro extraction under controlled agitation conditions for rapid on-site sampling of organic pollutants in water. J Chromatogr A 1216:6979–6985
Azenha MA, Nogueira PJ, Silva AF (2006) Unbreakable solid-phase micro extraction fibers obtained by sol–gel deposition on titanium wire. Anal Chem 78:2071–2074
Budziak D, Martendal E, Carasek E (2008) New poly (ethylene glycol) solid-phase micro extraction fiber employing zirconium oxide electrolytically deposited onto a NiTi alloy as substrate for sol–gel reactions. J Chromatogr A 1198–1199:54–58
Iroh JO, Williams C (1999) Formation of thermally stable polypyrrole-naphthalene/benzene sulfonate-carbon fiber composites by an electrochemical process. Synth Met 99:1–8
Wang LX, Li XG, Yang YL (2001) Preparation, properties and applications of polypyrroles. React Funct Polym 47:125–139
Bagheri H, Babanezhad E, Khalilian F (2009) An interior needle electropolymerized pyrrole-based coating for headspace solid-phase dynamic extraction. Anal Chim Acta 634:209–214
Bagheri H, Mohammadi A (2003) Pyrrole-based conductive polymer as the solid-phase extraction medium for the preconcentration of environmental pollutants in water samples followed by gas chromatography with flame ionization and mass spectrometry detection. J Chromatogr A 1015:23–30
Sahin Y, Ercan B, Sahin M (2008) In situ electrochemical solid-phase extraction of anions and cations using polypyrrole and over oxidized sulfonated polypyrrole. Talanta 75:369–375
Svetlicic V, Schmidt AJ, Miller LL (1998) Conductometric sensors based on the hypersensitive response of plasticized polyaniline films to organic vapors. Chem Mater 10:3305–3307
Lakard B, Segut O, Lakard S, Herlem G, Gharbi T (2007) Potentiometric miniaturized pH sensors based on polypyrrole films. Sensors Actuators B 122:101–108
Liljegren G, Nyholm L (2003) Electrochemically controlled solid-phase micro extraction and preconcentration using polypyrrole coated microarray electrodes in a flow system. Analyst 128:232–236
Yu JCC, Lai EPC (2005) Polypyrrole modified stainless steel frits for on-line micro solid phase extraction of ochratoxin A. Anal Bioanal Chem 381:948–952
Sumida T, Wada Y, Kitamura T, Yanagida S (2000) Electrochemical preparation of acroporous polypyrrole films with regular arrays of interconnected spherical voids. Chem Commun 17:1613–1614
Iroh JO, Su W (2002) Adhesion of electrochemically formed polypyrrole coatings to low carbon steel. J Appl Polym Sci 85:2757–2763
Mohammadi A, Yamini Y, Alizadeh N (2005) Dodecylsulfate-dopedpolypyrrole film prepared by electrochemical fiber coating technique for headspace solid phase micro extraction of polycyclic aromatic hydrocarbons. J Chromatogr A 1063:1–8
Cheng FL, Zhang ML, Wang H (2005) Fabrication of polypyrrole nanowire and nanotube arrays. Sensors 5:245–249
Mehdinia A, Bashour F, Roohi F, Jabbari A (2012) A strategy to enhance the thermal stability of a nanostructured polypyrrole-based coating for solid phase micro extraction. Microchim Acta 177:301–308
Sakkopoulos S, Vitoratos E, Dalas E (1998) Conductivity degradation due to thermal aging in conducting polyaniline and polypyrrole. Synth Met 92:63–67
Sadki S, Schottland P, Brodie N, Sabouraud G (2000) The mechanisms of pyrrole electropolymerization. Chem Soc Rev 29:283–293
Skotheim TA (1986) Handbook of conducting polymers. vols. 1 and 2, 2nd ed. Marcel Dekker, New York
Bagheri H, Ayazi Z, Naderi M (2013) Conductive polymer-based micro extraction methods. Anal Chim Acta 767:1–13
Ming Z, Markus P, Beate G, Jurgen HJ (2002) Electropolymerization of pyrrole and electrochemical study of polypyrrole. 5. Controlled electrochemical synthesis and solid-state transition of well-defined polypyrrole variants. Phys Chem B 106:10065–10073
Qian R, Qiu J (1987) Electrochemically prepared polypyrroles from aqueous solutions. Polym J 19:157–172
Ge M, Wallace GG (1992) Ion exchange properties of polypyrrole. React Polym 18:133–140
Mollahosseini A, Noroozian E (2009) Polyphosphate-doped polypyrrole coated on steel fiber for the solid-phase micro extraction of organo chlorine pesticides in water. Anal Chim Acta 638:169–174
Aziz-Zanjani MO, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase micro extraction. Microchim Acta 181:1169–1190
He Y, Wang Y, Lee HK (2000) Trace analysis of ten chlorinated benzenes in water by headspace solid-phase micro extraction. J Chromatogr A 847:149–154
Bagheri H, Aghakhani A (2011) Novel nanofibers coatings prepared by electro spinning technique for headspace solid-phase micro extraction of chlorobenzenes from environmental samples. Anal Methods 3:1284–1289
Grueiro-Noche G, Fernández Laespada M, Pérez Pavón J, Moreno Cordero B, Muniategui Lorenzo S (2013) Determination of chlorobenzenes in water samples based on fully automated micro extraction by packed sorbent coupled with programmed temperature vaporization-gas chromatography–mass spectrometry. Anal Bioanal Chem 405:6739–6748
Acknowledgments
The Research Council and Graduates School of Sharif University of Technology (SUT) are acknowledged for supporting the project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 211 kb)
Rights and permissions
About this article
Cite this article
Bagheri, H., Roostaie, A. & Allahdadlalouni, M. A polypyrrole film with dual counter ions as a highly efficient medium for headspace solid-phase extraction of chloro-organic compounds. Microchim Acta 182, 617–624 (2015). https://doi.org/10.1007/s00604-014-1368-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1368-5