Abstract
Purpose
The prognostic nutritional index (PNI) is considered useful for predicting the prognosis of patients with gastric cancer (GC). This retrospective study investigated the relationship between both the pre- and postoperative PNI and the prognosis of GC patients.
Methods
The subjects of this study were 254 patients who underwent curative surgery for histopathologically diagnosed GC.
Results
Patients were divided according to their pre- and postoperative PNI as follows: preoperative PNI of ≥ 52 (pre-PNIHigh), preoperative PNI of < 52 (pre-PNILow), postoperative PNI of ≥ 49 (post-PNIHigh), and postoperative PNI of < 49 (pre-PNILow). Both pre- and postoperative PNI were significantly associated with GC prognosis. Patients were then divided as follows: group A, patients with pre-PNIHigh and post-PNIHigh; group B, patients with either pre-PNIHigh and post-PNILow or pre-PNILow and post-PNIHigh; and group C, patients with pre-PNILow and post-PNILow. The 5-year survival rates were 100.0, 83.0, and 67.1% for groups A, B, and C, respectively. Multivariate analysis indicated that the combination of pre- and postoperative PNI was an independent prognostic indicator.
Conclusions
Combined analysis of the pre- and postoperative PNI offers accurate information about the prognosis of patients with GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common cancers. In 2015, there were an estimated 1,300,000 new cases and 819,000 deaths from GC worldwide, ranking it fifth in cancer incidence and the third-leading cause of cancer death [1, 2]. Thus, predicting the postoperative prognosis of patients with GC is extremely important. Many studies have indicated that the depth of invasion and the presence or absence of lymph node metastasis is the most important prognostic factors for GC [3, 4]. Furthermore, there is accumulating evidence of the usefulness of blood analytes to predict the prognosis of patients with GC. Serum tumor markers are easy to measure and helpful in the diagnosis, prediction of survival rates, and monitoring of recurrence following surgery [5, 6]. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) are the most frequently measured tumor markers in GC, although their positivity rates are lower in the serum of patients with GC than in those of patients with other cancers. Because blood analytes can be measured easily in routine clinical practice, the identification of useful markers is urgently required.
GC often causes lumen obstruction, dysphagia, and bleeding, with resulting malnutrition and increased risk of postoperative complications. Therefore, the evaluation of a patient’s preoperative nutritional status is important to ensure that they are sufficiently nourished before surgery. Several assessment tools are used for nutritional evaluation, including the subjective global assessment [7], mini nutritional assessment [8], and nutritional risk screening 2002 [9]. The prognostic nutritional index (PNI) as described by Onodera et al. [10] is a simple index calculated by the serum albumin (Alb) concentration and total lymphocyte count (LC) to evaluate a patient’s nutritional status. Nozoe et al. [11] recently reported that the preoperative PNI can be used to predict the prognosis and biological aggressiveness of GC. However, because gastrectomy decreases the stomach volume, some patients’ postoperative nutritional status becomes worse than their preoperative nutritional status. A poor nutritional status is reportedly associated with impaired immunity. Furthermore, Aoyama et al. [12] demonstrated that body weight loss, which is an indicator of malnutrition, is the most important risk factor for noncompliance with S-1 adjuvant chemotherapy in patients with stage 2/3 GC, who have undergone D2 gastrectomy. Therefore, it is likely that poor postoperative nutritional status is related to a poor prognosis in patients with GC; however, the prognostic significance of the postoperative nutritional status of patients with GC remains unclear. The aim of the current study was to evaluate the prognostic significance of the perioperative PNI in patients with GC.
Materials and methods
Patients
This study was based on a retrospective analysis of 254 patients with gastric adenocarcinoma, who underwent gastrectomy at our institution between January, 2001 and December, 2013. All patients underwent distal partial, proximal partial or total gastrectomy with regional dissection of the lymph nodes. Institutional review board approval was obtained and the informed consent requirement was waived for this study. The clinicopathologic findings were assessed according to the Japanese Classification of Gastric Carcinoma [13]. We collected data from blood tests performed preoperatively and 1 month postoperatively, including the serum Alb concentration and total LC of the peripheral blood from the patients’ records. The PNI was then calculated as follows: 10 × Alb concentration + 0.005 × total LC [10].
Patients were checked periodically for early recurrence by diagnostic imaging, including chest X-ray, double-contrast barium meal study, upper gastrointestinal endoscopy, ultrasonography, and computed tomography. Causes of death and patterns of recurrence were determined by reviewing the medical records, including laboratory data, ultrasonography, computed tomography, scintigraphy, peritoneal puncture, and laparotomy or by direct inquiry with family members. In some cases, postmortem examinations were undertaken to determine the cause of death.
Statistical analysis
For statistical analyses, differences between the two groups were evaluated using the Mann–Whitney U test. The Youden index was calculated using a receiver operating characteristic (ROC) analysis to calculate an optimal cut-off value for the preoperative and postoperative PNI to the overall survival status. Survival curves were calculated according to the Kaplan–Meier method. Differences between survival curves were examined with the log-rank test. We performed multivariate analysis of factors considered prognostic for overall survival, using a Cox proportional hazards model and a stepwise procedure. The covariates included in this analysis were age, gender, tumor size, histology, depth of invasion, lymph node metastasis, lymphatic vessel invasion, blood vessel invasion, postoperative complication, and the combination of preoperative and postoperative PNI. A P value of < 0.05 was considered significant. GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) and StatView (Abacus Concepts, Inc., Berkeley, CA, USA) were used for the statistical analyses.
Results
The mean preoperative PNI was 48.9 (range 30.6–63.6). Table 1 shows the correlation between the preoperative PNI and clinicopathologic factors. The preoperative PNI was significantly lower in elderly patients (≥ 70 years) than in younger patients (< 70 years); and in patients with large tumors (≥ 4 cm) than in those with small tumors (< 4 cm), respectively. Furthermore, a significantly lower PNI was observed in patients with advanced GC, those with blood vessel invasion, and those with stage II/III GC, than in patients with early GC, those without blood vessel invasion, and those with stage I GC, respectively. The mean postoperative PNI was 46.9 (range 24.2–61.7). The postoperative PNI was significantly lower in elderly patients (≥ 70 years) than in younger patients (< 70 years); in patients with large tumors (≥ 4 cm) than in those with small tumors (< 4 cm); and in patients with postoperative complications than in those without postoperative complications, respectively. There was a significant positive correlation between the preoperative and postoperative PNI (Fig. 1).
ROC analysis showed that the optimal cut-off value of the preoperative and postoperative PNI was 52 [area under the curve (AUC), 0.695; P < 0.0001] and 49 (AUC, 0.679; P < 0.0001), respectively. The patients were divided into the following two groups based on the preoperative PNI: preoperative PNI of ≥ 52 (pre-PNIHigh, n = 82) and preoperative PNI of < 52 (pre-PNILow, n = 172). The 5-year overall survival rates of patients with either pre-PNIHigh or pre-PNILow were 95.8 and 70.0%, respectively, and this difference was significant (P < 0.0001; Fig. 2a). The patients were then divided into the following two groups based on the postoperative PNI: postoperative PNI of ≥ 49 (post-PNIHigh, n = 95) and postoperative PNI of < 49 (pre-PNILow, n = 159). The 5-year overall survival rates of patients with either post-PNIHigh or post-PNILow were 91.4 and 70.1%, respectively, and this difference was significant (P < 0.0001; Fig. 2b).
There were 57, 25, 38, and 134 patients in the pre-PNIHigh and post-PNIHigh, pre-PNIHigh and post-PNILow, pre-PNILow and post-PNIHigh, and pre-PNILow and post-PNILow groups, respectively. Table 2 shows the relationships between postoperative PNI and clinicopathological variables in patients with gastric cancer according to the status of preoperative PNI. In patients with pre-PNILow, the frequency of postoperative complications was significantly lower in patients with post-PNIHigh than in those with post-PNILow. Therefore, patients in the pre-PNILow and post-PNIHigh groups could be characterized by a low frequency of postoperative complications. In patients with pre-PNIHigh, post-PNILow was observed more frequently in elderly patients, patients with lymphatic vessel invasion, and patients with postoperative complications than in younger patients, patients without lymphatic vessel invasion, and patients without postoperative complications, respectively. Therefore, patients with pre-PNIHigh and post-PNILow could be characterized by older age and a high frequency of lymphatic vessel invasion and postoperative complications. In comparing patients with pre-PNIHigh and post-PNILow and those with pre-PNILow and post-PNIHigh, a significant difference was observed only in the incidence of postoperative complications (P = 0.0003), but not in other clinicopathological characteristics. The overall 5-year survival rates were 80.1 and 67.1% for patients with pre-PNILow and post-PNIHigh and those with pre-PNILow and post-PNILow, respectively, and this difference was significant (P = 0.031) (Fig. 3a). Furthermore, the overall 5-year survival rates were 100% and 83.4% for patients with pre-PNIHigh and post-PNIHigh and those with pre-PNIHigh and post-PNILow, respectively, and this difference was significant (P = 0.0021) (Fig. 3b).
The patients were then divided into group A (those in the pre-PNIHigh and post-PNIHigh groups), group B (those in either the pre-PNIHigh and post-PNILow or pre-PNILow and post-PNIHigh groups), and group C (those in the pre-PNILow and post-PNILow groups). Group B contains two subgroups because the 5-year overall survival rates were almost the same (80.1% in patients with pre-PNILow and post-PNIHigh and 83.4% in those with pre-PNIHigh and post-PNILow) as mentioned above. The patients in groups A, B, and C were assigned 0, 1, and 2, respectively, according to a previous study [14]. ROC curves were constructed for the survival status, and AUC values were compared to assess the discrimination ability of the preoperative PNI, postoperative PNI, and combination of the preoperative and postoperative PNI (Fig. 4). Among the three prognostic scores, the combination of the pre- and postoperative PNI had the highest AUC value (0.703), followed by the preoperative PNI (AUC 0.695) and postoperative PNI (AUC 0.679). These findings indicate that the combination of pre- and postoperative PNI was more useful for predicting the prognosis of patients with GC than was single use of either the pre-PNI or post-PNI.
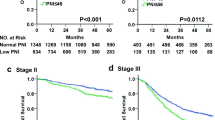
The overall 5-year survival rates were 100.0, 83.0, and 67.1% for groups A, B, and C, respectively, these differences being significant (P < 0.0001; Fig. 5a). Furthermore, the disease-specific 5-year survival rates were 100.0, 88.6, and 84.2% for groups A, B, and C, respectively, and these differences were also significant (P = 0.0091; Fig. 5b). Multivariate analysis revealed that the combination of the pre- and postoperative PNI was an independent prognostic indicator (Table 3). Since age was also an independent prognostic indicator and influenced postoperative PNI in this study, we assessed the correlation between the combination of the pre- and postoperative PNI and prognosis in elderly (≥ 70) vs. younger (< 70) patients. The overall 5-year survival rates were 100.0, 92.4, and 78.3% for groups A, B, and C, respectively, and the difference was significant for the younger patients (P = 0.017) (Fig. 6a). Furthermore, the overall 5-year survival rates were 100.0, 75.1, and 59.0% in groups A, B, and C, respectively, and this difference was also significant for the elderly patients (P = 0.0029; Fig. 6b).
Discussion
Recent reports suggest that the outcomes of patients with cancer are determined not only by tumor-related factors, but also by patient-related factors. Inflammation, nutrition, and immune status are the patient-related factors closely associated with the prognosis of patients with GC. The findings of the current study established the prognostic significance of the PNI, which is thought to reflect the patient’s nutritional status. The PNI was originally designed to assess the perioperative nutritional conditions and postoperative complications in patients with colorectal cancer in Japan [10]. It is simple to calculate and easily implemented in clinical practice. During the past few years, the ability of this PNI to predict both morbidity and long-term outcomes of patients with various malignancies has been recognized. Sakurai et al. [15] recently performed a multivariate analysis of 5-year overall and disease-specific survival and found that a low PNI was independently associated with unfavorable outcomes for patients with GC. We also demonstrated in the present study that the preoperative PNI is closely associated with the prognosis of GC patients.
The detailed mechanism by which PNI is associated with the prognosis of patients with cancer remains unclear. A low PNI reflects a decreased serum Alb concentration or a decreased LC. Serum Alb is the most abundant blood plasma protein. It is produced in the liver and constitutes a large proportion of all plasma proteins. Serum Alb is the traditional standard factor used to assess a patient’s nutritional status. The LC, which is another indicator used to measure the PNI, also reflects a patient’s nutritional status. Therefore, the PNI is likely to reflect the patient’s nutritional status better than just either the serum Alb concentration or LC alone. The LC is also believed to reflect the patient’s immune status. Lymphopenia is common in patients with advanced cancer and several studies have shown that a low preoperative LC is related to poor prognosis for patients with various types of cancer, including pancreatic cancer [16], esophageal cancer [17], renal cancer [18], and sarcoma or lymphoma [19]. These findings suggest that the LC in peripheral blood reflects immune activity against cancer cells. Furthermore, peripheral lymphocytes include natural killer cells, gamma-delta T cells, natural killer T cells, and B cells. A close correlation between decreased numbers of these immune cells and poor prognosis has been demonstrated in both the peripheral blood and cancer tissue of patients with certain cancers [20,21,22]. Therefore, the peripheral LC might be a good indicator of the cell-mediated immune status, including both acquired and adaptive immunity, and the humoral immune status against GC. The detailed mechanism of lymphopenia in patients with GC remains unclear. In this regard, we reported previously that upregulated Fas expression in CD8+ T cells is largely involved in increased apoptosis of circulating CD8+ T cells in patients with GC [23]. This might be one of the mechanisms responsible for the lymphopenia. Overall, the PNI is possibly an effective patient-related factor that includes both the nutritional and immune status of patients with GC.
Most studies demonstrating the correlation between the PNI and prognosis of patients who have undergone surgery for cancer focus mainly on the preoperative PNI. However, in the current study, we demonstrated that both the pre- and postoperative PNI were closely associated with the prognosis of patients with GC. Shibutani et al. [24] also revealed that the combination of the pre- and postoperative PNI was an independent prognostic factor for patients with colorectal cancer, indicating the importance of the postoperative nutritional status in their prognosis. Importantly, they observed a significant correlation between the pre- and postoperative PNI, consistent with our results. However, in the present study, there were 25 and 38 patients in the pre-PNIHigh/post-PNILow groups and the pre-PNILow/post-PNIHigh groups, respectively, indicating that the patients’ nutritional status changed before and after the operation. Our results indicated that this change is mainly due to age and the presence of postoperative complications. Therefore, we hypothesized that the combination of the pre- and postoperative PNI might be more useful to predict the prognosis of patients with GC than the individual use of either the preoperative PNI or the postoperative PNI. In fact, the AUC of their combination was higher than that of either the preoperative PNI or postoperative PNI alone. Furthermore, the prognosis of patients with post-PNIHigh was significantly better than that of patients with post-PNILow among those with pre-PNIHigh and those with pre-PNILow. These results show clearly that the combination of pre- and postoperative PNI was more useful to predict the prognosis of patients with GC than the individual use of either the preoperative PNI or the postoperative PNI. In fact, the combination of the pre- and postoperative PNI was significantly associated with both overall and disease-specific survival in the current study. Moreover, the combination of the pre- and postoperative PNI was significantly associated with overall survival regardless of age. Multivariate analysis revealed that the combination of the pre- and postoperative PNI was an independent prognostic indicator.
Because the pre- and postoperative PNI was closely related to the prognosis of GC patients, nutritional support, such as enteral nutrition might improve the prognosis of those patients with poor nutritional status. Furthermore, since postoperative complications were significantly associated with the post-PNIlow status, every effort should be made to prevent their development.
This study has a few limitations. First, some bias was present because it was retrospective. Second, we measured the PNI 1 month after the operation and used it as the postoperative parameter; however, the best timing to measure the postoperative PNI remains unclear. Third, the number of patients included in the current study was small; therefore, a large-scale, prospective randomized controlled trial is needed to confirm the results.
In conclusion, the combination of the pre- and postoperative PNI appears to be useful for predicting the prognosis of patients with GC. Because blood analytes can be measured easily in routine clinical practice and noninvasive assays, the pre- and postoperative PNIs may be useful biological markers in the routine clinical setting to help physicians plan optimal treatment strategies.
References
Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–49.
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990–2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48.
Bozzetti F, Bonfanti G, Morabito A, Bufalino R, Menotti V, Andreola S, et al. A multifactorial approach for the prognosis of patients with carcinoma of the stomach after curative resection. Surg Gynecol Obstet. 1986;162:229–34.
Maruyama K. The most important prognostic factors for gastric cancer patients. Scand J Gastroenterol. 1987;22:63–8.
Park HJ, Ahn JY, Jung HY, Lim H, Lee JH, Choi KS, et al. Clinical characteristics and outcomes for gastric cancer patients aged 18–30 years. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2014;17:649–60.
Nam DH, Lee YK, Park JC, Lee H, Shin SK, Lee SK, et al. Prognostic value of early postoperative tumor marker response in gastric cancer. Ann Surg Oncol. 2013;20:3905–11.
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enter Nutr. 1987;11:8–13.
Vellas B, Villars H, Abellan G, Soto ME, Rolland Y, Guigoz Y, et al. Overview of the MNA–Its history and challenges. J Nutr Health Aging. 2006;10:456–63 (discussion 63–5).
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr (Edinburgh, Scotland). 2003;22:321–36.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai zasshi. 1984;85:1001–5.
Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–3.
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000–6.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Haruki K, Shiba H, Shirai Y, Horiuchi T, Iwase R, Fujiwara Y, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg. 2016;40:2254–60.
Sakurai K, Ohira M, Tamura T, Toyokawa T, Amano R, Kubo N, et al. Predictive potential of preoperative nutritional status in long-term outcome projections for patients with gastric cancer. Ann Surg Oncol. 2016;23:525–33.
Clark EJ, Connor S, Taylor MA, Madhavan KK, Garden OJ, Parks RW. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB Off J Int Hepato Pancreato Biliary Assoc. 2007;9:456–60.
Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine. 2014;93:e257.
Saroha S, Uzzo RG, Plimack ER, Ruth K, Al-Saleem T. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol. 2013;189:454–61.
Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Can Res. 2009;69:5383–91.
Qiu H, Xiao-Jun W, Zhi-Wei Z, Gong C, Guo-Qiang W, Li-Yi Z, et al. The prognostic significance of peripheral T-lymphocyte subsets and natural killer cells in patients with colorectal cancer. Hepatogastroenterology. 2009;56:1310–5.
Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11:7322–7.
Berntsson J, Nodin B, Eberhard J, Micke P, Jirstrom K. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int J Cancer. 2016;139:1129–39.
Yoshikawa T, Saito H, Osaki T, Matsumoto S, Tsujitani S, Ikeguchi M. Elevated Fas expression is related to increased apoptosis of circulating CD8+ T cell in patients with gastric cancer. J Surg Res. 2008;148:143–51.
Shibutani M, Maeda K, Nagahara H, Ohtani H, Iseki Y, Ikeya T, et al. The prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancer. BMC Cancer. 2015;15:521.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no commercial interest in this subject to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Murakami, Y., Saito, H., Kono, Y. et al. Combined analysis of the preoperative and postoperative prognostic nutritional index offers a precise predictor of the prognosis of patients with gastric cancer. Surg Today 48, 395–403 (2018). https://doi.org/10.1007/s00595-017-1599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1599-2