Abstract
Purpose
The purpose of this study was to assess whether the anesthetic type is associated with the prognosis of pathological stage I non-small cell lung cancer (NSCLC).
Methods
Clinicopathological data from 431 consecutive patients who underwent lobectomy for NSCLC between 2010 and 2016 were collected. Patients were classified into groups according to the type of anesthesia: propofol-based total intravenous anesthesia (TIVA) or inhalation anesthesia (INHA). We investigated the prognostic differences between these two groups.
Results
A total of 72 patients in the TIVA group and 158 patients in the INHA group were eligible for the analysis. Recurrence was observed in 4 (5.6%) patients in the TIVA group and 19 (12.0%) patients in the INHA group (P = 0.159), and all-cause death occurred in 4 (5.6%) patients in the TIVA group and 24 (15.2%) patients in the INHA group (P = 0.049). The 5-year recurrence-free survival (RFS) and overall survival rates of the TIVA/INHA groups were 91.7%/77.4% and 94.4%/83.5%, respectively. TIVA was associated with a significantly better prognosis. A multivariable analysis of factors associated with RFS revealed that the type of anesthesia as a significant prognostic factor (P = 0.047).
Conclusion
Propofol-based TIVA was associated with a better prognosis in comparison to INHA in patients with surgically resected pathological stage I NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although surgical resection is the mainstay of therapy for potentially curable non-small cell lung cancer (NSCLC), postoperative recurrence even occurs in patients with early-stage disease; the recurrence rate has been reported to be 18.4% after lobectomy for patients with clinical T1 (≤ 3 cm) N0 NSCLC [1]. The 5-year overall survival (OS) rates according to pathological (p-) stage (UICC TNM classification, 8th edition) were reported to be 77–92% in patients with stage IA disease and 68% in those with stage IB disease [2]. Even in p-stage I patients, approximately 20% of patients develop recurrence after surgery [3, 4]. Therefore, the development or improvement of surgical techniques and/or perioperative management is needed to improve the prognosis of patients with p-stage I NSCLC.
In recent years, remarkable attention has been paid to the impact of the anesthetic choice on the cancer prognosis [5,6,7,8,9]. A large retrospective analysis found that propofol-based total intravenous anesthesia (TIVA) was associated with a better prognosis in comparison to inhalation anesthesia (INHA) in patients undergoing surgical treatment for solid malignant tumors [5], and similar results have been observed in patients with esophageal, gastric, colorectal, and breast cancers [6,7,8,9]. In vitro, animal model and clinical studies have supported these results; briefly, propofol appears to suppress tumor proliferation and invasiveness and retain antimetastatic host defenses, such as natural killer (NK) cells and cell-mediated immunity [10,11,12,13,14,15].
With respect to surgically resected NSCLC, Oh et al. reported that TIVA was associated with a lack of prognostic benefit in comparison to INHA [16]. Although the study included > 25% patients with lymph node metastasis, the authors did not assess the different impacts of TIVA on the cancer prognosis according to p-stage. It is expected that any favorable effects of TIVA were largely offset by the poor prognosis in advanced NSCLC patients. The heterogeneity of these patients might make it difficult to independently evaluate the impact of propofol on the prognosis. Thus, we hypothesized that propofol-based TIVA may be associated with a better prognosis in patients with p-stage I NSCLC. The aim of this study is to assess the postoperative recurrence and survival rates according to anesthetic choice among p-stage I patients who underwent complete resection for NSCLC in a single-institution retrospective cohort study.
Methods
Ethical approval
The ethics committees of our institute approved this study (No. 154, 2020) and waived the need for informed consent from patients because all patient data remained anonymous.
Study design and patient selection
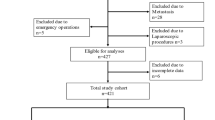
This is a single-institution retrospective cohort study. We used our institution’s NSCLC database, which contained information about clinicopathological, anesthetic and surgical factors, clinical outcomes, and adjuvant treatment. The pathological TNM classification was described according to the 8th edition of the UICC Staging Manual [17]. We reviewed the data of consecutive patients who underwent lobectomy with complete resection at the Yamagata Prefectural Central Hospital between January 2010 and December 2016 (n = 431). Patients with metachronous lung cancer, those who received chemotherapy or radiotherapy prior to surgery, those with p-stage II or III disease, and those with adenocarcinoma in situ or minimally invasive adenocarcinoma were excluded. After the application of the exclusion criteria, 230 p-stage I patients remained and were included in the present analysis (Fig. 1).
Surgical procedure and anesthesia technique
We usually conducted lobectomy and mediastinal lymph node dissection as a treatment for NSCLC with a minimally invasive approach; posterolateral incision sparing anterior serratus muscle. Lobectomy combined with wedge resection and bilobectomy were considered lobectomy procedures in this study.
Anesthesia was induced with fentanyl and propofol and maintained using propofol or inhalation agents. Patients were divided into TIVA (continuous propofol infusion using target-controlled infusion) and INHA (sevoflurane or desflurane) groups. Patients who maintained the use of both inhalation agents and propofol were assigned to the INHA group. The anesthetic choice was left to the discretion of anesthetists during the study period. In both groups, intravenous remifentanil and/or fentanyl continuous infusion during surgery were administered. Patients in both groups usually received continuous regional anesthesia, such as epidural anesthesia or thoracic paravertebral block and patient-controlled intravenous analgesia with fentanyl for 1–2 days. Non-steroid anti-inflammatory drugs and/or acetaminophen were taken orally for postoperative pain control. The painkiller use protocol did not change during the study period.
Postoperative complications, surveillance, and the evaluation of recurrence
Postoperative complications of grade ≥ II in the Clavien-Dindo classification system [18] and of grade ≥ 2 using the Common Terminology Criteria for Adverse Events (CTCAE) were evaluated in this study. Postoperative complications were classified into three categories: pulmonary complications included prolonged air leak, hypoxemia, acute respiratory failure, pneumonia, atelectasis, empyema, and pleural effusion; cardiovascular complications included atrial fibrillation and supraventricular arrhythmia; and infectious complications included pneumonia, empyema, surgical site infection, and urinary tract infection.
Postoperative surveillance consisted of outpatient clinic visits at 1 or 2 weeks after surgery and every month thereafter for up to 3 months. Follow-up was continued every 3 to 6 months for 5 years. Chest x-ray and laboratory tests, including the assessment of serum tumor markers, were conducted every 3 months. During the first 3 years of follow-up, patients underwent computed tomography (CT) every 6 months; thereafter, CT was performed annually. Brain CT or magnetic resonance imaging and positron-emission tomography/CT were performed if recurrence was suspected. The date of histologic confirmation or the radiologic diagnosis of recurrence was defined as the date of recurrence. Recurrence was diagnosed by the cancer treatment board.
Statistical analysis
Patient characteristics were evaluated using the Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables. OS was defined as the interval from the date of surgery to the date of death due to any cause or the date of last hospital visit. Recurrence-free survival (RFS) was defined as the interval from the date of surgery to the date of first recurrence, date of death due to any cause, or date of the last contact with the patient. Observations were censored at the last hospital visit when the patient was alive or lost to follow-up. The OS and RFS rates were established by the Kaplan–Meier method and evaluated by the log-rank test. Univariable Cox proportional-hazards regression analyses were used to identify prognostic factors for OS and RFS. Backward–forward stepwise variable selection by the Akaike Information Criterion (AIC) was conducted for a multivariable analysis. In this study, we set RFS as a primary endpoint to evaluate whether the anesthetic choice affects the recurrence as well as survival. The secondary endpoints included OS, recurrence rates and patterns, and postoperative complications. All statistical analyses were performed using JMP® Pro 15.1.0 (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-sided, and P values of < 0.05 were considered statistically significant.
Results
Patient characteristics
Table 1 shows the relationship between the clinicopathological factors and the type of anesthesia. Females and patients who underwent surgery more recently tended to receive TIVA; no significant differences were observed between the groups in the other preoperative, surgical, anesthetic, and pathological factors.
Anesthetic type and postoperative prognosis and complications
In the 230 eligible patients, the median length of follow-up for censored cases was 54 months (range 18–88 months) in the TIVA group and 64 months (19–108 months) in the INHA group. No patients died within 30 days after surgery. During follow-up, 23 patients (10.0%) developed recurrences. Patients in the TIVA group had a lower rate of recurrence (4 patients, 5.6%) in comparison to the INHA group (19 patients, 12.0%); however, this difference was not statistically significant (P = 0.159). The recurrence patterns did not differ between the groups (Table 2). A total of 28 patients (12.2%) died; 9 died from lung cancer and 19 died from other diseases. Significantly fewer deaths occurred in the TIVA group (4 patients, 5.6%) in comparison to the INHA group (24 patients, 15.2%) (P = 0.049). The causes of death did not differ between the groups (P = 1.000).
Figure 2a shows the RFS curves according to the type of anesthesia; the 5-year RFS rates in the TIVA and INHA groups were 91.7% and 77.4%, respectively. RFS was significantly longer in the TIVA group than in the INHA group (P = 0.020). The OS curves are shown in Fig. 2b. The 5-year OS rates in the TIVA and INHA groups were 94.4% and 83.5%, respectively; this difference was not statistically significant (P = 0.061). A stratified analysis by the date of surgery revealed that patients in the TIVA group experienced significantly longer RFS in comparison to those in the INHA group (P = 0.016) from 2014–2016. However, no significant differences in RFS were observed from 2010–2013 (P = 0.401) (Fig. 3).
Table 3 shows the association between the type of anesthesia and postoperative complications. No significant differences in cardiovascular or infectious complications were observed between the groups. Patients in the TIVA group experienced fewer pulmonary complications (3 patients, 4.2%) in comparison to those in the INHA group (18 patients, 11.4%); however, this difference was not statistically significant (P = 0.088).
Table 4 shows the clinicopathological factors that affected RFS and OS. With respect to RFS, the univariable analyses identified type of anesthesia as well as sex, p-stage and postoperative pulmonary complications as being significantly associated with the prognosis. The multivariable analysis revealed that INHA was significantly associated with a poor prognosis (P = 0.047). On the other hand, the OS did not differ between the anesthesia types to a statistically significant extent either before or after multivariable adjustment.
Discussion
The results of this study revealed that propofol-based TIVA is associated with significantly longer RFS in comparison to INHA in patients with surgically resected p-stage I NSCLC. This result is unique and suggests a new approach to improving the prognosis of patients with surgically resected p-stage I NSCLC.
There are several possible explanations for the favorable effects of propofol-based TIVA on the prognosis. First, anesthetic agents may directly influence cancer cell progression. Propofol has been shown to induce growth inhibition via apoptosis [10] and to inhibit the invasive ability of cancer cell lines [11, 12]. In contrast, in vitro, sevoflurane was shown to increase breast cancer cell proliferation, migration, and invasion [19] and to enhance sphere-forming activity in glioma stem cells [20]. Sevoflurane and desflurane alter the expression of metastasis-related genes [21]. These volatile agents also upregulate hypoxia-inducible factors (HIFs), which are involved in increased proliferation, angiogenesis, and metastasis in cancer cells, whereas propofol suppresses the synthesis and activation of HIFs [22]. Second, anesthetic agents may influence the immune response to cancer. Although it is well established that neuroendocrine and cytokine responses after surgery induce immunosuppression, propofol does not reduce the activity of NK cells and does not increase lung tumor retention in rats [13]. In the clinical setting, the counts of CD3 + cells, CD4 + cells, and NK cells and ratios of CD4 + /CD8 + after surgery significantly decreased in patients who underwent isoflurane anesthesia versus those who received propofol anesthesia [14]. Furthermore, the postoperative pro-inflammatory cytokine levels were significantly lower and anti-inflammatory cytokine levels were higher in patients who received propofol-based TIVA and remifentanil in comparison to those who received inhalational anesthesia with isoflurane [15]. Taken together, along with the results of previous studies, propofol-based TIVA appears to be more appropriate for patients undergoing cancer surgery.
Even complete surgical resection cannot completely eradicate minimal residual disease (MRD), which may be defined as small numbers of cancer cells that remain in the body after treatment, either due to dissemination during surgery or preexisting micrometastasis. These residual cancer cells are clinically undetectable and may develop into metastatic recurrences [23]. It is believed that the period between postoperative immunosuppression and the administration of adjuvant treatment is a possible window of opportunity for MRD to flourish [23]. Therefore, minimizing perioperative immunosuppression via propofol-based TIVA could be a new treatment strategy.
A previous study in patients with surgically resected NSCLC patients did not reveal any prognostic benefits of TIVA [17]. In the study, > 25% patients had lymph node metastasis, so any favorable effects of TIVA might have been offset by the poor prognosis of these advanced NSCLC patients. Another interpretation is that p-stage II or III patients are more heterogeneous with respect to the adjuvant chemotherapy and treatments they receive after recurrence, making it difficult to independently evaluate the impact of propofol on their prognosis. Therefore, propofol-based TIVA could be a novel treatment strategy for achieving a better prognosis in patients with stage I NSCLC.
We observed that patients in the TIVA group experienced fewer postoperative pulmonary complications in comparison to patients in the INHA group, although this difference was not statistically significant. A previous study showed that patients developed significantly fewer pulmonary complications after TIVA in comparison to INHA [24]. Isoflurane and sevoflurane have both been shown to induce bronchodilation in patients with chronic obstructive pulmonary disease (COPD). We expected that INHA would have been selected for patients with COPD; however, the frequency of COPD, smoking history, and results of respiratory function tests were similar between the patients in the TIVA and INHA groups. Although animal studies have suggested that INHA triggers alveolar inflammatory processes [24], resulting in a higher risk of pulmonary complications, the underlying mechanism has remained unclear. A previous study demonstrated that postoperative pulmonary complications were independent negative prognostic factors in patients with surgically resected lung cancer [25] and this association was also confirmed in the present study. Propofol anesthesia also reduced surgical stress and postoperative complications, resulting in the enhancement of recovery from esophageal cancer surgery, in comparison to those who received inhalational sevoflurane [26]. These data possibly explain the better prognoses of patients who received TIVA.
In the present study, patients in the TIVA group were treated more recently. The stratified analysis did not reveal any favorable effects of TIVA on the prognosis of patients who underwent surgery in the earlier study period. To clarify the underlying reasons for this result, we examined differences in patient backgrounds and the incidence of recurrence or death according to the date of surgery; however, no explanatory factors were identified. We assume that this was due to the small sample size and event count. One also would expect a more recent group of patients to have improved outcomes, however, the univariable analysis did not show the relationship between survival and the date of surgery.
The present study was associated with some limitations. First, this was a retrospective cohort study and no clear criteria were established regarding the selection of anesthesia. There would be unstudied and subtle clinical factors that influenced the choice of anesthetic and clinical outcomes, although we carefully examined the patients characteristics, including age, sex, smoking history, comorbidities, respiratory function, performance status and pathological factors, and revealed that sex was the only significant factor. There may also be inherent differences in the anesthesiologists who preferentially used TIVA, which could have had an impact on the outcomes of the patients whom they treated. Second, this was a single-institution study and the sample size was relatively small. The present study did not have enough power to reveal significant differences in OS between the groups, although a significant difference was observed in RFS. The small sample size also did not allow adjustment for a variety of potential confounding factors in multivariable analysis. Finally, although we obtained data on the duration of anesthesia, the use of regional anesthesia, and the anesthetic type, data on other aspects of anesthetic management, such as acute pain or opioid use, were unavailable. These factors might influence the possibility of metastatic recurrence [23]; however, the surgical procedure and painkiller use protocol did not change during the study period. There are studies reporting the negative effects of opioids on the anti-tumor immune response [23]. Previous studies showed that the amount of opioids administered during the surgical periods was significantly higher in a TIVA group than in an INHA group [27] and the postoperative opioid use did not differ between these groups [28]. Collectively, we hypothesize that a difference in acute pain or perioperative opioid use-related immunosuppression is unlikely to play a role in the better prognosis that was seen in the TIVA group in the present study.
In conclusion, the present study revealed that propofol-based TIVA is associated with a better prognosis in comparison to INHA in patients with surgically resected p-stage I NSCLC. Therefore, surgeons need to pay more attention to the prognostic impact of the anesthetic choice on survival in NCSLC patients.
References
Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22.
Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51.
al-Kattan K, Sepsas E, Fountain SW, Townsend ER. Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg. 1997; 12: 380–4.
Tsuchiya T, Akamine S, Muraoka M, Kamohara R, Tsuji K, Urabe S, et al. Stage IA non-small cell lung cancer: vessel invasion is a poor prognostic factor and a new target of adjuvant chemotherapy. Lung Cancer. 2007;56:341–8.
Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79.
Jun IJ, Jo JY, Kim JI, Chin JH, Kim WJ, Kim HR, et al. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep. 2017;7:14020.
Zheng X, Wang Y, Dong L, Zhao S, Wang L, Chen H, et al. Effects of propofol-based total intravenous anesthesia on gastric cancer: a retrospective study. Onco Targets Ther. 2018;11:1141–8.
Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS, Lin KT, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. 2018;129:932–41.
Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The choice of anaesthetic–sevoflurane or propofol–and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. 2014;119:251–61.
Tsuchiya M, Asada A, Arita K, Utsumi T, Yoshida T, Sato EF, et al. Induction and mechanism of apoptotic cell death by propofol in hl-60 cells. Acta Anaesthesiol Scand. 2002;46:1068–74.
Mammoto T, Mukai M, Mammoto A, Yamanaka Y, Hayashi Y, Mashimo T, et al. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184:165–70.
Miao Y, Zhang Y, Wan H, Chen L, Wang F. GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed Pharmacother. 2010;64:583–8.
Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97:1331–9.
Liu S, Gu X, Zhu L, Wu G, Zhou H, Song Y, et al. Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Medicine (Baltimore). 2016;95:e5479.
Ke JJ, Zhan J, Feng XB, Wu Y, Rao Y, Wang YL. A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care. 2008;36:74–8.
Oh TK, Kim K, Jheon S, Lee J, Do SH, Hwang JW, et al. Long-term oncologic outcomes for patients undergoing volatile versus intravenous anesthesia for non-small cell lung cancer surgery: a retrospective propensity matching analysis. Cancer Control. 2018;25:1073274818775360.
Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 8th ed. Hoboken: Wiley-Blackwell; 2017.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:1871–96.
Ecimovic P, McHugh B, Murray D, Doran P, Buggy DJ. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res. 2013;33:4255–60.
Shi QY, Zhang SJ, Liu L, Chen QS, Yu LN, Zhang FJ, et al. Sevoflurane promotes the expansion of glioma stem cells through activation of hypoxia-inducible factors in vitro. Br J Anaesth. 2015;114:825–30.
Iwasaki M, Zhao H, Jaffer T, Unwith S, Benzonana L, Lian Q, et al. Volatile anaesthetics enhance the metastasis related cellular signalling including CXCR2 of ovarian cancer cells. Oncotarget. 2016;7:26042–56.
Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–50.
Niwa H, Rowbotham DJ, Lambert DG, Buggy DJ. Can anesthetic techniques or drugs affect cancer recurrence in patients undergoing cancer surgery? J Anesth. 2013;27:731–41.
Soltanizadeh S, Degett TH, Gogenur I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: a systematic review. J Clin Anesth. 2017;42:19–25.
Wang S, Li X, Li Y, Li J, Jiang G, Liu J, et al. The long-term impact of postoperative pulmonary complications after video-assiste d thoracic surgery lobectomy for lung. J Thorac Dis. 2017;9:5143–52.
Tsuchiya M, Shiomoto K, Mizutani K, Fujioka K, Suehiro K, Yamada T, et al. Reduction of oxidative stress a key for enhanced postoperative recovery with fewer complications in esophageal surgery patients: Randomized control trial to investigate therapeutic impact of anesthesia management and usefulness of simple blood test for prediction of high-risk patients. Medicine (Baltimore). 2018;97:e12845.
Lai HC, Chan SM, Lu CH, Wong CS, Cherng CH, Wu ZF. Planning for operating room efficiency and faster anesthesia wake-up time in open major upper abdominal surgery. Medicine (Baltimore). 2017;96:e6148.
Song JG, Shin JW, Lee EH, Choi DK, Bang JY, Chin JH, et al. Incidence of post-thoracotomy pain: a comparison between total intravenous anaesthesia and inhalation anaesthesia. Eur J Cardiothorac Surg. 2012;41:1078–82.
Funding
There are no funding sources to be disclosed in relation to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hayasaka, K., Shiono, S., Miyata, S. et al. Prognostic significance of propofol-based intravenous anesthesia in early-stage lung cancer surgery. Surg Today 51, 1300–1308 (2021). https://doi.org/10.1007/s00595-020-02216-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02216-y