Abstract
Purpose
In order to analyze the changes of glucose metabolism by maximum standardized uptake value (SUVmax) of 18F-FDG PET/CT in patients with rectal cancer submitted to neoadjuvant radiochemotherapy (nRCT) and to correlate SUV changes with tumor regression grade (TRG).
Methods and material
Three sequential 18F-FDG PET/CT studies were performed in 31 patients with rectal cancer at the following time point: before starting the treatment (PET/CT1), during the treatment (PET/CT2), and after completion of neoadjuvant treatment (PET/CT3). The SUVmax values of the rectal lesion in the PET/CT1 (SUV1), PET/CT2 (SUV2), and PET/CT3 (SUV3) were obtained; deltaSUV1 [(SUV1 − SUV2)/SUV1] and deltaSUV2 [(SUV1 − SUV3)/SUV1] were also calculated. Metabolic parameters were compared to TRG.
Results
Significant differences in pathologic responder and non-responder patients were found only for SUV2 (6.4 ± 2.9 in responder and 10.7 ± 4.8 in non-responder patients, respectively; P = 0.006) and SUV3 (3.6 ± 1.4 in responder and 6.6 ± 2.1 in non-responder patients, respectively; P = 0.0009). The best predictor for TRG response was SUV3 (threshold of 4.4) with sensitivity, specificity, accuracy, negative predictive value, and positive predictive value of 77.3%, 88.9%, 80.7%, 61.5%, and 94.4%, respectively.
Conclusion
18F-FDG PET/CT is a reliable and accurate technique to assess the response to nRCT in rectal cancer. In our population, the absolute value of SUVmax after treatment was the best predictor of pathological response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neoadjuvant radiochemotherapy (nRCT) in rectal cancer is an established standard procedure since the results of randomized multi-center trials [1–3]; it has been shown to produce a significant downsizing and downstaging of locally advanced rectal cancer (LARC), to increase the rate of complete surgical resection and to reduce the local recurrence [2–5]. Despite these advances, up to 38% of patients will eventually die because of recurrent disease [6, 7]. With respect to management of primary disease, accurate evaluation of the response to neoadjuvant treatment can potentially help to optimize surgical therapy, predict long-term outcome, or modify chemo-radiation treatment in those patients with less than optimal response. The current standard method for discriminating responders from non-responders patients is conventional histopathologic analysis, measuring the extent of the residual tumor. This method, however, is applicable only in a postoperative setting and consequently can be used neither for the preoperative selection of personalized treatment (as sphincter-saving surgery in deep-seated tumors, less aggressive resection in minimally advanced tumors) nor for planning of intraoperative radiation therapy or modulating the preoperative treatment. Considering these issues, there is the need for reliable noninvasive methods suitable for the prediction of response to neoadjuvant RCT.
Endorectal ultrasound (ERUS), computed tomography (CT), and magnetic resonance imaging (MRI) generally provide useful information about invasion of the intestinal wall (T staging), lymph node metastases (N), and distant metastases (M). Nevertheless, when applied to assess tumor response to neoadjuvant therapy, purely morphological imaging methods can yield equivocal results, with accuracy values ranging from 30% to 60% [8–12]. This is mainly due to the difficulties in distinguishing fibrotic regressive changes from viable tumor cells in residual masses.
18F-FDG PET is a powerful, noninvasive tool for imaging tumor metabolic activity, particularly suitable to assess changes in tumor glucose metabolism after neoadjuvant treatment. The semiquantitative assessment of glucose metabolism by evaluating the standardized uptake value (SUV) has been shown to have clinical relevance in evaluating response to nRCT in several tumor types, including esophageal and gastric cancer [13–16]. Recently, this functional imaging technique has been shown to have an emerging role in the evaluation of treatment response in patients with rectal cancer [17–22].
The objective of this study is to analyze the change in glucose metabolism measured by maximum standardized uptake value (SUVmax) of 18F-FDG PET/CT in patients with rectal cancer submitted to neoadjuvant treatment and to correlate metabolic change with tumor regression grade (TRG) [23] considered as standard reference for treatment response.
Methods and material
Population
From May 2006 to March 2009, 31 patients, 8 female and 23 male (mean age: 67 years; range: 43–82) with rectal cancer were prospectively enrolled in this study, after giving informed consent. The eligibility criteria were the presence of a biopsy proven rectal cancer, the absence of distant metastases at clinical staging, no other tumor in the clinical history of the patient and no contraindications to nRCT.
All the patients were submitted to staging procedure including recto-colonoscopy, contrast enhanced CT of the chest and abdomen and ERUSs and/or MRI of the lower abdomen.
Within 12 weeks after the completion of nRCT and before surgery, all patients were re-evaluated with ERUS and/or MRI of the pelvis for defining the surgical approach.
18F-FDG-PET/CT acquisition protocol and image analysis
All studies were performed with a PET/CT scanner (Discovery ST—GE Healthcare, Milwaukee, WI, USA) consisting of a PET scanner coupled with a multi-detector CT scanner allowing the acquisition of co-registered CT and PET images at the same time.
For our acquisition protocol, patients fasted for at least 6 h before the intravenous administration of 3.7 MBq/kg body weight of 18F-FDG. Blood glucose level was checked before tracer administration and patients with glucose level above 170 mg/dL were excluded from the study. All patients were orally hydrated (500 mL of water) during the FDG uptake period and were asked to empty their bladder before positioning for the scan. About 60 min after the injection of the tracer, the PET/CT study was started. CT was acquired first, during shallow breathing, with 140 kV, 60 mA, 0.8 s each rotation, pitch 1.65 and 3.75 mm of slice thickness, without oral or intravenous contrast media. PET was acquired in 3D mode, 3 min per each bed position; images were reconstructed with ordered subsets expectation–maximization (OSEM) algorithm, 128 × 128 matrix size, attenuation, random, and scatter corrected. Attenuation correction was performed on the basis of CT scan data. The CT pixel values measured in Hounsfield units were transformed into linear attenuation coefficients for the 511-keV energy radiation. CT and PET images were then matched and fused into transaxial, coronal, and sagittal images.
Timing of PET/CT acquisition
The first PET/CT scan (PET/CT1) was performed within 4 weeks before starting the treatment in all 31 patients enrolled; the second scan (PET/CT2), available for 28/31 patients enrolled, was performed within 3 weeks after the beginning of the treatment. The third scan (PET/CT3) was obtained in all 31 patients within 12 weeks after the completion of therapy and before surgery.
Image interpretation
PET, CT, and fused PET/CT images were displayed on Xeleris workstation (GE Medical Systems, Milwaukee, WI). Images were interpreted by two experienced nuclear medicine physicians without knowledge of clinical data, but the presence of rectal cancer. Lesion uptake was identified as an area of pathologically increased 18F-FDG uptake, excluding causes of nonspecific or physiologic accumulation of the tracer. Regions of interest (ROIs) were drawn over the region of pathological uptake in the baseline scan (PET/CT1) for the calculation of SUV1. At subsequent PET/CT (2 and 3) images were co-registered with the baseline study by means of the anatomical CT and the ROIs were drawn in the same positions of the PET/CT1. In three patients increased uptake were present in the rectum in PET/CT2 or PET/CT3 outside the primary tumor site detected on PET/CT1; this was not considered for SUV definition and it was interpreted as inflammation.
SUV values were calculated using the maximum activity values within each ROI on the transaxial slices, normalized to the injected dose and patient’s body weight.
The SUVmax values in the PET/CT1 (SUV1), PET/CT2 (SUV2), and PET/CT3 (SUV3) were used to define deltaSUV1 and deltaSUV2 in percentage as follows:
Neoadjuvant treatment
Radiation treatment
Pelvic irradiation was performed with treatment volumes defined accordingly to the distance of the lesion from anal verge. For caudal rectal lesions (from 0 to 5 cm), the inferior limit was 1 cm below the anal radiopaque marker put on the anal verge during the simulation and including perineum; the superior limit was defined on a plane passing between L5 and S1; the lateral borders of Antero-Posterior (AP) fields were 2 cm beyond the bony margin of minor pelvis to include iliac lymph nodes. The lateral fields (gantry 90° and 270°) had the same superior and inferior limits of AP; the anterior margin was just behind the pubic symphysis; the posterior fields covered the whole sacrum to the posterior margin. For a lesion of the middle rectum (5–12 cm from the anal verge), only inferior limit of AP and lateral fields were modified: the treatment volume included, in this case, the pelvis just below the ischiatic tubers.
The technique is called Box 3: three fields, at gantry 90°, 270°, and 0° and lateral wedges; the patient is in prone position, with a belly-board device. During CT simulation, performed without contrast media, a radiopaque marker was located on the anal verge to radiologically define the inferior border of the treatment volume. The prescribed dose was 50.4 Gy at the isocenter, in 28 fractions (daily dose: 1.8 Gy), with 15 MeV photons.
Chemotherapy
During radiotherapy period, concurrent chemotherapy was performed in 26/31 patients with 225 mg/m2/die of 5-Fluorouracil with continuous venous infusion (cvi). One patient received Capecitabine at the dose of 1700 mg/m2/day and four patients received 225 mg/m2/day of 5-Fluorouracil c.v.i. plus Oxaliplatinum at the dose of 60 mg/m2/week.
Radio-chemotherapy was well tolerated and there was no need to suspend the treatment due to toxicity. Hematologic and gastroenteric toxicity were <G2 (WHO classification).
Surgery
All patients underwent surgery within 12 weeks after nRCT completion. The surgical approach was defined considering the clinical and radiological response to neoadjuvant RCT after conventional restaging. PET/CT findings were never used to modify the surgical approach.
Histopathology and TRG definition
Resected specimens were analyzed according to Wang et al. [24]. Each specimen was fixed in 10% buffered formalin for at least 48 h and inked. Serial transversal tissue blocks were cut at 5 mm intervals from the distal portion. Each block, consisting of the full thickness of the rectal wall and the mesorectum, was embedded in paraffin. Whole-mount sections were obtained and stained with hematoxylin and eosin. All the specimens were examined by a single pathologist, expert in gastrointestinal pathology.
Pathologic response to the treatment was defined according to TRG defined by Mandard et al. [23] as following: TRG 1 was defined as complete regression without neoplastic cells and only fibrosis; TRG 2 presence of rare residual cancer cells scattered through fibrotic tissue; TRG 3 increased number of residual cancer cells but fibrosis still predominant; TRG 4 residual cancer outgrowing fibrosis; and TRG 5 no regressive changes detectable. The TRG 1 and 2 scores were considered as a response, while TRG from 3 to 5 as non-response to treatment [25]. Pathological stages was also define according to (y)TNM definition of International Union Against Cancer (UICC) [26] and compared to clinical stage before treatment.
Statistical analysis
Stata software 9.0 (Stata Corporation, 1999, Texas-US) was used to perform the statistical analysis. A level of P < 0.05 was adopted for significance. First, time interval between PET/CT studies, radio-chemotherapy and surgery in pathologic responder and non-responder patients were compared. In particular, the time interval deltaT1 (days from the start of therapy to PET/CT2), deltaT2 (days from the end of therapy to PET/CT3), and deltaT3 (days from PET/CT3 to surgery) in responder vs. non-responder patients were considered.
Mean, median, and standard deviation of the SUV1, SUV2, and SUV3 were calculated and the comparison between SUV1 and SUV2 and between SUV1 and SUV3 was done using paired t Student test. The comparison of the same metabolic parameter was also performed between histopathologic responders and non-responders patients with the non-parametric Mann–Whitney U test. A linear regression analysis was performed to investigate the association between TRG scores and SUV and deltaSUV; then a multiple linear regression was also calculated to find the independent variables in predicting the pathologic TRG response.
Receiver operating characteristic (ROC) analysis was performed to define the best accuracy of the metabolic parameters in predicting the response to treatment.
Results
Patients
Before neoadjuvant treatment, the clinical stage (cTNM) was I in three patients, IIA in four patients, IIB in one patient, IIIA in three patients, IIIB in 16 patients, and IIIC in four patients. For surgical treatment, anterior resection of the rectum was performed in 24 patients, Miles resection in five patients, and total mesorectal excision (TME) in two patients. The histology type from resected specimens was adenocarcinoma in all patients, one with G1 grading, 15 with G2, and four with G3; in 11 patients the histologic grading of the lesion were not available.
According to UICC classification [26], the pathologic stage [(y)pTNM] obtained from the resected specimens after the neoadjuvant treatment was I in eight patients, IIA in six patients, IIIA in two patients, IIIB in two patients, and IIIC in three patients; in nine patients neoplastic tissue was not found (T0N0M0) with complete regressive changes at histology (TRG 1).
In comparison to clinical stage (cTNM), pathologic downstage after therapy was found in 24/31 (77.4%).
The histologic TRG score was as follows: TRG 1 was found in four patients, TRG 2 in 13 patients, TRG 3 in six patients, TRG 4 in two patients, and TRG 5 in one patient. Considering responder those patients with TRG 1–2 and non-responder those with TRG 3–5, overall 22/31 (71%) responder patients and 9/31 (29%) non-responder patients were found.
18F-FDG PET/CT results
Mean SUVmax and deltaSUV values of the rectal lesion for each PET/CT study are given in Table 1. SUV1 was found significantly higher than both SUV2 and SUV3 (paired t Student test: P < 0.0001 in both cases); the same result was obtained comparing SUV2 and SUV3, with the latter parameter significantly lower than the former (paired t Student test: P < 0.0001). As for deltaSUV1, a marked increase of FDG uptake (58%) at PET/CT2 was observed in one patient.
Mean SUV values of pathologic responder and non-responder patients are given in Table 2. A statistically significant difference for SUV2 (P = 0.006) and SUV3 (P = 0.0009) between the two groups was found. No statistically significant differences were found for all the other parameters and in particular for deltaSUV1 and deltaSUV2.
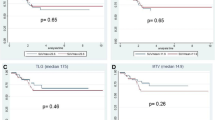
Results from univariate linear regression analysis comparing metabolic parameters to TRG groups are shown in Fig. 1. Among all the metabolic parameters considered in our study, a direct significant correlation with TRG groups was found for SUV2 (P = 0.048) and mostly for SUV3 (P = 0.009), but only the latter parameter was significantly correlated to TRG in a multivariate analysis (P = 0.002).
In order to find out the most accurate metabolic variable predicting pathologic response to treatment, ROC curves were calculated (Table 3, Fig. 2). The highest accuracy in predicting the response to treatment was obtained with a SUV3 cut-off value of 4.4. With this threshold, FDG uptake at PET/CT3 was true positive (TP) for TRG response in 17 patients, true negative (TN) in eight patients, false positive (FP) in one patient, and false negative (FN) in five patients, resulting in a sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of 77.3%, 88.9%, 80.7%, 94.4%, and 61.5%, respectively. As shown in Table 3, a similar value of accuracy (78.6%), but with a lower PPV (88.2%) was obtained with a SUV2 and a threshold value of 7.3. In Figs. 3 and 4 an example of metabolic responder and non responder patient is shown respectively.
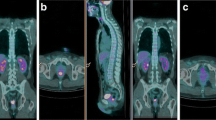
An example of PET/CT studies in a patient with metabolic response is reported. There is a significant decrease of SUV values from 11.2 in PET/CT1 study (first row) to 7.0 and 2.2 in PET/CT2 and PET/CT3, respectively (second and third rows). deltaSUV1 and deltaSUV2 were 37.5% and 80.4%, respectively. TRG on rectal specimen was 2.
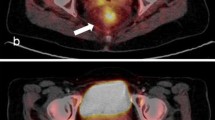
An example of PET/CT studies in a metabolic non-responder patient is reported. The SUV value of the rectal lesion (white arrows) was 22.8 in PET/CT1 study (first row) and decreased to 13.9 and 5.5 in PET/CT2 and PET/CT3, respectively (second and third rows). deltaSUV1 and deltaSUV2 were 39.0% and 75.9%, respectively. TRG on rectal specimen was 4.
As for the effect of timing, no statistically significant differences were found in responder and non-responder patients for each deltaT parameter considered, with only a trend to significance for deltaT3, as showed in Table 4.
Discussion
Neoadjuvant therapy is considered a valid approach in patients with rectal cancer for preventing local recurrence and it may be a successful treatment for less aggressive surgery technique, in particular for sphincter-preserving surgery [27, 28].
Aim of this study was to evaluate if the changes of glucose metabolism in rectal cancer measured by 18F-FDG PET/CT performed before, during, and after neoadjuvant therapy, can be used to predict therapy response in comparison to TRG considered as the standard reference.
In our study, a significant decrease of SUV from 16.3 ± 8.6 to 8.1 ± 5.9 and to 4.5 ± 2.1 was found, in agreement with previously published results. Rosenberg et al. [22] in 30 patients with rectal cancer submitted to PET/CT before, during, and after neoadjuvant treatment, showed a significant decrease of SUV in rectal lesion from 9.5 to 5.5 (P < 0.001) and finally to 3.5 (P < 0.001). Similarly, Cascini et al. [18] in 33 patients with rectal cancer submitted to 18F-FDG PET at baseline and 12 day after the start of therapy found a significant change of SUVmax values that decreased from 11.20 at baseline to 6.0 after 12 days (P < 0.0001). In 17 out of 33 patients a PET scan was also performed after the end of therapy and before surgery; a further reduction of SUV was found with a median value of 2.7 (P = 0.012).
The correlation between therapy-related changes in FDG uptake and tumor response in rectal cancer has been previously reported by several groups [8, 18, 20, 22]. In these studies the percentage of SUV reduction, both early during the treatment and late after its completion, was found to be the parameter best related to pathologic response. In our study, SUV3 was statically different between histopathologic responders (TRG 1–2) and non-responder (TRG 3–5) patients with a mean value of 3.6 ± 1.4 and 6.6 ± 2.1, respectively (P = 0.0009). On the contrary, no statistical differences were found for SUV1 (P = 0.151), deltaSUV1 (P = 0.147), and deltaSUV2 (P = 0.151). Regarding early metabolic response, Cascini et al. [18] showed that early responder patients, evaluated with PET/CT 12 days after the start of therapy, had an higher decrease of SUV than non-responder patients (62% vs. 28%, respectively; P < 0.0001). Rosenberg et al. [22] did not obtain the same result but showed a percentage of early SUV reduction with a trend to be different in responder and non-responder patients (44.3% and 29.6%, respectively; P = 0.085). In our patients population the deltaSUV1 and deltaSUV2 values neither were correlated with TRG nor were able to discriminate histopathologic responder from non-responder patients. We have to consider that in our patients the deltaSUV values showed a wide range of fluctuation ranging from an increase of 58%, in one patient with clinical signs of proctitis, to a decrease of 89.4%. Beside this, as a matter a fact, in the only patients with TRG 5 we found deltaSUV1 and deltaSUV2 of 51% and 77%, respectively, with absolute value SUV2 and SUV3 of 20.4 and 9.8, respectively; this patient was correctly classified as non-responder with both SUV2 and SUV3 threshold (7.2 and 4.4, respectively) but not with deltaSUV1 and deltaSUV2 threshold (49.1% and 60%, respectively—Table 3). Similarly, in another patients with TRG 4, we found SUV2 and SUV3 13.9 and 5.5, respectively; according to our threshold values, patient was considered as metabolic non-responder for both parameter. At the opposite deltaSUV1 was 39% matching as metabolic non-responder patients, whereas deltaSUV2 was 76% scoring the patient as metabolic responder. These observations were confirmed by linear regression analysis where SUV2 and mostly SUV3 were found to be the only parameters significantly correlated to TRG response.
In our study, ROC curves analysis (Fig. 2, Table 3) have shown that SUV3 has the best accuracy (80.7%) in predicting response to neoadjuvant treatment with a threshold value of 4.4. Similar accuracy (78.6%) was obtained for SUV2 with a threshold of 7.3, but with a lower specificity and PPV. It is of interest to note that in our study the PPV of SUV3 in predicting response was very high (94.4%) suggesting more conservative surgical approaches only in patients with evidence of lower glucose uptake at the end of neoadjuvant treatment. On the other hand, NPV was quite low (61.5%), due to the presence of high SUV values, ranging from 5.5 to 6.9, in five patients with TRG 2 (FN). At histology, even if not homogeneously, an inflammatory component was found in almost all patients and this could explain the persistent metabolic activity after the radiation therapy, as previously addressed by other authors [29, 30].
In our study, both deltaSUV1 and deltaSUV2 were found inadequate to define the response to treatment, although a trend to be statistically different in responder and non-responder patients was present. As in our protocol PET/CT and surgery timing was given a larger range than in other studies [18–20, 22] a statistical analysis was performed to explore the possible correlation between the time course of PET/CT, surgery, and pathological response (Table 4). No significant differences were found in the two groups of patients depending on time interval between therapy starting and PET2, but a trend could be observed for the time between PET3 and surgery. This result may be an indication toward a more rigorous timing in performing the different phases of the protocol in order to observe in each patient the same functional event at the same time.
This study protocol is still ongoing, and the patient sample needs to be increased, particularly non-responder patients. This might increase the diagnostic accuracy of other metabolic parameters as deltaSUV in defining the response to treatment.
Conclusion
Metabolic imaging with 18F-FDG PET/CT can be reliably used to assess the response to neoadjuvant treatment in patients with rectal cancer. In our experience, the absolute value of SUVmax of rectal lesion after the treatment was the best predictor of TRG response. Finally, the high PPV of SUV3 in predicting response to treatment may suggest more conservative surgical approaches in patients with lower metabolic activity at the end of neoadjuvant therapy.
References
Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Sauer R, Becker H, Hohenberger W, et al. (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Bosset JF, Collette L, Calais G, et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123
Minsky BD, Cohen AM, Kemeny N, et al. (1992) Enhancement of radiation-induced downstaging of rectal cancer by fluorouracil and high-dose leucovorin chemotherapy. J Clin Oncol 10:79–84
Rodel C, Grabenbauer GG, Schick C, et al. (2000) Preoperative radiation with concurrent 5-fluorouracil for locally advanced T4-primary rectal cancer. Strahlenther Onkol 176:161–167
Guillem JG, Chessin DB, Cohen AM, et al. (2005) Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 241:829–836 ((discussion 36–38))
Jemal A, Tiwari RC, Murray T, et al. (2004) Cancer statistics, 2004. CA Cancer J Clin 54:8–29
Amthauer H, Denecke T, Rau B, et al. (2004) Response prediction by FDG-PET after neoadjuvant radiochemotherapy and combined regional hyperthermia of rectal cancer: correlation with endorectal ultrasound and histopathology. Eur J Med Mol Imaging 31:811–819
Hoffmann KT, Rau B, Wust P (2002) Restaging of locally advanced carcinoma of the rectum with MR imaging after preoperative radio-chemotherapy plus regional hyperthermia. Strahlenther Onkol 178:386–392
Deneke T, Rau B, Hoffmann KT (2005) Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol 15:1658–1666
Chen CC, Lee RC, Lin JK, Wang LW, Yang SH (2005) How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum 48:722–728
Rau B, Hunerbein M, Barth C, et al. (1999) Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc 13:980–984
Weber WA, Ott K, Dittler HJ, et al. (2001) Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 19:3058–3065
Ott K, Fink U, Becker K, et al. (2003) Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 21:4604–4610
Ott K, Weber W, Lordick F, et al. (2006) Metabolic imaging predicts response, survival, and recurrence in adenocarcinoma of the esophagogastric junction. J Clin Oncol 24:4692–4698
Brucher BL, Weber W, Bauer M, et al. (2001) Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg 233:300–309
Kalff V, Duong C, Drummond EG, et al. (2006) Findings on 18FFDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med 47:14–22
Cascini GL, Avallone A, Delrio P, et al. (2006) 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med 47:1241–1248
Capirci C, Rubello D, Chierichetti F, et al. (2006) Long-term prognostic value of 18F-FDG PET in patients with locally advanced rectal cancer previously treated with neoadjuvant radiochemotherapy. AJR 187:202–208
Capirci C, Rampin L, Erba AP, et al. (2007) Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging 34:1583–1593
Calvo FA, Domper M, Matute R, et al. (2004) 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys 58:528–535
Rosenberg R, Herrmann K, Gertler R, et al. (2009) The predictive value of metabolic response to preoperative radiochemotherapy in locally advanced rectal cancer measured by PET/CT. Int J Colorectal Dis 24:191–200
Mandard AM, Dalibard F, Mandard JC, et al. (1994) Pathologic assessment of tumor regression after preoperative CRT of esophageal carcinoma. Clinicopathologic correlations. Cancer 73:2680–2686
Wang Z, Zhou Z, Wang C, et al. (2005) Microscopic spread of low rectal cancer in regions of the mesorectum: detailed pathological assessment with whole-mount sections. Int J Colorectal Dis 20:231–237
Vecchio FM, Valentini V, Minsky BD (2005) The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 62:752–760
Sobin L, Gospodarowicz M, Wittekind C (eds) (2009) International union against cancer: TNM classification of malignant tumors, 7th edn. New York: Wiley/Blackwell
Mohiuddin M, Regine WF, Marks J, et al. (1998) High-dose preoperative radiation and the challenge of a sphincter-preservation surgery for cancer of the distal 2 cm of the rectum. Int J Radiat Oncol Biol Phys 40:569–574
Valentini V, Coco C, Cellini N (2001) Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer. Acute toxicity, tumor response and sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys 51:371–383
Kao PF, Chou YH, Lai CW (2008) Diffuse FDG uptake in acute prostatitis. Clin Nucl Med 33:308–310
Becker K, Mueller JD, Schumacher C, et al. (2003) Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98:1521–1530
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guerra, L., Niespolo, R., Di Pisa, G. et al. Change in glucose metabolism measured by 18F-FDG PET/CT as a predictor of histopathologic response to neoadjuvant treatment in rectal cancer. Abdom Imaging 36, 38–45 (2011). https://doi.org/10.1007/s00261-009-9594-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-009-9594-8