Abstract
Purpose
Clinically relevant postoperative pancreatic fistulas (CR-POPF) occurring after distal pancreatectomy often cause intra-abdominal infections. We monitored the presence of bacterial contamination in the ascitic fluid after distal pancreatectomy to clarify the bacterial origin of intra-abdominal infections associated with CR-POPF.
Methods
In 176 patients who underwent distal pancreatectomy, ascitic fluid bacterial cultures were performed on postoperative days (POD) 1–4 and when the drainage fluid became turbid. The association between postoperative ascitic bacterial contamination and CR-POPF incidence was investigated.
Results
CR-POPF occurred in 18 cases (10.2%). Among the patients with CR-POPF, bacterial contamination was detected in 0% on POD 1, in 38.9% on POD 4, and in 72.2% on the day (median, day 9.5) when the drainage fluid became turbid. A univariate analysis revealed a significant difference in ascitic bacterial contamination on POD 4 (p < 0.001) and amylase level on POD 3–4 (p < 0.001). A multivariate analysis revealed the amylase level and ascitic bacterial contamination on POD 4 to be independent risk factors.
Conclusions
In the CR-POPF group, ascitic bacterial contamination was not observed in the early postoperative stage, but the bacterial contamination rate increased after pancreatic juice leakage occurred. Therefore, CR-POPF-related infections in distal pancreatectomy may be caused by a retrograde infection of pancreatic juice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative pancreatic fistula (POPF) is the most important complications after pancreatic surgery, with a frequency of 5–20% [1,2,3]. The International Study Group of Pancreatic Surgery introduced a standardized POPF definition [4], and Grade B/C is categorized as clinically relevant POPF (CR-POPF), which involve bacterial infection and intra-abdominal abscess formation. Some reports have shown that bacterial contamination in the ascitic fluid is detected from the first day after surgery in patients undergoing pancreaticoduodenectomy (PD), which is associated with intra-abdominal abscess formation following pancreatic leakage. Preoperative cholangitis, which often develops in patients undergoing PD, reportedly causes early bacterial contamination in the ascitic fluid after surgery [5,6,7]. On the other hand, CR-POPF, which is also associated with bacterial infection, occur in 9.0–11% of the patients undergoing distal pancreatectomy (DP) [8, 9]. However, the bacterial origin of the infections remains unclear in patients undergoing DP.
To clarify the origin of bacterial infection in patients with CR-POPF after DP, we monitored the bacterial contamination of ascitic fluid after DP. This study aimed to investigate factors that cause bacterial infection associated with CR-POPF in patients undergoing DP.
Materials and methods
Patients
A total of 176 patients who underwent DP at Tokyo Medical University Hospital from November 2011 to October 2018 were enrolled in the study. Patients with gastrectomy and/or colectomy data were excluded to avoid the effect of bacterial contamination from the intestinal juice. Their diagnoses were as follows: intraductal papillary mucinous neoplasm (n = 22, 12.5%); pancreatic neuroendocrine tumor (n = 15, 8.5%); solid pseudopapillary neoplasm (n = 5, 2.8%); pancreatic adenocarcinoma (n = 81, 46.0%); mucinous cystic neoplasm or serous cystic neoplasm (n = 27, 15.3%); and others (n = 26, 14.8%). We retrospectively reviewed the patients’ clinical data. This study was approved by the institutional review board of Tokyo Medical University (SH4036), and the need for informed consent was waived because of the retrospective study design.
Surgical procedure and postoperative management
The pancreas was basically transected using a stapler (Echelon 60 blue cartridge: Ethicon Endo-Surgery, Cincinnati, OH, USA, or iDrive Ultra Powered Stapler purple cartridge: Medtronic Japan Co. Ltd, Tokyo, Japan). If the pancreatic parenchyma was too thick to use the stapler, then the pancreas was dissected using an ultrasonic dissection device, and the main pancreatic duct was sutured with a non-absorbable monofilament thread. The stump was sealed with soft coagulation and was not sutured [10].
After resection, the abdominal cavity was washed with 1000–3000 ml of saline before inserting the drain. A 10-mm flat drain and closed-suction drains (10-mm, flat J-VAC BLAKE Silicone drain; Ethicon Inc., Somerville, NJ, USA) were inserted longitudinally into the stump of the pancreas. All patients were administered an intraoperative antibiotic prophylaxis of second-generation cephem every 3 h. In addition, second-generation cephem was routinely administered as antibiotic prophylaxis for 3 days after surgery. The drain was removed on POD 4 if the drainage fluid was clear and the amylase level of the drainage fluid was less than three times that of the serum amylase level. Bacterial cultures and amylase level analyses in the ascitic fluid from the pancreatic stump drain were performed on POD 1–4 and on the day when the drainage fluid became turbid. We defined turbid fluid as serous fluid that changed to non-serous fluid. The drainage fluid was placed in a test tube and judged by its translucency. An amylase level in the ascitic fluid more than three times that in the serum was defined as positive for pancreatic juice leakage. The International Study Group on Pancreatic Surgery definition and grading system of POPF were used to define POPF [4]. We defined grade B and C POPF as CR-POPF.
Statistical analysis
The analysis results are shown as the median ± maximum values. The Mann–Whitney U test or chi-squared test was used for comparisons between the two groups. The cutoff value for continuous variables was calculated using the receiver operating characteristic curve. The risk factors were analyzed using logistic regression analysis. A p value <0.05 was considered to be significant. The IBM SPSS Statistics 26 software program (IBM Corp., Armonk, NY, USA) was used for the statistical analyses.
Results
Pancreatic juice leakage and bacterial contamination of ascitic fluid in patients with and without CR-POPF
CR-POPF occurred in 18 patients (10.2%). There was no significant difference in the patient factors, tumor factors, or transection method between the CR-POPF (−) and CR-POPF (+) groups (Table 1).
In the CR-POPF (−) group, pancreatic juice leakage occurred in 90.5% of the patients on POD 1; however, the rate gradually decreased to 25.9% on POD 4. Bacterial contamination of the ascitic fluid was detected in only 3.8% of the patients between POD 1 and POD 4. In the CR-POPF (+) group, the incidence of pancreatic juice leakage continued to be high from POD 1 to POD 4, ranging from 88.9% to 100%. The detection rate of the bacterial contamination on POD 1 was 0%; however, it was 38.9% on POD 4 day (Table 2). The detected bacteria were Staphylococcus sp. (n = 4, 22.0%), Pseudomonas sp. (n = 2, 11.0%), and Corynebacterium sp. (n = 1, 5.5%) (Table 3).
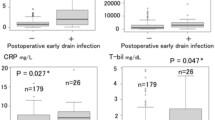
Turbid drainage fluid developed in all patients with CR-POPF between POD 4 and 14 (median, 9.5 days). Seventeen patients (94.4%) had pancreatic juice leakage. The median amylase level of ascitic fluid was 3563 (17–358,400) U/L. Thirteen patients (72.2%) had bacterial contamination on the day when the turbid drainage fluid was noticed (Staphylococcus sp., 38.9%; Corynebacterium sp., 16.7%; Pseudomonas sp., 11.1%; and, Streptococcus sp., 5.6%); 53.8% of the detected bacteria were the same species as those detected on POD 4 (Table 3). Only one type of bacterium was detected in each case.
Risk factors for CR-POPF
The development rate of pancreatic juice leakage and the bacterial contamination rate of ascitic fluid were compared between the CR-POPF (−) and CR-POPF (+) groups. A univariate analysis revealed that a significant difference was observed in pancreatic juice leakage on POD 3 (p < 0.001) and POD 4 (p < 0.001), and bacterial contamination on POD 4 (p < 0.001) (Table 3). A multivariate analysis revealed that the pancreatic juice leakage on POD 4 (OR, 8.206; 95% CI, 1.420–47.424; p = 0.019) and the ascitic bacterial contamination on POD 4 (OR, 6.076; 95% CI, 1.413-26.132; p = 0.015) were independent risk factors for CR-POPF (Table 4).
Discussion
In this study, we investigated the presence of bacterial contamination in the ascitic fluid after DP to clarify the bacterial origin of infections associated with CR-POPF. Although some patients had CR-POPF without bacterial infection, 77.2% of CR-POPF patients had bacterial contamination of ascitic fluid when drainage fluid changed turbid. In these patients, bacterial positivity of drainage fluid increased daily during the postoperative course, and the amylase level remained high. Staphylococcus was the most frequently detected causative bacterium.
Despite reports that bacterial infection is involved in POPF after PD, and there are few reports regarding POPF after DP [1,2,3]. Biliary drainage associated with preoperative cholangitis may increase infectious complications after PD [5, 6, 11]. Postoperative ascitic infection and surgical site infection may be caused by preoperative bile duct bacterial infection and intraoperative bile exposure during surgery. According to one study, approximately 1000 colony forming units (CFUs) of bacteria per 1 g of duodenal contents and about 10,000 CFUs of bacteria inhabit the jejunum in the upper small intestine [12]. A gastrointestinal anastomosis is created in PD, and the peritoneal cavity may be exposed to bacteria owing to leakage of digestive juice during surgery. In our previous reports, positive ascites culture on day 1 after PD was determined to be an independent risk factor for POPF. More than half of the bacteria were of the Enterococcus sp. [2, 6]. This suggests that the bacterium causing POPF following PD originated from the digestive juice during surgery. In DP, preoperative cholangitis is extremely rare in the absence of common bile duct stenosis. There is no bile exposure during surgery. In addition, since no gastrointestinal anastomosis is used, the possibility of bacterial exposure from the gastrointestinal tract is low.
In DP, it has been reported that pancreatic thickness, age, obesity, malnutrition, and perioperative hemorrhaging are risk factors for POPF [8, 9, 13,14,15]. However, there have been few reports on the relationship between POPF and postoperative ascitic infection in DP, and no reports have evaluated ascitic bacterial contamination over time. In this study, we examined whether early postoperative bacterial infection in DP affects CR-POPF. The ascitic bacterial culture results on POD 1–3 were not found to be a risk factor for CR-POPF. The ascitic bacterial infection rate gradually increased with each postoperative day, and ascitic infection on POD 4 became an independent risk factor for CR-POPF. In the present study, postoperative ascitic bacterial culture after DP did not detect enterococci such as Enterococcus or E. coli in the POPF group, but the incidence of Pseudomonas and Staphylococcus increased during the postoperative course. According to a report by Marchegiani et al., the ascitic culture on POD 5 after DP showed positive bacterial results in 84.8% of cases, and mucosal bacteria accounted for 24.8% of the positive cases. In the positive cases, 84.8% were from the skin flora [16]. In addition, Yang et al. reported a positive ascites culture rate of 7.9% by the 3rd postoperative day and a 29% positive rate of ascites culture from the fourth to seventh days, with Staphylococcus the most frequent isolate (29%) [17]. From these results, it is speculated that long-term retention of the postoperative drain increases the rate of positive ascites culture. In this study as well, the rate of positive ascites culture increased during the postoperative course, to 4.4% and 7.7% on the third and fourth days after surgery, respectively. In the CR-POPF (+) group, the ascitic amylase level remained high over time. An infection of the fluid collected at the pancreatic stump may increase pancreatic juice leakage. In the case of DP, unlike PD, postoperative drainage may cause retrograde infection and pancreatic juice infection, thus resulting in Grade B POPF.
In conclusion, in DP cases, ascitic bacterial contamination was not observed in the early postoperative period, but the bacterial contamination rate increased after the occurrence of pancreatic juice leakage. Therefore, CR-POPF-related infections in DP may be caused by a retrograde infection of the pancreatic juice during the postoperative course, thus suggesting that the removal of the drain may reduce the CR-POPF rate after distal pancreatectomy.
References
Ohgi K, Sugiura T, Yamamoto Y, Okamura Y, Ito T, Uesaka K. Bacterobilia may trigger the development and severity of pancreatic fistula after pancreatoduodenectomy. Surgery. 2016;160:725–30.
Nagakawa Y, Matsudo T, Hijikata Y, Kikuchi S, Bunso K, Suzuki Y, et al. Bacterial contamination in ascitic fluid is associated with the development of clinically relevant pancreatic fistula after pancreatoduodenectomy. Pancreas. 2013;42:701–6.
Yamashita K, Kato D, Sasaki T, Shiwaku H, Ishii F, Naito S, et al. Contaminated drainage fluid and pancreatic fistula after pancreatoduodenectomy: a retrospective study. Int J Surg. 2018;52:314–9.
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2016;161:584–91.
Kitahata Y, Kawai M, Tani M, Hirono S, Okada K, Miyazawa M, et al. Preoperative cholangitis during biliary drainage increases the incidence of postoperative severe complications after pancreaticoduodenectomy. Am J Surg. 2014;208:1–10.
Akashi M, Nagakawa Y, Hosokawa Y, Takishita C, Osakabe H, Nishino H, et al. Preoperative cholangitis is associated with increased surgical site infection following pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2020;27:640–7.
Sivaraj SM, Vimalraj V, Saravanaboopathy P, Rajendran S, Jeswanth S, Ravichandran P, et al. Is bactibilia a predictor of poor outcome of pancreaticoduodenectomy? Hepatobiliary Pancreat Dis Int. 2010;9:65–8.
Kawaida H, Kono H, Watanabe M, Hosomura N, Amemiya H, Fujii H. Risk factors of postoperative pancreatic fistula after distal pancreatectomy using a triple-row stapler. Surg Today. 2018;48:95–100.
Ecker BL, McMillan MT, Allegrini V, Bassi C, Beane JD, Beckman RM, et al. Risk factors and mitigation strategies for pancreatic fistula after distal pancreatectomy: analysis of 2026 resections from the international, multi-institutional distal pancreatectomy study group. Ann Surg. 2019;269:143–9.
Nagakawa Y, Hijikata Y, Osakabe H, Matsudo T, Soya R, Sahara Y, et al. Why does postoperative pancreatic fistula occur after hand-sewn parenchymal closure and staple closure in distal pancreatectomy? Surg Laparosc Endosc Percutan Tech. 2019;29:e15–9.
Povoski SP, Karpeh MS Jr, Conlon KC, Blumgart LH, Brennan MF. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg. 1999;230:131–42.
Lankelma JM, Nieuwdorp M, de Vos WM, Wiersinga WJ. The gut microbiota in internal medicine: implications for health and disease. Neth J Med. 2015;73:61–8.
Nagakawa Y, Tsuchida A, Saito H, Tohyama Y, Matsudo T, Kawakita H, et al. The VIO soft-coagulation system can prevent pancreatic fistula following pancreatectomy. J Hepatobiliary Pancreat Surg. 2008;15:359–65.
Tjaden C, Hinz U, Hassenpflug M, Fritz F, Fritz S, Grenacher L, et al. Fluid collection after distal pancreatectomy: a frequent finding. HPB (Oxford). 2016;18:35–40.
Nahm CB, de Reuver PR, Hugh TJ, Pearson A, Gill AJ, Samra JS, et al. Intra-operative amylase concentration in peri-pancreatic fluid predicts pancreatic fistula after distal pancreatectomy. J Gastrointest Surg. 2017;21:1031–7.
Marchegiani G, Perri G, Pulvirenti A, Sereni E, Azzini AM, Malleo G, et al. Non-inferiority of open passive drains compared with closed suction drains in pancreatic surgery outcomes: a prospective observational study. Surgery. 2018;164:443–9.
Yang F, Jin C, Hao S, Fu D. Drain contamination after distal pancreatectomy: incidence, risk factors, and association with postoperative pancreatic fistula. J Gastrointest Surg. 2019;23:2449–58.
Acknowledgements
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osakabe, H., Nagakawa, Y., Kozono, S. et al. Causative bacteria associated with a clinically relevant postoperative pancreatic fistula infection after distal pancreatectomy. Surg Today 51, 1813–1818 (2021). https://doi.org/10.1007/s00595-021-02287-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02287-5