Abstract
Purpose
To present a real-world experience of the elective treatment of abdominal aortic aneurysms (AAAs) using both open repair (OR) and endovascular repair (EVAR).
Methods
Data from patients treated consecutively between January 1, 2000 and December 31, 2014 were collected retrospectively and reviewed. The primary outcomes were 30-day mortality and complication rates, freedom from reintervention, and survival in the long-term.
Results
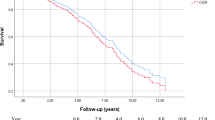
We analyzed data on 1112 patients (660 EVAR, 452 OR). The 30-day mortality and complications rates were higher after OR than after EVAR (2.9 vs. 1.1%, P = .03 and 24.7 vs. 1.1%, P < .0001, respectively). At 10 years, survival was 66.1 ± 3.2% after OR and 78.1 ± 2.2% after EVAR (P = .0006) and freedom from reintervention was 93.5 ± 1.8% after OR and 88.4 ± 1.8% after EVAR (P = .005). The preoperative aneurysm diameter was significantly associated with the development of type Ia endoleaks after EVAR (P < .0001) and of a proximal pseudoaneurysm after OR (P < .0001).
Conclusion
In the long-term, EVAR was associated with higher reintervention rates, but better survival than OR. The preoperative AAA diameter was the most important predictor of the development of endoleaks after EVAR and proximal pseudoaneurysm after OR.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the introduction of endovascular repair (EVAR) as an alternative to open repair (OR) for abdominal aortic aneurysms (AAAs), many trials have been published comparing the two treatments, most of all questioning long-term durability [1]. Data emerging from those trials show a superiority of EVAR in terms of perioperative mortality and complications; however, concerns remain about long-term reintervention rates, which are reported to be higher after EVAR than after OR. One of the main limitations of these trials is represented by poor reproducibility, given the presence of obvious selection bias compared with the “real-world”. Many randomized clinical trials enrolled patients who were fit enough for either EVAR repair or OR, being relatively healthy and probably with lower surgical risk than patients than one would encounter in clinical practice. Moreover, sometimes it is difficult to understand the real rate of postoperative complications if researchers do not use a standardized method to evaluate those complications, such as the Japan Clinical Oncology Group (JCOG) Postoperative Complication criteria [2], for a more precise comparison of the frequency of surgical complications among trials.
The aim of this study is to present our retrospective evaluation of a “real-world experience” about the elective treatment of AAAs with both EVAR and OR in two centers over 14 years, and highlight some considerations arising from this experience.
Materials and methods
In Italy, both EVAR and OR procedures are covered by the National Health Insurance and are performed routinely without any need for clinical trials. This retrospective “real-world” study was approved by the local Institutional Review Board. In our Research Hospital, all subjects on admission gave informed consent to the anonymous processing of their data for research purposes.
Data from patients consecutively treated between January 1, 2000 and December 31, 2014 were collected retrospectively from a database.
Indications for elective treatment included a saccular morphology of the aneurysm, a diameter greater than 5 cm in men and 4.5 cm in women, or evidence of aneurysmal growth of more than 1 cm per year [3]. All patients underwent preoperative computed tomography angiography (CTA) for evaluation of the thoracic–abdominal aortic anatomy and proper indication for either open repair or EVAR. All procedures were performed by vascular surgeons in the operating room. At the beginning of our experience, EVAR was performed only for patients who were considered “unfit for OR” because of a high surgical risk for either comorbidities or hostile abdomen [4]. However, EVAR has since become the treatment of choice for the treatment of AAAs in most patients, reserving OR for patients “unfit for EVAR”, especially because of poor general condition. For example, patients presenting with a very short proximal neck or excessive proximal neck angulation, as well as those with aortoiliac occlusive disease, were usually treated with OR. When EVAR was performed, different types of endografts were used, mainly according to the instructions for use (IFU; Fig. 1). There was a small proportion of patients (4%) who were treated outside the IFU for an angulated neck or an AAA shorter than 15 mm; however, if aortoiliac anatomy was considered prohibitive for EVAR, the patient preferably underwent OR. Bilateral femoral surgical access was performed in most patients.
Local and spinal anesthesia were used whenever possible, but OR was always performed under general anesthesia through a transperitoneal approach, either via median laparotomy or bilateral subcostal access, except for inflammatory aneurysms, which were generally treated via an extraperitoneal route. Aorto-aortic grafts, as well as aorto-bisiliac and aorto-bifemoral grafts, were used as appropriate, according to the extension of the disease. The proximal anastomosis was always performed on what was thought to be an optimal aortic neck, based on both preoperative CT scan and intraoperative findings. In our experience the neck was considered appropriate for the proximal anastomosis when its diameter was less than 25 mm and calcification/thrombotic apposition did not cover more than 50% of its circumference.
Patient records were reviewed for demographics, medical history, and aortoiliac morphology via computed tomography angiography. Procedural data included operation time, blood loss, and any intraprocedural complications. Primary success for EVAR was defined as successful delivery of the graft without any intraprocedural endoleak or graft thrombosis and without need for conversion to OR. Follow-up data were obtained from outpatient visits, CT or duplex scan results, and telephone interviews. Imaging was performed at 1, 6, and 12 months during the first year after the operation, and annually thereafter, using mainly duplex scans. A CT scan was performed at 1 year and if a duplex scan showed inconclusive findings (such as hostile abdomen), or sac enlargement, graft occlusion, stenosis, endoleaks, or pseodoaneurysm.
Primary outcomes were 30-day mortality and complication rates, long-term survival, and freedom from reintervention in the long-term. Factors associated with the need for reintervention and graft-related complications in the long-term were also analyzed. Statistical analysis was performed using JMP 5.1.2 software with Kaplan–Meier, t test, logistic regression, and the Cox proportional hazards regression model, as appropriate. Univariate and multivariate analysis was performed. P values <0.05 were considered significant.
Results
A total of 1135 patients (1056 men, 93%) underwent either EVAR (662) or OR in our two hospitals. During the period between 2000 and 2014, the percentage of patients undergoing EVAR grew steadily, from 43.8% in the first 5 years, to 58.2% in the middle period and finally, to 80.5% in the last 5 years. The median age of the patients was 72 years (IQR 67–68 years, range 49–98 years). Patients who underwent OR were younger than those who underwent EVAR (median age, 71 vs. 73 years; IQR, 65–77 years vs. 67–79 years, respectively, P = .01). Patients were mainly affected by smoking history/ status (50.4%), hypertension (42.2%), and dyslipidemia (38.5%). Chronic renal failure was coexistent preoperatively in 5.6% of patients who underwent EVAR and 11.8% of those who underwent OR (<0.0001).
Most aneurysms were located in the infrarenal segment of the aorta; however, in the EVAR group, two patients had an aortic aneurysm involving one or both renal arteries. These two patients were deemed too high a surgical risk for OR, by the anesthesiologist, and eventually underwent endovascular exclusion of the pararenal AAA using a Multilayer Flow Modulator stent (Table 1a). Both patients had an uneventful postoperative recovery, but were excluded from the analysis.
In the OR group, there were 5 pararenal aneurysms, 11 juxtarenal aneurysms, and 5 thoracoabdominal aneurysms type IV according to Crawford’s criteria. Aortic cross-clamping was performed at the diaphragm for the thoracoabdominal aneursyms. The juxtarenal aneurysms required aortic cross-clamping between the superior mesenteric artery (SMA) and the renal arteries, whereas the pararenal aneurysms required aortic cross-clamping between the celiac trunk and the SMA. Infrarenal cross-clamping was done for all others. Renal revascularization was performed for 12 patients, being all of those with pararenal aneurysms, all of those with thoracoabdominal aneurysms, and two of those with juxtarenal aneurysm. All these patients had an uneventful postoperative course and no reinterventions were needed during follow-up. Nevertheless, these 21 patients were excluded from the analysis, leaving a total of 1112 patients treated for an infrarenal abdominal aortic aneurysm (660 EVAR, 452 OR) included in the intention-to-treat analysis of 30-day and long-term outcomes.
There were no significant differences between the two cohorts of patients in terms of aneurysm diameters, (Table Ib; EVAR group: median 53 mm, IQR 52–55 mm, range 45–91 mm; OR group: median 51 mm, IQR 45–51.5 mm, range 35–120 mm; P = .58). However, there were significant differences between the distribution of both small-medium (45–60 mm) and large (≥60 mm) aneurysms. In particular, 28.5 and 15% of AAAs were greater than 60 mm in the OR and EVAR group, respectively (P < .0001). On the other hand, the proportion of “very small AAAs”, defined as those with a diameter <45 mm, was similar between the two groups, being 2.7% in the EVAR group vs. 3.5% in the OR group (P = .62). Notably, all these “very small AAAs” had a saccular morphology with an aneurysmal neck longer than 20 mm.
The morphology of the aneurysmal neck was significantly different between the two groups in terms of length and angulation on the coronal axis, reflecting the indication for the different types of procedure. No differences were recorded between the groups in the number of AAAs with a very long aneurysmal neck (≥20 mm). In the EVAR group, primary success, defined as the successful delivery of the endograft without any intraoperative endoleaks, graft thrombosis, or conversion to OR, was achieved in 98.9%. We recorded three cases of immediate type Ia endoleak, which was corrected with a proximal aortic cuff. Another patient required endovascular relining of the graft because of immediate disconnection between the main body and the iliac limb (type III endoleak). One patient required implantation of a conical endograft with a femoro-femoral right-to-left bypass for occlusion of the contralateral gate during the cannulation. Immediate conversion to OR was necessary in three patients: for aortic rupture in two and for persistent occlusion of both iliac limbs in one.
Intraoperative complications occurred in 34 (7.5%) of the OR group patients, the most common complication being acute limb ischemia, which was resolved by embolectomy in all. Three patients required a splenectomy, three required partial intestinal resection, and one required right nephrectomy. There were three cases of myocardial infarction during the operation and two cases of intraoperative aortic rupture. In seven patients, the inferior mesenteric artery was re-implanted onto the graft because of poor back-flow. In all except three patients, both the hypogastric arteries received either direct or reverse vascularization, and in those patients with one occluded hypogastric artery, there was no need for additional revascularization.
The median length of in-hospital stay, calculated only for the patients discharged from hospital, was 9 days after OR (IQR 8–12 days), which was significantly longer than that after EVAR (median 3 days, IQR 3–5 days, P = .001). Four and seven patients died in hospital after EVAR and OR, respectively (0.6 vs. 1.5%, P = .02).Thirty-day mortality was higher after OR than EVAR (2.9% vs. 1.1%, odds ratio = 2.6, 95% CI 1.1–6.6, P = .03). Complications in the first 30 days were much more likely to occur after OR than after EVAR (24.7% vs. 1.1%, odds ratio = 29.2, 95% CI 14.5–70, P < .0001). The causes of 30-day mortality were similar in the two groups (Table 2).
The most frequent complications reported within 30 days after OR were respiratory failure (9.9%) mainly caused by pneumonia, and wound complications (6.6%). There was one case of early graft infection resulting in fatal sepsis.
Median follow-up was 63.7 months (range 1–197.8 months). At 5 and 10 years, the estimated survival was 83.9 ± 1.9% and 66.1 ± 3.2%, respectively, after OR and 87.1 ± 1.5% and 78.1 ± 2.2%, respectively, after EVAR (P = .0006, Fig. 2). Univariate analysis showed that age was the only factor significantly associated with death in the long-term for both EVAR and OR, being HR 2.02 for a 1-year increase after EVAR (CI 95% 1.04–3.09) and 2.15 for a 1-year increase after OR (CI 95% 1.21–4.81); both P = .002. Multivariate analysis also showed that age was significantly associated with the different survival of the two groups (HR 1.89, CI 95% 1.01–2.23, P = .03). Deaths in the long-term were mainly not graft-related, but were most frequently related to neoplasms and trauma. One patient died of an aortic graft infection and one, of an aorto-oesophageal fistula, 8 and 1.7 years after OR, respectively. In the long-term, 98 patients died after EVAR and 131 died after OR.
In the long-term, 13 of the EVAR patients required OR, for persistent proximal type Ia endoleaks (n = 6), endotensions with progressive enlargement of the sac (n = 3), stent-graft infections (n = 2), type III endoleak with aortic rupture (n = 1), and complete graft thrombosis (n = 1). Estimated freedom from rupture at 10 years was 97.9 ± 0.7% after EVAR and 96.5 ± 0.4% after OR (P = .11). Aortic rupture occurred in two patients after EVAR and in two after OR in the long-term. Freedom from reintervention at 5 and 10 years was 97.7 ± 0.8% and 93.5 ± 1.8%, respectively, after OR, and 93.4 ± 1.1% and 88.4 ± 1.8%, respectively, after EVAR (P = .005, Fig. 3). The main reasons for reintervention were any type of endoleak (EL) after EVAR (7.1%), type Ia EL being the most frequent (18 out of 47, 38.3%), and a proximal pseudoaneurysm (PSA) after OR (3.8%). Among all the factors analyzed, preoperative aneurysm diameter was the most important factor associated with the occurrence of these complications (both P < .0001; Table 3), with a Hazard Ratio of 1.1 and 1.17, respectively, for OR and EVAR per 1 cm increase.
None of the patients with a type Ia EL or a proximal PSA had a proximal aortic neck shorter than 15 mm or more angulated more than 60° on the coronal axis. However, nearly all these patients had an AAA that was larger than 60 mm preoperatively (94.4% of patients with a type Ia EL and 100% of those with a proximal PSA). Receiver Operating Characteristic (ROC) analysis showed that a preoperative AAA diameter greater than 59 mm in the EVAR group and greater than 57 mm in the OR were significantly predictive of these complications (Area Under Curve: 99% and 87.8%, respectively, for EVAR and OR). Neither the saccular morphology of the AAA nor the neck length were associated with the occurrence of EL or PSA after EVAR or OR, respectively. A separate assessment of the outcome results was performed by the two treatment centers. The hospital performing each type of treatment was included as a covariate in all models (Hospital 1 vs. Hospital 2), but no significant difference was recorded.
Discussion
EVAR for elective infrarenal AAA repair accounts for up to 80% of all AAA repairs done in the United States [5]. In fact, according to the most recent SVS guidelines, EVAR should be considered as first line therapy in an emergent setting, if the AAA is suitable for endovascular repair. Similarly, we recorded a paradigm shift from “EVAR in patients unfit for OR” towards “OR in patients unfit for EVAR”, with anatomical condition being the most important determining factor. Patients with very short proximal neck or excessive proximal neck angulation, as well as those with aortoiliac occlusive disease, preferably underwent OR. This change in clinical practice will require more skilled vascular surgeons who are familiar with supraceliac aortic cross-clamping and must also be able to face challenging open conversion after EVAR.
One consideration arising from our real-world experience was linked to this paradigm shift, which in our clinical practice led to a redefinition of “high surgical risk”. This new definition applied to patients who were unfit for both EVAR and OR. The second consideration was the evolution of EVAR devices. Over 14 years, we observed that EVAR evolved technically more than OR, reflecting improvements in the developing technologies linked to the “EVAR world”. These developments aimed to achieve a graft with a better fixation and to expand the cohort of patients who could be treated with EVAR; for example, through the introduction of ultra-low profile endografts. The trend; however, will be towards greater use of fenestrated and branched devices for more complex aneurysms involving renal and visceral vessels, beyond the anatomical limits considered so far. There is much scientific evidence [1] that EVAR has an immediate and mid-lasting advantage in terms of mortality over OR, but loses the comparison in the long-term reintervention rates. The only trial that did not show any differences in long-term reoperations was the OVER trial [6], which took into consideration the wound complications after OR within a 2-year follow-up.
As expected, 30-day mortality was higher after OR than after EVAR in this series, with rates that compared favorably to those of randomized trials. However, in contrast to what was reported in the trials, long-term mortality was better after EVAR than OR. Recently, Chang et al. [7] analyzed the longitudinally linked California Office of Statewide Health Planning and Development inpatient database, reporting results on 23 670 patients treated between 2001 and 2009 by either EVAR or OR. Similarly, they found a survival advantage for EVAR repair, maintained for 3 years postoperatively. However, deaths in the long-term were not generally related to the procedure, but to neoplasms. In both groups, age was the only factor significantly associated with long-term mortality.
Toya et al. [8] found that aneurysm-related death was more likely in patients with postoperative chronic kidney disease (CKD) progression. The presence of a shaggy aorta, absence of oral beta-blocker administration, and an elevated preoperative creatinine level were independent predictors of early postoperative CKD progression. According to Haga et al. [9], pre- and postoperative adequate hydration, postoperative diuretics, and low-dose dopamine could help reduce the risk of CKD progression. On the other hand, we noted a higher reintervention rate in the long-term after EVAR than after OR, in accordance with all data reported in the literature. Reinterventions were needed irrespective of the type of endograft used. In our experience, all main endografts were placed according to specific IFUs and we did not claim to assess the superiority of one device over another. Interestingly, a similarity in reinterventions was found in both groups, since the occurrence of type Ia endoleak and proximal anastomotic pseudoaneurysm were the main reasons for long-term reintervention after EVAR and OR, respectively. The preoperative diameter of the aneurysm was the most important predictor of the development of type Ia endoleaks after EVAR and proximal pseudoaneurysm after OR in the long-term. This could be the most important finding in our analysis and, to our knowledge, has not been reported before. In our experience, OR is generally the preferred procedure for larger AAAs; however, that complication was associated with the preoperative diameter of the AAA in both groups, with a Hazard Ratio of 1.1 and 1.17, respectively, for OR and EVAR, per 1-cm increase in diameter. The robustness of these findings was supported by the fact that the differences observed were not simply due to a comparison of fusiform to saccular aneurysms or very long necks vs. average length necks.
Our findings suggest that repairing AAAs before their diameters become too large may reduce the chance of PSA/type Ia endoleaks in the long-term, as small and medium size AAAs were less prone to that complication. Moreover, regardless of the type of procedure and endograft used, we must bear in mind that aneurysmal disease is an evolving pathology, which usually involves the aortic wall structure in its entirety. In the EVAR group, even when an endograft with active proximal fixation was used, the proximal aortic neck, which had been considered “healthy”, degenerated over time, with loss of the proximal sealing. The proximal neck dilated also dilated after OR, but in considerably fewer patients. When OR was performed, the proximal anastomosis was always performed on what was thought to be “the best” aortic neck, based on both the preoperative imaging and intraoperative findings. These speculations obviously need further investigation, although aortic neck dilation has been described after EVAR with self-expanding devices [10]. Moreover, De Donato et al. [11] reported no aortic neck evolution after EVAR in the mid-term when an endograft with no chronic outward force was used.
One of the advantages of our study was the ability to report a “real-world” experience of patients with infrarenal AAAs over a long-term follow-up period after treatment, without exclusion criteria, apart from acutely ruptured AAAs, which are a subject of separate discussion. Moreover, both EVAR and OR were offered to the patients by vascular urgeons with experience and expertise in both procedures. In contrast to a database analysis, a “real-world” experience allows all clinical variables to be collected and analyzed as appropriate. However, the observational and retrospective nature of the study also represents its main limitation.
Conclusion
Both EVAR and OR achieved satisfactory immediate and long-term results for the elective treatment of an AAA. Patients treated with EVAR had a higher reintervention rate, but better survival than those treated with OR in the long-term. The preoperative diameter of the aneurysm was the most important predictor of endoleaks after EVAR and proximal pseudoaneurysm after OR in the long-term.
References
Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014;1:CD004178.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46(6):668–85.
Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50:S2–49.
Dalainas I, Nano G, Casana R, Tealdi Dg. Mid-term results after endovascular repair of abdominal aortic aneurysms: a four-year experience. Eur J Vasc Endovasc Surg. 2004;27:319–23.
Vallabhaneni R, Farber MA, Schneider F, Ricco JB. Debate: whether young, good-risk patients should be treated with endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2013;58:1709–15.
Kyriakides TC, Lederle F, Freischlag J. Open versus endovascular repair of abdominal aortic aneurysms: the OVER trial. Clin Trials. 2004;1(Suppl 1):212.
Chang DC, Parina RP, Wilson SE. Survival after endovascular vs open aortic aneurysm repairs. JAMA Surg. 2015;150:1160–6.
Toya N, Ohki T, Momokawa Y, Shukuzawa K, Fukushima S, Tachihara H, et al. Risk factors for early renal dysfunction following endovascular aortic aneurysm repair and its effect on the postoperative outcome. Surg Today. 2016;46(12):1362–9.
Haga M, Hoshina K, Shigematsu K, Watanabe T. A perioperative strategy for abdominal aortic aneurysm in patients with chronic renal insufficiency. Surg Today. 2016;46(9):1062–7.
Diehm N, Dick F, Katzen BT, Schmidli J, Kalka C, Baumgartner I. Aortic neck dilatation after endovascular abdominal aortic aneurysm repair: a word of caution. J Vasc Surg. 2008;47:886–92.
de Donato G, Setacci F, Bresadola L, Castelli P, Chiesa R, Mangialardi N, et al; TriVascular Ovation Italian Study. Aortic neck evolution after endovascular repair with TriVascular Ovation stent graft. J Vasc Surg. 2016;63:8–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Daniela Mazzaccaro and her co-authors have no conflicts of interest.
Additional information
D. Mazzaccaro and G. Nano shared first authorship.
P. G. Settembrini: SVS Member.
Rights and permissions
About this article
Cite this article
Mazzaccaro, D., Nano, G., Settembrini, A.M. et al. Open and endovascular elective treatment of abdominal aortic aneurysms: a real-world experience. Surg Today 47, 1347–1355 (2017). https://doi.org/10.1007/s00595-017-1525-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1525-7