Abstract
Background
Pylorus-preserving gastrectomy (PPG) has the postoperative advantages of a better quality of life and less weight loss than distal gastrectomy. However, postoperative delayed gastric emptying (DGE) due to antral hypomotility can be a problem. Although preserving the infra-pyloric vein (IPV) is reported to improve congestion of the antrum and prevent DGE, the benefits of this procedure have not been confirmed. The present study aimed to clarify the preventive effect on DGE of preserving the IPV.
Methods
A total of 148 patients [IPV-preserved (IPVP): 78 patients and IPV-non-preserved (IPVN): 70 patients] who underwent laparoscopic and robotic PPG (LRPPG) for early gastric cancer were enrolled in this study. The clinicopathologic characteristics and incidence of DGE were compared between the groups. The nutritional risk index (NRI) at 1, 2, and 3 years after the operation and the relapse-free survival (RFS) were also compared.
Results
There were no significant differences in the clinicopathological characteristics between the two groups. DGE was observed in 15 of 148 patients (10.1%). The incidence of DGE did not differ markedly between the 2 groups (IPVP vs. IPVN; 11.5% vs. 8.6% p = 0.596). There were no significant differences in other complications between the groups either (IPVP vs. IPVN; 19.2% vs. 21.4%; p = 0.838). The NRI and 3-year RFS were not significantly different between the two groups.
Conclusion
Regarding LRPPG, preserving the IPV did not help prevent DGE and resulted in no significant difference in the outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pylorus-preserving gastrectomy (PPG), first reported by Maki et al. [1], was originally performed on patients who suffered from benign gastric ulcer. In 1991, Kodama et al. introduced this procedure for the treatment of early gastric cancer in order to prevent post-gastrectomy syndrome [2]. Recently, PPG has been conducted on patients with early gastric cancer located mainly in the middle or lower third of the stomach [3, 4]. By preserving the pyloric function, PPG is considered to have great advantages over conventional distal gastrectomy, such as preventing gastritis due to bile reflex, dumping syndrome, diarrhea, and excessive body weight loss [5, 6]. In order to preserve the pyloric function, a pyloric antrum cuff length > 4 cm, preserving the hepatic and pyloric branches of the vagus nerve and preserving the right gastric artery and infra-pyloric artery (IPA), have been reported to be important [7,8,9].
Nevertheless, early postoperative delayed gastric emptying (DGE) has been reported to be a serious disadvantage of PPG [8, 10,11,12,13,14]. The incidence of DGE has been reported to range from 6.3 to 40%. Several studies have reported that preserving the infra-pyloric vessels, including the infra-pyloric vein (IPV) and IPA, can help prevent DGE by supplying a sufficient amount of blood and ensuring appropriate drainage in the antrum [14, 15]. Based on these findings, several surgeons in Japan have carefully preserved these vessels during PPG. Recently, the demand for laparoscopic surgery has increased because of its low invasiveness and better postoperative course than that for open gastrectomy [12,13,14,15]. However, laparoscopic gastrectomy remains technically demanding, and preserving the IPV in laparoscopic surgery requires a high degree of surgical skill. More meticulous operations, such as robotic surgery, make it easier to perform IPV preservation. However, whether or not this technically demanding procedure is actually useful for preventing DGE after PPG has yet to be confirmed.
The aim of this study was to evaluate the role of IPV preservation in addition to preserving the IPA on preventing DGE in patients undergoing laparoscopic or robotic PPG (LRPPG).
Patients and methods
Patients
A total of 153 patients who underwent LRPPG for gastric cancer from January 2010 to December 2014 in Shizuoka Cancer Center were enrolled. Five patients who underwent a delta-shaped anastomosis were excluded from the study. Therefore, we reviewed a total of 148 patients in this study. In accordance with the Japanese Gastric Cancer Treatment Guidelines (ver.4) [4], the surgical indications of LRPPG were clinical intra-mucosal or submucosal carcinoma without lymph nodes metastasis in the middle or lower third of the stomach. An antral cuff was required when a 2-cm distal margin was secured. The clinical diagnosis of tumor depth and metastasis of lymph nodes were determined based on the findings of preoperative imaging, including computed tomography (CT), upper gastrointestinal series, and endoscopy.
The clinical and surgical records of all patients were retrieved from a prospectively collected database or electronical medical records. The pathologic findings were determined according to the seventh edition of the American Joint Committee on Cancer (AJCC) TNM classification [16]. Postoperative morbidity was evaluated using the Clavien–Dindo classification [17].
This study was approved by the Human Ethics Review Committee of Shizuoka Cancer Center (29-J157-29-1-3).
Surgical procedure
LRPPG with D1+ lymphadenectomy was performed via laparoscopy or by the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) with four robotic arms. We routinely performed endoscopic marking at both the proximal and distal side of the tumor with 1-ml injections of India ink and clipping with endoclips at 1 to 3 days before surgery.
Laparoscopic surgery was performed using 5-port methods as described previously [18]. Pneumoperitoneum was established with carbon dioxide (pressure: 10 mmHg). Robotic surgery was also performed as previously described [19]. The greater omentum was preserved and the gastrocolic ligament was divided at least 4 cm from the gastroepiploic vessels. The lymph nodes at stations 1, 3, 4sb, 4d, 6 and 7, 8a, and 9 (according to Japanese Classification of Gastric Carcinoma [4]) were dissected. The lymph nodes of the left side, along the right gastric artery (station 5), were usually resected in order to preserve the supra-pyloric artery vein and pyloric branch of the vagal nerve.
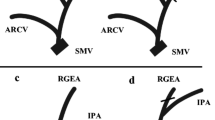
Initially, we did not preserve the IPV; however, from 2010, we changed the procedure in order to preserve the IPV whenever possible. Patients with IPV preservation were defined as the IPVP group, and those without were defined as the IPVN group.
When preserving the IPV, the adipose tissues, including the station 6 lymph nodes, were dissected along with the right gastroepiploic vein (RGEV). The RGEV was then divided after the branching of the IPV. In the IPVN group, the RGEV was divided at the root after the branching of the anterior superior pancreaticoduodenal vein (ASPDV). In both groups, the right gastroepiploic artery (RGEA) was divided after the branching of the IPA. The right gastric artery was preserved and divided after the branching of one or two stomach branches. In principle, the hepatic branch and pyloric branch of the anterior vagus nerve were preserved. The celiac branch of the vagus nerve was preserved whenever possible.
After lymph node dissection and the mobilization of the stomach, the distal transecting margin (guided by distal marking) was confirmed in order to secure at least 4 cm from the pylorus.
In cases of intra-corporeal anastomosis, both the distal and proximal sides of the stomach were transected with linear staplers. Regarding gastro–gastro-anastomosis, the hybrid technique using a linear stapler and manual suturing, as reported by Koeda et al. [13], was used for intra-corporeal anastomosis. In brief, an entry hole for the stapler was made at the greater curvature end. The posterior walls of both remnant stomachs were stapled with a 60-mm linear stapler. The anterior wall was then sutured via a layer-to-layer hand-sewn technique using absorbable barbed sutures.
For extra-corporeal anastomosis, a 6-cm midline incision was made on the epigastrium. Both the distal and proximal parts of the stomach were then clamped with forceps and resected. Subsequently, gastro–gastro-anastomosis was performed using vertical mattress suturing to close the posterior wall and the Gambee suture technique to close the anterior wall.
The definition and evaluation of postoperative complications, including DGE
The grade of postoperative complication was assessed using the Clavien–Dindo classification [17]. Complications of Clavien–Dindo classification grade ≥ II occurring within 30 postoperative days were regarded as postoperative complications. DGE was also determined by the Clavien–Dindo classification. Patients with postprandial symptoms, such as upper abdominal distension, nausea, or vomiting, accompanied by retention of the remnant stomach on abdominal X-ray were diagnosed with DGE if they required oral medications with gastrointestinal motility regulators.
Nutritional assessments
In order to assess the postoperative nutritional status, the nutrition risk index (NRI) was evaluated at 12 and 36 months after gastrectomy in accordance with a previous description [20]. The NRI was calculated using the following formula: NRI = (1.519 × serum albumin, g/L) + (41.7 × current weight/usual weight). The serum albumin level and current or usual weight were recorded preoperatively and at 1, 2, and 3 years after gastrectomy.
Survival analyses
The relapse-free survival (RFS) was calculated from the date of gastrectomy to the date on which recurrence was first diagnosed or the death of the patient due to some cause.
Statistical methods
The categorical variables of patient’s characteristics were analyzed using Fisher’s exact test. Continuous variables were evaluated by Mann–Whitney’s U test and expressed as the median and interquartile range (IQR). The risk factors of DGE were analyzed utilizing Fisher’s exact test. P < 0.05 was regarded as statistically significant. The RFS was calculated by the Kaplan–Meier method. An analysis of variance (ANOVA) was applied to the NRI. Multiple comparisons were corrected using Bonferroni’s correction. All statistical analyses were conducted using the R Statistics software program, version 3.2.3 [R Foundation, Vienna, Austria].
Propensity score matching was performed to remove covariates associated with the presence of postoperative complications. The patients’ propensity scores were calculated using a logistic regression model based on age, sex, surgical approach (laparoscopic or robotic assisted), ASA-PS, and whether or not the celiac branch of the vagus nerve was preserved by the SAS 9.4 software program (SAS Institute, Cary NC). Patients in the IPVP and IPVN groups were matched 1:1 using the nearest propensity score on the logit scale.
Results
Patient characteristics
A total of 148 patients were included in this study: 78 of whom received IPVP, while the remaining 70 received IPVN. The characteristics of these patients are presented in Table 1. There were no significant differences in the patients’ characteristics between the two groups.
Surgical treatment and pathological results
Operative data are shown in Table 2. Celiac branches of the vagus nerve were more frequently preserved in IPVP than in IPVN. The operation time was longer in IPVP than in IPVN. Blood loss was significantly larger in IPVN than in IPVP. The pathological findings are listed in Table 3. The total number of harvested lymph nodes was significantly larger in IPVP than in IPVN; however, the number of harvested lymph nodes in station 6 was not markedly different between the groups.
Incidence of early surgical complications
The incidence of postoperative complications of Clavien–Dindo classification grade ≥ II was similar between the groups (Table 4). There were also no significant differences in the incidence of DGE between the groups. Consequently, the median postoperative stay was almost the same between the groups. After propensity score matching, there were no significant differences in the characteristics (Suppl. Table 1) or surgical outcomes (Suppl. Table 2) of the two groups of patients. Furthermore, there was no difference between the two groups in the incidence of postoperative complications, including DGE (Suppl. Table 3).
The risk factors of DGE were investigated (Table 5). No factors were found to be associated with the occurrence of DGE, including IPV preservation.
Nutritional status after gastrectomy
Changes in the NRI at pre-operation and 1, 2, and 3 years after the operation are shown in Fig. 1. The NRI declined after the operation and remained unchanged until 3 years after the operation in both groups. There were no significant differences in the NRI between the IPVP and IPNV groups.
Survival analyses
The median follow-up period was 55 months (range 6–97 months). There were no patients who died of gastric cancer by 3 years after gastrectomy. Three patients died of other cancer in the IPVN group, while two patients (one each) died of cerebrovascular disease and a drowning accident in the bath in the IPVP group. There were no marked differences in the 3-year RFS rates between the groups (Fig. 2). Two patients suffered from remnant gastric cancer over 3 years after gastrectomy in the IPVP group.
Discussion
We found no significant effect of IPV preservation on preventing DGE. Regarding the role of the preservation of the IPA, Nunobe et al. showed that preserving the IPA as well as the right gastric artery and vein have contributed to a low incidence (6% and 8%) of DGE [11]. However, the role of preserving the IPV is controversial. Kiyokawa et al. reported that preserving the IPV to avoid venous stasis was effective in preventing DGE, as edema of the antrum due to venous stasis and inflammation may be one cause of DGE [14]. However, Nishizawa et al. reported that there was no marked difference with respect to the incidence of DGE in patients with and without IPV preservation [21]. Therefore, preserving the blood stream of the antrum by preserving the IPA might be important for preventing DGE in patients who receive PPG. However, further preventive effects by preserving the IPV are questionable.
Anatomically, the peripheral side of IPV accompanies the IPA along the proximal gastric wall. However, the central side of IPV is positioned apart from the IPA in front of the pancreatic head and flows into the right gastric epiploic vein or anterior superior pancreatic duodenal vein [21,22,23]. Given this anatomical situation, the vein surrounding the stomach wall appears to have many variations and a complicated network. As a result, the vein may have drained through other venous branches or into the intramural blood flow of the remnant stomach even if the main trunk of IPV was not preserved.
In addition, we evaluated the influence of IPV preservation on the postoperative nutritional status and survival. If IPV preservation does indeed affect the postoperative gastric motility and engenders a positive effect on the digestive function after gastrectomy, the nutritional status would be expected to be better in the IPVP group than in the IPVN group. However, the postoperative nutritional status was not markedly different between the groups, with both showing postoperative NRI scores of > 97.5, which is considered the cut-off level for malnutrition [20]. This result suggests that the postoperative gastric function was well-preserved regardless of the preservation of the IPV. Furthermore, in the survival analysis, no patients died of gastric cancer, indicating the oncological feasibility of both methods.
From the perspective of pathophysiology, remnant gastric hypomotility is a general cause of DGE [24,25,26,27,28,29]. In a normal stomach, gastric motility propagation with food trituration is mediated through the smooth muscle cells, which control stomach contraction; the interstitial cells of Cajal, which regulate the gastric pacemaker activity; and the intramural nerves, which initiate the smooth muscle cell activity. However, when the stomach wall is segmented, as in distal gastrectomy or PPG, gastric motility propagation is disturbed.
The pyloric branch of the vagus nerve plays an important role in controlling tonic and phasic pyloric contractions. Therefore, preserving the pyloric branch of the vagal nerve is necessary for ensuring the pyloric function [24]. Generally, if the right gastric artery is preserved, the pyloric branch of the vagal nerve is necessarily preserved. Lu et al. compared two animals’ models of resected and preserved pyloric branch and found that the pyloric sphincter did not work without the pyloric branch [24]. Regarding symptoms after PPG, Namikawa et al. compared two groups of patients with and without preservation of the pyloric branch. Although no significant differences were noted, the patients without preservation tended to experience late dumping symptoms [27].
Regarding the role of preservation of the celiac branch of the vagus nerve for post-gastrectomy syndrome, Kim et al. found that preserving this branch was associated with lower rates of diarrhea and appetite loss after surgery [30]. In order to prevent post-gastrectomy syndrome, we aimed to preserve this branch as far as possible. While Furukawa et al. concluded that preserving this branch did not prevent the incidence of post-gastrectomy syndrome, including DGE after LPPG [31]. In this study, although the celiac branch of the vagus nerve was more frequently preserved in the IPVP group than in the IPVN group, the incidence of DGE in the two groups did not differ to a statistically significant extent. In addition, the incidence did not differ after propensity score matching according to celiac branch preservation well. Thus, it is suggested that preserving the celiac branch of vagus nerve did not contribute to the avoidance of DGE.
Concerning the antral cuff length, we carefully left at least 4 cm. In their initial report, Maki et al. described leaving an antral cuff length of 1.5 cm [1]. However, Nakane et al. reported that the incidence of DGE was lower in the patients with an antral cuff length of 2.5 cm than in those with a shorter antral cuff [25]. In addition, Namikawa et al. found that the post-gastrectomy symptoms, including symptoms of DGE, were least frequent in the patients with an antral cuff length of 3.0–5.0 cm [27]. Therefore, the length of the antral cuff must be at least 3 cm in order to prevent the DGE.
When we applied IPV preservation, we expected that incidence of DGE would be reduced. However, the incidence of DGE was not markedly different, regardless of IPV preservation, despite a relatively complicated procedure with a prolonged operation time. Furthermore, the amount of blood loss was significantly smaller in the IPVP group than in the IPVN group. The apparent inconsistency of these results may be due to the different time periods in which these procedures were performed. IPVP was performed in the later period of our experience, so improvements in the technique may have reduced the amount of blood loss. In this sense, the operation time was not decreased despite improvements in the technique. While using the laparoscopic scope and robotic surgical scope, we can visually recognize the detailed anatomical structures with a magnified view and thus are able to perform minute operations. Nevertheless, preservation of the IPV remains a technically demanding and complicated procedure. The findings of the present study do not support the preservation of the IPV in LRPPG.
Based on the results of the present study, our current procedure for PPG involves preserving the IPA and supra-pyloric artery, vein and nerves but not preserving the IPV. Preserving the celiac branch of the vagal nerve is optional.
From an oncological perspective, the number of station 6 nodes dissected was almost the same in the IPVP and IPVN groups. Kiyokawa et al. [14] also reported that the number of harvested lymph nodes in station 6 was not markedly different between procedures with and without IPV preservation. The total number of lymph nodes harvested was higher in the IPVP group than in the IPVN group, possibly due to the different time periods in which these procedures were performed, as was suggested to explain the differences in the volume of blood loss.
Finally, in this study, DGE was observed in 15 of 148 patients (10.1%). In general, the incidence of DGE has been reported to range from 6.3 to 40% [8, 10,11,12,13,14]. This relatively wide range of reported incidences of DGE may be mainly due to differing definitions of DGE rather than to different operative procedures, such as preservation (or lack thereof) of the IPA, IPV or pyloric branch of the vagal nerve. Some investigators diagnosed DGE based on symptoms of nausea, epigastric fullness, or a poor oral intake, while others made diagnoses based on radiological findings, including upper gastrointestinal series or scintigraphy, or the presence or absence of therapeutic intervention [8, 10,11,12,13,14]. Kumagai et al. [32] defined DGE based on the presence of the following three conditions: (1) upper abdominal distension, (2) remnant stomach fullness on radiography imaging, and (3) a period of starvation exceeding 24 h. Under this definition, it has been reported that the incidence of DGE was 10% (10/60). However, Morita et al. [8] defined DGE in patients with specified symptoms requiring fasting and intravenous fluid support, and they reported an incidence of DGE of 8.0% (49/611). In order to benchmark the incidence of DGE, a comprehensive definition of DGE must be established. The Clavien–Dindo classification is a well-known and well-established classification system for evaluating the severity of postoperative complications mainly based on the treatment required. We applied Clavien–Dindo classification to evaluate DGE in the present study. Grade ≥ II severity means the patients required medication or supportive care with intravenous fluid replacement. We believe that this definition is reasonable for evaluating the DGE in consideration of the pathophysiology of this status.
Several limitations associated with the present study warrant mention. First, this was a retrospective study performed at a single center, so several biases may exist.
Second, robot-assisted surgery was more frequent in the IPVP group than in the IPVN group. There is a possibility that this bias may influence the results of operation time, blood loss, number of harvested lymph node, and the occurrence of DGE.
In conclusion, IPV preservation had no impact on preventing DGE in either laparoscopic or robotic PPG for early gastric cancer.
References
Maki T et al (1967) Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery 61(6):838–845
Kodama M, Koyama K (1991) Indications for pylorus preserving gastrectomy for early gastric cancer located in the middle third of the stomach. World J Surg 15(5):628–633 discussion 633-4
Kodera Y et al (2001) Lymph node metastasis in cancer of the middle-third stomach: criteria for treatment with a pylorus-preserving gastrectomy. Surg Today 31(3):196–203
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20(1):1–19
Isozaki H et al (1996) Postoperative evaluation of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg 83(2):266–269
Nishikawa K et al (2002) Functional characteristics of the pylorus in patients undergoing pylorus-preserving gastrectomy for early gastric cancer. Surgery 131(6):613–624
Shibata C et al (2012) Current status of pylorus-preserving gastrectomy for the treatment of gastric cancer: a questionnaire survey and review of literatures. World J Surg 36(4):858–863
Morita S et al (2008) Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg 95(9):1131–1135
Kodama M et al (1995) Early postoperative evaluation of pylorus-preserving gastrectomy for gastric cancer. World J Surg 19(3):456–460 discussion 461
Tomita R, Fujisaki S, Tanjoh K (2003) Pathophysiological studies on the relationship between postgastrectomy syndrome and gastric emptying function at 5 years after pylorus-preserving distal gastrectomy for early gastric cancer. World J Surg 27(6):725–733
Nunobe S et al (2007) Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer 10(3):167–172
Jiang X et al (2011) Long-term outcome and survival with laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc 25(4):1182–1186
Koeda K et al (2016) Intracorporeal reconstruction after laparoscopic pylorus-preserving gastrectomy for middle-third early gastric cancer: a hybrid technique using linear stapler and manual suturing. Langenbecks Arch Surg 401(3):397–402
Kiyokawa T et al (2017) Preserving infrapyloric vein reduces postoperative gastric stasis after laparoscopic pylorus-preserving gastrectomy. Langenbecks Arch Surg 402(1):49–56
Suh YS et al (2014) Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg 259(3):485–493
Edge SB (2010) The American Joint Committee on Cancer: the 7th of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240(2):205–213
Hikage M et al (2018) Comparison of surgical outcomes between robotic and laparoscopic distal gastrectomy for cT1 gastric cancer. World J Surg 42(6):1803–1810
Tokunaga M et al (2014) Early phase II study of robot-assisted distal gastrectomy with nodal dissection for clinical stage IA gastric cancer. Gastric Cancer 17(3):542–547
Fujiya K et al (2018) Impact of malnutrition after gastrectomy for gastric cancer on long-term survival. Ann Surg Oncol 25(4):974–983
Nishizawa N et al (2016) Anatomical knowledge for the infra-pyloric vein preservation during the laparoscopy-assisted pylorus-preserving gastrectomy. Dig Surg 33(5):363–370
Sawai K et al (1995) Pylorus-preserving gastrectomy with radical lymph node dissection based on anatomical variations of the infrapyloric artery. Am J Surg 170(3):285–288
Haruta S et al (2015) Anatomical considerations of the infrapyloric artery and its associated lymph nodes during laparoscopic gastric cancer surgery. Gastric Cancer 18(4):876–880
Lu YF et al (1999) Vagus effect on pylorus-preserving gastrectomy. World J Gastroenterol 5(2):177–178
Nakane Y et al (2002) Length of the antral segment in pylorus-preserving gastrectomy. Br J Surg 89(2):220–224
Le Blanc-Louvry I et al (2003) An impaired accommodation of the proximal stomach to a meal is associated with symptoms after distal gastrectomy. Am J Gastroenterol 98(12):2642–2647
Namikawa T et al (2015) Factors that minimize postgastrectomy symptoms following pylorus-preserving gastrectomy: assessment using a newly developed scale (PGSAS-45). Gastric Cancer 18(2):397–406
Parkman HP (2015) Idiopathic gastroparesis. Gastroenterol Clin North Am 44(1):59–68
Stein B, Everhart KK, Lacy BE (2015) Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 49(7):550–558
Han DS et al (2015) Comparison of surgical outcomes of robot-assisted and laparoscopy-assisted pylorus-preserving gastrectomy for gastric cancer: a propensity score matching analysis. Ann Surg Oncol 22(7):2323–2328
Furukawa H et al (2018) Preservation of the celiac branch of the vagal nerve for pylorus-preserving gastrectomy: is it meaningful? Gastric Cancer 21(3):516–523
Kumagai K et al (2015) Totally laparoscopic pylorus-preserving gastrectomy for early gastric cancer in the middle stomach: technical report and surgical outcomes. Gastric Cancer 18(1):183–187
Acknowledgement
We are grateful to Dr. Naotake Yanagisawa for his help in the statistical analyses, particularly propensity score matching.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Sanae Kaji, Rie Makuuchi, Tomoyuki Irino, Yutaka Tanizawa, Etsuro Bando, Taiichi Kawamura, Hayato Omori, Keiichi Fujiya, Noriyuki Nishiwaki, Kenichiro Furukawa, Kenichi Nakamura, Yusuke Koseki, Yuhei Waki, Raito Asaoka, and Masanori Terashima have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaji, S., Makuuchi, R., Irino, T. et al. Preventive effect on delayed gastric emptying of preserving the infra-pyloric vein in laparoscopic pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc 34, 3853–3860 (2020). https://doi.org/10.1007/s00464-019-07151-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-07151-9