Abstract
Purpose
While regarded as function-preserving gastrectomy, few prospective longitudinal clinical trials have addressed the postoperative quality of life (QOL) after pylorus-preserving gastrectomy (PPG). We prospectively compared chronological changes in postoperative body weight and the QOL between PPG and distal gastrectomy (DG) for pathological Stage I gastric cancer (GC).
Methods
We conducted a multi-institutional prospective study (CCOG1601) to evaluate patients who underwent DG and PPG. The QOL was examined using the European Organization for Research and Treatment of Cancer Quality of life questionnaire-C30 (EORTC QLQ-C30) and the Post-Gastrectomy Syndrome Assessment Scale-37 (PGSAS-37). A total of 295 patients were enrolled from 15 institutions, and propensity score matching was performed to adjust for the essential variables for comparison analyses.
Results
After propensity score matching, 25 pairs of patients were identified. In the first postoperative month, DG achieved a superior nausea and vomiting score (EORTC QLQ-C30) and meal-related distress, indigestion, and dumping scores (PGSAS-37). No significant differences were noted between DG and PPG in the long-term QOL. Postoperative body weight loss was similar in both groups.
Conclusions
This prospective observational study failed to demonstrate the superiority of PPG over DG in terms of postoperative body weight changes and the QOL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although the incidence of gastric cancer (GC) continues to decline in Japan, it remains the main cause of cancer-related death worldwide [1, 2]. The improvements in cancer screening programs and diagnostic techniques enable early detection and a prolonged survival after gastrectomy, facilitating the application of less invasive and more function-preserving procedures to maintain postoperative body weight and improve the quality of life (QOL) [3,4,5].
Function-preserving pylorus-preserving gastrectomy (PPG) aims to preserve the QOL of patients with early cancer of the middle portion of the stomach [6]. PPG was first reported by Maki et al. as an option for treating benign gastric disease [7]. Preservation of the pylorus and vagal nerve branches confers several advantages over distal gastrectomy (DG) with Billroth I reconstruction (DGB1), such as prevention of dumping syndrome, lowering the incidence of bile reflux, and facilitating recovery from body weight loss [8]. Theoretically, less invasive laparoscopic PPG (LPPG) should achieve favorable outcomes.
However, the disadvantages of PPG include delayed gastric emptying or gastric stasis, which causes nausea, vomiting, and postprandial fullness during the early postoperative period in 3%–8% of patients [3, 6, 9]. Furthermore, PPG is technically more demanding than DG, particularly when a laparoscopic approach is employed, because of the requirements for identifying and preserving the hepatic and celiac branches of the vagal nerve, infrapyloric artery, and vein. Nevertheless, limited evidence is available to support the implementation of PPG to achieve a good long-term postoperative QOL and sufficient body composition.
Therefore, we designed a multi-institutional prospective, longitudinal study to evaluate the hypothesis that PPG confers a greater benefit than DG in mitigating postoperative body weight loss and improving the QOL. PPG is only recommended for clinical stage I GC according to the Japanese Treatment Guidelines; therefore, we limited our analysis to this subset of patients.
Methods
Study design and ethics
We conducted a multi-institutional prospective study to compare the postoperative QOL between patients who underwent DG and PPG for pathological Stage I GC. The internal review boards of all participating institutions reviewed and approved the scientific and ethical validity of the protocol (University Hospital Medical Information Network (UMIN) Clinical Trial Registry, UMIN000021131 [(http://www.umin.ac.jp/ctr/index.htm]).
Patient selection
The patient selection criteria were as follows: (1) histologically confirmed stomach adenocarcinoma; (2) clinical Stage IA (T1N0) or IB (T1N1, T2N0) with a tumor as defined by the Japanese Classification of Gastric Carcinoma [10]; (3) planned DG or PPG as defined by the Japanese Gastric Cancer Treatment Guidelines; (4) not indicated for endoscopic mucosal resection or endoscopic submucosal dissection (EMR/ESD); (5) age range 20–80 years old; (6) Eastern Cooperative Oncology Group Performance Status 0 or 1; (7) no history of gastrointestinal surgery; and (8) written informed consent provided.
The exclusion criteria were as follows: (1) patients undergoing salvage surgery after EMR or ESD, (2) patients with other active malignancies, (3) simultaneous surgery other than cholecystectomy, and (4) any other condition judged unsuitable for inclusion at the investigator’s discretion. Patients diagnosed with pathological Stages II–IV were excluded. The eligibility criteria were amended as of March 2017 to include patients with pathological stage II-III GC.
Surgical procedure and perioperative treatment
The surgical procedure was selected based on institutional policy as well as the patient’s choice. PPG was performed for cT1N0 tumors located in the middle portion of the stomach when the distal tumor border was ≥ 4 cm proximal to the pyloric ring according to the Japanese Gastric Cancer Treatment Guidelines in all institutions The final decision regarding the application of DG or PPG was made at the surgeon’s discretion during surgery. D1 + lymphadenectomy was performed for clinical T1N0 GC, and D2 lymphadenectomy was performed for clinical T2N0 or T1N1 GC, as defined by the Japanese Gastric Cancer Treatment Guidelines 2021 (ver.6) [11]. In the case of PPG, the infrapyloric artery (IPA) and vein (IPV), right gastric artery (RGA) and vein (RGV), and hepatic and pyloric branches of the vagal nerve (IPN) were preserved.
Preoperative blood transfusions were not administered, and the study protocol did not include oral intake or postoperative nutritional support. Follow-up included physical examinations, blood cell counts, biochemical blood tests, tumor marker analyses, and diagnostic imaging. Patients underwent ultrasonography or computed tomography and endoscopy at 6- and 12-month intervals, respectively, until postoperative year 5.
Assessing the body weight and QOL
Body weight was measured before surgery and annually for three years after surgery. Patients who required conversion from DG to PPG were included in the PPG group. We used the EORTC QLQ-C30 and PGSAS-37 questionnaires to assess the baseline and postoperative QOL. Patients were asked to complete the questionnaires before surgery and at 1, 3, 6, 12, and 36 months after surgery. The postoperative QOL was compared according to changes in preoperative values. Postoperative QOL surveillance was performed at the registration center. Questionnaires were sent directly from the data center to all patients after surgery and returned to the registration center after completion.
The EORTC QLQ-C30 comprises the global health status, functional scales, and symptom scales [12]. All raw scales were converted into scores ranging from 0 to 100 [12]. A high score on a functional scale represents a high and healthy functional level, and a high score for the global health status and QOL represents a favorable QOL. In contrast, a high score on a symptom scale or item indicates a high level of symptoms and associated problems [12].
PGSAS-37 is an integrated questionnaire specifically designed to assess the postoperative symptoms and QOL after gastrectomy. Main outcome measures of the PGSAS-37 comprise symptom subscales, living-status scales, and QOL scales [13,14,15]. The total symptom score was calculated based on the average of the seven symptom scales. High PGSAS-37 questionnaire scores represent favorable outcomes regarding ingested amount of food per meal and the quality of ingestion subscale, whereas low scores on symptom subscales, such as the necessity for additional food, the ability to work, and QOL scales, indicate favorable outcomes.
Propensity score matching (PSM)
We used PSM to adjust for the essential variables for the comparative analyses that followed. Propensity scores were estimated using a logistic regression model based on age, sex, tumor localization (L or other), clinical T (T1 or others), clinical N (0 or others), and reconstruction method (Billroth I or others). One-to-one matching without replacement was performed with a 0.1 caliper width, and the resulting score-matched pairs were used in the subsequent analyses.
Study endpoints
The primary objective was an exploratory comparison between the PPG and DG of the dumping subscale after surgery using the PGSAS-37 questionnaire. The other exploratory endpoints included the following variables: other patient-related outcomes evaluated through the QLQ-C30 and PGSAS-37, body weight loss rates compared with baseline, operating time, blood loss, postoperative complication rates stated in the Clavien-Dindo classification [16], and the number of harvested lymph nodes.
Sample size
The present exploratory study employed a 30/group sample size, which we considered the maximum number that could be acquired during the enrollment period.
Statistical analyses
Comparisons of continuous variables were conducted using Student’s t-test, and Fisher’s exact test was used to compare the values of categorical variables. Statistical adjustments for multiplicity were not performed because this was a hypothetical exploratory analysis. P < 0.05 indicated a significant difference between the datasets. Cohen’s d was calculated according to the guidelines of the PGSAS program. Interpretation of effect sizes was as follows: < 0.2 = small, 0.2 to < 0.5 = medium, and 0.5 to 0.8 = large in Cohen’s d.
Statistical analyses were performed using the JMP software program (ver. 16; SAS Institute, Inc., Cary, NC, USA) and SAS (ver. 9.4; SAS Institute, Inc., Cary, NC, USA) as well as R version 4.2.2.
Results
Patients
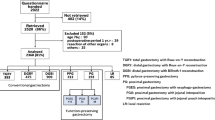
Between March 2016 and September 2019, 295 patients from 15 institutions were enrolled, including 263 and 32 patients planned for DG or PPG, respectively (Fig. 1). We excluded 49 patients from those planned for DG for the following reasons: 19 were diagnosed with pathological stages II–IV before the protocol amendment, 17 did not submit questionnaires, 4 experienced disease recurrence, 2 were converted to total gastrectomy, 2 received postoperative adjuvant chemotherapy, and 5 did not fulfill the eligibility criteria. In addition, surgery for three patients were converted to PPG. Six patients were excluded from the patients who were planned for PPG (three did not submit questionnaires, data were not collected for two, and one patient experienced disease recurrence). Thus, we analyzed 211 patients who eventually underwent DG (the DG group) and 29 patients who underwent PPG (the PPG group). Table 1 summarizes the baseline characteristics of the patients before and after PSM. After matching, patients with tumors located in the L region, cT1/cN0, and reconstruction using the Billroth I method were included in the DG group, and 25 pairs of patients were used in subsequent analyses (Fig. 1, Table 1 and Supplemental Table 1).
Perioperative factors
Supplemental Table 1 shows the surgical approach, intraoperative findings, postoperative outcomes, and pathological stages after matching. All patients underwent laparoscopic surgery, and D1 + lymphadenectomy was performed in 24 patients (96%) in both the groups. The operation time was longer in the PPG group than in the DG group (303.4 ± 60.3 min and 265.2 ± 63.6 min, P = 0.034, respectively). There were no significant differences in blood loss, resected lymph nodes, postoperative complications, or postoperative hospital stay between the two groups (Supplemental Table 1). Operative PPG data were collected for 23 patients (92%). Regarding the size of the proximal gastric remnant, 4 patients (17%) retained more than half of the stomach, 18 (78%) retained approximately one-third of the stomach, and 1 (5%) retained less than one-quarter of the stomach. In terms of the length of the pyloric cuff, 4 patients (17%) had ≤ 3.0 cm, 11 (48%) had 3.1–4.9 cm, and 8 (35%) had ≥ 5.0 cm. The celiac branch of the vagal nerve was preserved in 3 patients (13%), and the hepatic branch of the vagal nerve was preserved in 17 patients (74%) (Supplemental Table 2).
Postoperative changes in body weight
Figure 2 shows the postoperative changes in body weight before and after PSM. Body weight loss was more prominent among patients in the DG group before matching than PPG, and the differences were significant at postoperative years (POY) 1 and 2. The body weights of both groups declined to their lowest levels on POY 3. After matching, the body weights of both groups declined to the lowest levels at POY 1 (POY 1; − 4.7% ± 6.7% and − 5.7% ± 5.8%, P = 0.624, respectively) and recovered to the preoperative level at POY 2. No significant postoperative differences were observed between the groups at any time point.
A comparison of postoperative changes in the QOL after matching
Figure 3 shows that the nausea and vomiting scores of the EORTC QLQ-C30 declined to their lowest levels on POM 1 and recovered by POY 3 in both groups. The PGSAS-37 scores of the esophageal reflux and meal-related distress subscales declined to their lowest levels on POM 1. The scores subsequently recovered, although they did not reach the baseline level on POY 3 in either group (Fig. 3). Table 2 summarizes the comparison of the scores of the EORTC QLQ-C30 parameters after PSM at POM 1, 6, and POY 3. The EORTC QLQ-C30 scores for social and emotional functioning on POM 1 tended to be higher (i.e. a better QOL) in the DG group than in the PPG group. Nausea and vomiting scores on POM 1 were lower (i.e. a better QOL) in the DG group than in the PPG group. There were no significant postoperative differences between the groups at 6 months or 3 years. The results of the PGSAS-37 symptom survey after PSM at POM 1, 6, and POY 3 are summarized in Table 3. The meal-related distress, indigestion, and dumping subscales were higher (i.e. a worse QOL) in the PPG group at POM 1 than in the DG group. The esophageal reflux subscale score tended to be higher (i.e. a worse QOL) in the PPG group at POM 1 than in the DG group (P = 0.069).
Discussion
Recent advances in the management of GC have led investigators in the Far East to seek improvements in QOL outcomes after curative surgery for GC, as well as safety and oncological issues. Park et al. reported that postoperative complications and mortality were comparable in patients who underwent laparoscopic PPG (LPPG) and laparoscopic DG (LDG) in a multicenter RCT trial [17]. In the present study, we employed the EORTC QLQ-C30 and PGSAS-37 assessment questionnaires to investigate the benefit of PPG, a function-preserving surgery, compared with conventional DG, through the evaluation of the postoperative QOL. However, we failed to detect a benefit of PPG associated with various QOL-related scores, including dumping subscale scores, which were a major interest of the current study, particularly in view of the current understanding that PPG preserves the pyloric function and prevents symptoms associated with dumping syndrome.
According to the Japanese Gastric Cancer Treatment Guidelines, PPG is recommended for cT1N0 tumors located in the middle portion of the stomach when the distal tumor border is ≥ 4-cm proximal to the pyloric ring [11]. We expected to identify several advantages of PPG over DG, as the former is clearly a technically more demanding surgical procedure than the latter. In the present study, the operating time of PPG was 12 min longer than that of DG, although the incidence of postoperative complications (any grade) after PPG and DG did not differ significantly (7% and 8%, respectively). There were no significant differences in blood loss, resected lymph nodes, or length of postoperative hospital stay between the two groups. However, it was found that important clinical characteristics (e.g. tumor location and clinical stage) for assessing the postoperative QOL and body weight changes were unexpectedly different between the DG and PPG groups. Furthermore, it is reasonable to compare patients who underwent reconstruction using the same route of food passage. Therefore, we performed PSM to adjust for essential variables including age, sex, tumor localization (L or the other), clinical T (T1 or the others), clinical N (0 or the others), and reconstruction method (Billroth I or others) for the comparison analyses. Matching successfully enabled a comparison between the DG and PPG groups with similar clinical characteristics.
PPG generally involves preservation of the pyloric cuff, which helps the stomach maintain its role as a reservoir and prevents postgastrectomy syndrome that occurs owing to the rapid transport of ingested food into the jejunum [3]. However, the pylorus may not necessarily function as desired in the early postoperative phase. For example, Imada et al. reported that caloric intake after PPG significantly increased one year after surgery compared to one month postoperatively, suggesting that oral food intake may initially be adversely affected by delayed gastric emptying (DGE) inherent to PPG [18]. Furthermore, evidence indicates that the size of the remnant stomach and its reservoir function contribute to reduced body weight loss after PPG compared to DG [19]. However, in this study, there were no significant differences in the postoperative body weight changes between the PPG and DG groups at any time point. In addition, the superiority of the postoperative long-term QOL in PPG cases compared to DG has not been demonstrated.
The advantages of preserving the vagal nerve branches and infrapyloric vessels during PPG have been discussed in previous studies. Nunobe et al. revealed that preserving the vagal nerve helped avoid DGE [20]. In contrast, Furukawa et al. reported that the incidence of postoperative DGE, observed early after PPG, did not depend on whether or not the vagal nerve branches were preserved [18, 21]. Namikawa et al. reported that preservation of the pyloric branch of the vagal nerve leads to complaints of nausea, whereas patients who do not receive preservation tend to complain of late dumping symptoms [22]. Other retrospective studies postulate that preserving the infrapyloric vein avoids venous stasis and effectively prevents DGE because edema of the antrum due to venous stasis and inflammation causes DGE [23, 24].
Thus, many researchers agree that preservation of relevant nerves and vessels could contribute to the avoidance of postoperative gastric dysfunction, dumping syndrome, and other unpleasant symptoms [3]. In the present study, the IPA, IPV, and IPN were sacrificed in a significant proportion of patients who underwent PPG (data not shown), which induced DGE and likely resulted in the inferior scores associated with esophageal reflux and meal-related distress at POM 1 [25]. However, these symptoms are transient and do not manifest as differences in scores after postoperative month three.
If the preoperative scores of functions fluctuate, there is a risk that postoperative functions may be over- or underestimated. Therefore, we enrolled patients who underwent gastrectomy for clinical Stage I GC so that most patients were expected to be asymptomatic. Accordingly, we evaluated the QOL scores at each time point according to the changes compared with the preoperative values.
Several limitations associated with the present study warrant mention. First, this study was prospective and observational, and the surgical procedures were selected at the discretion of the surgeons. Second, PPG is indicated for clinical T1-stage cancers in the middle-third of the stomach and is only conducted in specialized centers, which may introduce potential selection bias because surgeons with greater expertise in GC surgery may favor PPG. Third, preservation of the hepatic and pyloric branches of the vagus, IPA, and IPV during PPG was not prescribed in the present study’s protocol. Consequently, the surgical technique for PPG may not be uniform, and some surgeries may have been suboptimal. Fourth, the nutritional status was evaluated based on body weight only. More detailed measurements are therefore required. Fifth, body weight data were collected only at baseline and one, two, and three years postoperatively in the CCOG1601 study. Finally, adjustments for multiplicity were not conducted because of the exploratory analysis. A larger sample size is required to perform this adjustment.
Conclusions
There was little benefit of PPG compared to DG regarding postoperative body weight loss and the long-term QOL, including dumping syndrome.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Kajiwara Y, Takahashi A, Ueno H, Kakeji Y, Hasegawa H, Eguchi S, et al; National Clinical Database. Annual report on National Clinical Database 2020 for gastroenterological surgery in Japan. Ann Gastroenterol Surg. 2023;7(3):367–406.
Terayama M, Ohashi M, Makuuchi R, Hayami M, Ida S, Kumagai K, et al. A continuous muscle-sparing advantage of pylorus-preserving gastrectomy for older patients with cT1N0M0 gastric cancer in the middle third of the stomach. Gastric Cancer. 2023;26(1):145–54.
Fujishiro M, Yoshida S, Matsuda R, Narita A, Yamashita H, SetoY. Updated evidence on endoscopic resection of early gastric cancer from Japan. Gastric Cancer. 2017;20(Suppl 1):39–44.
Wee HL, Canfell K, Chiu HM, Choi KS, Cox B, Bhoo-Pathy N, et al. Cancer screening programs in South-east Asia and Western Pacific. BMC Health Serv Res. 2024;24(1):102.
Hiki N, Sano T, Fukunaga T, Ohyama S, Tokunaga M, Yamaguchi T. Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. J Am Coll Surg. 2009;209:297–301.
Maki T, Shiratori T, Hatafuku T, Sugawara K. Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery. 1967;61(6):838–45.
Park DJ, Lee HJ, Jung HC, Kim WH, Lee KU, Yang HK. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg. 2008;32(6):1029–36.
Nunobe S, Hiki N, Fukunaga T, Tokunaga M, Ohyama S, Seto Y, et al. Laparoscopy-
assisted pylorus-preserving gastrectomy. preservation of vagus nerve and infrapyloric blood flow induces less stasis. World J Surg. 2007;31(12):2335–40.
Japanese Gastric Cancer Association: Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011 ;14(2):101–12.
Association JGC. Japanese Gastric Cancer Treatment Guidelines 2021 (ver 6). Gastric Cancer. 2023;26(1):1–25.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 3;85(5):365–76.
Nakada K, Ikeda M, Takahashi M, Kinami S, Yoshida M, Uenosono Y, et al. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer. 2015;18(1):147–58.
Ikeda M, Takiguchi N, Morita T, Matsubara H, Takeno A, Takagane A, et al. Quality of life comparison between esophagogastrostomy and double tract reconstruction for proximal gastrectomy assessed by Postgastrectomy Syndrome Assessment Scale (PGSAS)-45. Ann Gastroenterol Surg. 2022;7(3):430–40.
Tanaka C, Kanda M, Murotani K, Yoshikawa T, Cho H, Ito Y, et al. Long-term quality of life and nutrition status of the aboral pouch reconstruction after total gastrectomy for gastric cancer: a prospective multicenter observational study (CCOG1505). Gastric Cancer. 2019;22:607–16.
Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–26.
Park DJ, Kim Y-W, Yang H-K, Ryu KW, Han S-U, Kim H-H, et al. Short-term outcomes of a multicentre randomized clinical trial comparing laparoscopic pylorus-preserving gastrectomy with laparoscopic distal gastrectomy for gastric cancer (the KLASS-04 trial). Br J Surg. 2021;108(9):1043–9.
Imada T, Rino Y, Takahashi M, Hatori S, Tanaka J, Shiozawa M, et al. Gastric emptying after pylorus-preserving gastrectomy in comparison with conventional subtotal gastrectomy for early gastric carcinoma. Surg Today. 1998;28(2):135–8.
Tsujiura M, Hiki N, Ohashi M, Nunobe S, Kumagai K, Ida S, et al. Excellent Long-Term Prognosis and Favorable Postoperative Nutritional Status After Laparoscopic Pylorus-Preserving Gastrectomy Ann Surg Oncol. 2017;24(8):2233–40.
Nunobe S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer. 2007;10(3):167–72.
Furukawa H, Ohashi M, Honda M, Kumagai K, Nunobe S, Sano T, et al. Preservation of the celiac branch of the vagal nerve for pylorus-preserving gastrectomy: is it meaningful? Gastric Cancer. 2018;21(3):516–23.
Namikawa T, Hiki N, Kinami S, Okabe H, Urushihara T, Kawahira H, et al. Factors that minimize postgastrectomy symptoms following pylorus-preserving gastrectomy: assessment using a newly developed scale (PGSAS-45) Gastric Cancer. 2015;18(2):397–406.
Kiyokawa T, Hiki N, Nunobe S, Honda M, Ohashi M, Sano T. (2017) Preserving infrapyloric vein reduces postoperative gastric stasis after laparoscopic pylorus-preserving gastrectomy. Langenbecks Arch Surg. 2017;402(1):49–56.
Kaji S, Makuuchi R, Irino T, Tanizawa Y, Bando E, Kawamura T, et al. Preventive effect on delayed gastric emptying of preserving the infra-pyloric vein in laparoscopic pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc. 2020;34:3853–60.
Fukaya M, Abe T, Nagino M. Rapid progressive long esophageal stricture caused by gastroesophageal reflux disease after pylorus-preserving pancreatoduodenectomy. BMC Surg. 2016;18:16–9.
Acknowledgements
We thank Edanz (https://jp.edanz.com/ac) for editing this manuscript. We are also grateful to Shinya Koike (Atsumi Hospital), Katsuya Yamashita (Toyohashi Medical Center), and Ryoji Hashimoto (Nakatsugawa Municipal Hospital) for their participation in the CCOG1601 study.
Author information
Authors and Affiliations
Contributions
CT, MK, KM, and YK contributed to study design. TW, KI, SS, HT, AI, IN, and HM contributed to the data collection and management. KM, YM, and MH contributed to data collection, data management, and interpretation. KM contributed to the statistical analysis. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest (COIs).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanaka, C., Kanda, M., Misawa, K. et al. The long-term quality of life after distal and pylorus-preserving gastrectomy for stage I gastric cancer: A prospective multi-institutional study (CCOG1601). Surg Today (2024). https://doi.org/10.1007/s00595-024-02881-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00595-024-02881-3