Abstract
Aim
to assess the effects of advanced wound dressings (AWD) commonly used in the treatment of predominantly neuropathic diabetic foot ulcers (DFU) The present meta-analysis was designed to support the development of the Italian Guidelines for the Treatment of Diabetic Foot Syndrome (DFS).
Methods
A Medline and Embase search were performed up to April 1st, 2024 collecting all RCTs including diabetic patients or reporting subgroup analyses on diabetic patients with DFU comparing AWD with placebo/standard of care (SoC), with a duration of at least 12 weeks. Prespecified endpoints were: ulcer healing (principal), time-to-healing, frequency of dressings change, major and minor amputation, pain, and all-cause mortality. AWD assessed were: alginates; foam, hydrocolloids, hydrogels, hyaluronic acid, hemoglobin spray, silver-impregnated, sucrose octasulfate-impregnated, honey-impregnated, micro-organism-binding, and protease-modulating matrix dressings. Mantel-Haenzel Odds ratios and 95% confidence intervals (MH-OR, 95% CIs) were either calculated or extracted directly from the publications. Weighted mean differences (WMD) and 95% CIs were calculated for continuous variables.
Results
Fifteen studies fulfilled all inclusion criteria. Participants treated with AWD had a significantly higher ulcer healing rate and shorter time-to-healing in comparison with SoC/placebo (MH-OR 1.50 [0.80, 2.79], p = 0.20 and WMD:: − 24.38 [− 42.90, − 5.86] days, p = 0.010). No other significant effect on the above reported prespecified endpoints were observed. For the primary endpoint, the quality of evidence was rated as “moderate”.
Conclusions
In conclusion, AWD, particularly sucrose-octasulfate, hydrogels, hyaluronic acid, and honey dressings, can actively promote wound healing and shortening time-to-healing in patients with DFU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic foot ulcers (DFU) affect about 2–5% of patients with diabetes mellitus [1] and represent a challenging complication due to the higher associated risk of major adverse lower-limb events (MALE; i.e.: artery revascularization, sepsis, amputation, disability, and all-cause mortality) in comparison with diabetic patients without DFU [2].
DFU often caused by underlying conditions, such as peripheral artery disease, neuropathy, foot deformities, required timely treatments to reduce the risk of adverse events, recurrence, and ulcer healing failure [3, 4]. Gold standard treatments of DFU are therefore peripheral artery revascularization, management of infections, and plantar pressure relief. However, together with these therapeutic approaches, managed by a multidisciplinary team [5], local treatments, such as an appropriate debridement, exudate and local infection/inflammation management, and local active dressings, are equally important for promoting and accelerating ulcer healing [6]. Common local dressings have differential actions for managing exudate, infection, non-viable tissues, and promoting tissue granulation [6,7,8].
Several commonly used specialized wound dressings are available for DFU local treatment, including: alginates; hydrocolloids/hydrogels, silver-impregnated dressings, sucrose octasulfate- impregnated dressings, micro-organism-binding dressings, etc. Unfortunately, these widely used local advanced dressings have few evidence on their effectiveness and safety and their recommendation would require a formal evaluation on the basis of published randomized control trials.
This systematic review and meta-analysis of randomized control trials intends to assess the effects of local advanced dressings commonly used in the treatment of DFU. The aim of the prespecified analyses reported in this paper was to answer to a clinical question [9] (PICO):
"In patients with neuropathic (non-ischemic and non-infected) foot ulcers, is the use of an active dressing preferable to standard of care, to reduce adverse outcomes and increase the chance of ulcer healing” (PICO #9 [9])?
The present meta-analysis was, in fact, performed in the process of developing the Italian guidelines for the treatment of Diabetic Foot Syndrome (DFS) [9]. These guidelines, which have been promoted by the Italian Society of Diabetology (Società Italiana di Diabetologia, SID) and the Italian Association of Clinical Diabetologists (Associazione Medici Diabetologi, AMD), are being developed for inclusion in the Italian National Guideline System (INGS), designed as a standard reference for clinical practice in Italy, using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method [10].
Methods
We conducted this systematic review and meta-analysis in conformity with PRISMA checklist (Table S1) [11] and following a protocol previously published [9].
Search strategy and selection criteria
This meta-analysis is a part of a wider meta-analysis of studies on DFS conducted for the development of the Italian guidelines on the treatment of diabetic foot syndrome [9]. The present analysis includes all RCTs including diabetic patients or reporting subgroup analyses on diabetic patients with foot ulcers comparing advanced wound dressing with placebo/no therapy/standard of care, with a duration of at least 12 weeks. Animal studies were excluded, whereas no language or date restriction was imposed. A Medline and Embase search were performed up to April 1st, 2024 using the following search string: diabetic foot ulcer AND local AND treatment. Detailed information on the search strategy is reported in Table S2. Further studies were manually searched for references from retrieved papers.
Selection criteria
To be eligible, a study should enroll patients with diabetes and foot ulcers assessing the effects of advanced wound dressing compared either with placebo or standard of care.
The adjunctive treatments to be assessed were divided into two groups based on their main action and/or characteristics:
-
Advanced wound dressings
-
(1)
Alginate dressings: highly absorbent calcium alginate or calcium sodium alginate which can be combined with collagen. The alginate forms a gel at the wound surface level, which can increase absorbency.
-
(2)
Foam dressings: polyurethane foam dressings designed to absorb ulcer exudate and maintain moist wound surface.
-
(3)
Hemoglobin spray: topically application of hemoglobin to the wound bed to promote oxygen diffusion and increase granulation tissue formation.
-
(4)
Hydrocolloids: are occlusive dressings usually composed of a hydrocolloid matrix. This matrix forms a gel to provide a moist environment. Fibrous hydrocolloids have been developed for being more absorbent than standard hydrocolloid dressings.
-
(5)
Hydrogels: dressing made of insoluble polymers and designed to absorb wound exudate or rehydrate a wound. Hydrogels are supplied in either flat sheets or as beads.
-
(6)
Hyaluronic acid: is a naturally occurring, non-immunogenic, biodegradable polysaccharide acting with fibrin to support the influx of fibroblasts and endothelial cells into the wound site and the subsequent formation of granulation tissue. Interventions assessed: HA-impregnated inert pads, HA gel, or cream; pad or matrix composed entirely of HA.
-
(7)
Protease-modulating matrix dressings: capable of regulating the activity of proteolytic enzymes in chronic wounds.
-
(8)
Sucrose octasulfate-impregnated dressings (Technology Lipido-Colloid-Nano-OligoSaccharide Factor): the potassium salt contained in the sucrose octasulfate inhibits the excess of matrix metalloproteinase and interacts with growth factors promoting the restoration of their biological functions and contributing to new tissue formation.
-
(1)
-
Dressings with antimicrobial properties
-
(9)
Silver-impregnated dressings: different types (foam, hydrocolloids, etc.) of dressing containing silver ions usually used for treating infected ulcers.
-
(10)
Honey-impregnated dressings: medical-grade honey dressings proposed as an antimicrobial/anti-inflammatory local treatment which can be used both for acute and chronic ulcers.
-
(11)
Microorganism-binding dressings: are capable of binding microorganisms in a two water-repellent surfaces and subsequently removed at dressing change.
-
(9)
To be included in the present meta-analysis, all these dressing, assessed to be included in the Italian Guidelines for the treatment of DFS, should be EMA-approved.
Two independent reviewers (A.S. and M.A.) screened all titles and abstracts of the identified studies for inclusion. Discrepancies were resolved by a third, independent reviewer (M. M.).
Data extraction and collection
Variables of interest were ulcer healing, major and minor amputation, all-cause mortality, time-to-healing, frequency of dress changes, quality of life, and back-to-walk, as previously reported [9].
Data extraction was performed independently by two of the authors (B.R. and A.S.), and conflicts were resolved by a third investigator (M.M.).
Titles and abstracts were screened independently by the authors, and potentially relevant articles were retrieved in full text. For all published trials, results reported in published papers and supplements were used as the primary source of information. When the required information on protocol or outcomes was not available in the main or secondary publications, an attempt at retrieval was performed by consulting the clinicaltrials.gov website. The identification of relevant abstracts and the selection of studies were performed independently by all the authors. Data extraction and conflict resolution were performed by two investigators (A.S. and B.R.). The risk of bias in RCTs was assessed using the Cochrane recommended tool [12], which includes seven specific domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The results of these domains were graded as ‘low’ risk of bias, ‘high’ risk of bias, or ‘uncertain’ risk of bias.
Endpoints
The primary endpoint was ulcer healing at the endpoint. Secondary endpoints were major and minor amputation, all-cause mortality, time-to-healing, back-to-walk, pain, quality of life, and frequencies of dressing changes at the endpoint.
Statistical analyses
Heterogeneity was assessed by I2 test, whereas Funnel plots were used to detect publication bias for principal endpoints with at least 10 trials.
If data from more than one study on a given outcome were available, a meta-analysis using a random-effects model as the primary analysis was performed. Mantel-Haenzel Odds ratios and 95% confidence intervals (MH-OR, 95% CIs) were either calculated or extracted directly from the publications. Weighted mean differences (WMD) and 95% CIs were calculated for continuous variables.
For all the explored endpoints, active dressings were analyzed together and individually.
For the primary endpoint only, several pre-specified sub-group analysis was performed comparing trials with shorter (< 6 months) and longer duration (≥ 6 months) and those including and not including infected ulcers.
All analyses were performed using Review Manager (RevMan), Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) for RCT and Comprehensive Meta Analysis v. 2.
The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) methodology10 was used to assess the quality of the body of retrieved evidence, using the GRADEpro GDT software (GRADEpro Guideline Development Tool. McMaster University, 201,526. Available from gradepro.org).
Results
Retrieved trials
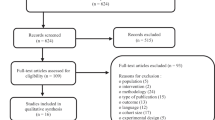
The study flow summary is reported in Fig. 1S of Supporting Information. The search of Medline and Embase databases allowed the identification of 257 items; after excluding studies by reading the title (n = 211), a further 31 studies were excluded after reviewing full-text (Table 3S of Supporting Information). Out of 15 studies [7, 13,14,15,16,17,18,19,20,21,22,23,24,25,26] fulfilling all inclusion criteria, only two [7, 15] were placebo-controlled trials; the remaining 13 trials were open-label in comparison with standard of care (see Table 1). All trials were short-term studies (follow-up < 24 weeks), with the exclusion of.
three trials: two versus hydrocolloids [21, 22] and one versus hydrogel [20].
No trials for alginates, foams, and microorganism-binding dressings have been retrieved preventing any reliable assessments.
The principal characteristics of the included studies, comparing active dressings (n = 540) with placebo or SoC (n = 468), are reported in Table 4S of Supporting Information. The mean age, proportion of women, and baseline HbA1c were 61 years, 24, and 7.6% (58 mmol/mol), respectively.
The quality of studies was heterogeneous and relatively low due to open-label design and possible relevant selection bias. Only one study7 was a high-quality trial with no relevant bias (Fig. 2S of Supporting Information).
Ulcer healing and time to ulcer healing
Only two studies did not report information on proportion of healed patients [18, 19]. Advanced dressing, considered together, were associated with a significantly higher ulcer healing rate (MH-OR: 1.82 [1.32, 2.51], p = 0.0003 I2 = 33%; Fig. 1) in comparison with SoC/Placebo. Funnel plot suggested a possible publication bias which downloads the strength of this association (Fig. 3S). Differences across individual dressing did not reach a fully statistical significance (test for subgroup differences: P = 0.050, I2 = 51.1%); however, some dressing reported only a trend toward reduction of healing rate (i.e., hemoglobin spray) or even neutral effects (i.e. hydrocolloids/hydrofibers, protease-modulating matrix, and silver dressings) in comparison with SoC.
Nine trials reported information on time to healing [14, 17, 18, 20,21,22,23, 26, 27] showing a significantly shorter time to healing in favor of active dressings (WMD: − 24.38 [− 42.90, − 5.86] days, p = 0.010, I2:100%).
A pre-specified analysis including only trails with a treatment duration of at least 6 months (n = 3 trials [20,21,22]) reported no significant between-group differences in healing rate (Fig. 4S, Panel A) and a nonsignificant trend toward reduction of time-to-healing (Fig. 4S, Panel B).
A further pre-specified analysis excluding trials enrolling infected ulcers [13,14,15, 17, 18, 24], revealed similar favorable effects of advanced wound dressings on both ulcer healing (Fig. 5S, Panel A) and time-to-healing (Fig. 5S, Panel B). A post-hoc analysis comparing AWD with different comparators has been performed in order to verify the possible role of different type of SoC in healing processes. No significant between-group differences were observed, suggesting no effects of different type of SoC in modulating AWD effectiveness (Fig. 6S of Supplementary Materials).
Amputations
Out of seven studies reporting information on major amputations, four reported at least one case with a MH-OR for active dressings of 0.72 [0.17, 2.97], p = 0.58, I2: 0%; Fig. 7S).
Only five trials [17, 18, 21, 22, 25] reported information on minor amputations. Three studies [17, 21, 22] reported at least one minor amputation among either patients allocated to active dressings or SoC/placebo, showing no between-group differences (MH-OR: 1.26 [0.50, 3.15], p = 0.62; I2: 0%; Fig. 8S) (Fig. 2).
All-cause mortality
Only four studies reported at least one event (n = 21 deaths) [7, 18, 25, 26] showing no effects of active dressing on all-cause mortality (MH-OR for active dressings vs SoC/placebo: 0.48 [0.19, 1.26], p = 0.14; I2 = 0%; Fig. 9S).
Other outcomes: back-to-walk, frequency of dressing changes, and pain
No studies reported information on back-to-walk and pain, which were prespecified endpoints. Frequency of dressing changes was reported for all the included studies, the majority of whom left the dressing change to the discretion of the investigators or followed a pre-specified protocol. Only two studies have assessed this parameter [7, 21], the first with sucrose octasulfate and the latter with a silver dressing, with no between group differences observed (WMD: − 0.16 [− 0.49, 0.17] times/week, p = 0.34, I2: 0%; Fig. 10S).
Quality of life
Only two trials reported information on quality of life [7, 21], using two different tools and therefore preventing any formal meta-analysis. Active dressings in both studies was not associated with better quality of life scores in comparison with SoC/placebo.
Quality of retrieved evidence
Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology [10] was used to assess the quality of the body of retrieved evidence. For the primary endpoint, the quality of evidence was rated as “moderate” (Table S4 of Supporting Information) due to the relatively low quality of the included studies (selection and performance bias) and a possible publication bias.
Discussion
Diabetic foot syndrome represents a clinical and economical challenge for any healthcare system. Patients with DFU are at higher risk for local and systemic complications, including infections/sepsis, amputations, need for revascularization, and all-cause mortality. As the prevalence of diabetes and diabetic foot ulcers continue to rise globally [28], the development of innovative and effective therapies, including local dressings, is an essential tool to increase the chance of ulcer healing and reducing the risk of MALE.
The panel responsible for the development of the Italian Guidelines for the treatment of Diabetic Foot Syndrome identified as relevant, a clinical question regarding the use of advanced wound dressings, choosing ulcer healing, time-to-healing, all-cause mortality, major and minor amputation, frequency of dressing changes, pain, and quality of life [9].
In the present systematic review, which was performed to support the development of these guidelines, the primary endpoint was ulcer healing, which were scored as the most important outcome by the guideline panel [9].
The novelty of the present paper consists in collecting all RCT exploring the efficacy of advanced wound dressings on top of standard of care for the treatment of DFU. In fact, with the exception of a retract network meta-analysis comparing nine different dressings, no previous meta-analyses assessed the effectiveness of common local dressings in patients with DFU.
We collected RCT performed on 11 commonly used medical dressings, retrieving few trials for each individual dressing, with the exception of alginates, foams, and microorganism-binding dressings, for whom no trials have been never performed in patients with DFU. Differently from other meta-analyses [29,30,31] which included both randomized and nonrandomized clinical studies, we decided to restrict our analyses only to RCT to reduce the risk of prescription and selection bias and reducing heterogeneity. We have also chosen to exclude bio-engineered dressing, such as growth factors, mesenchymal stem cells, skin grafts/substitutes (considered all together by other meta-analysys [32]) which will be the topic of a further PICO [9], and that are used for recalcitrant ulcers and not for routinely local treatments.
The results of our paper showed that taken overall, advanced dressings are more effective than standard of care (i.e., wet-to-moist dressing, saline-moistened or sterile gauze, etc.) in increasing the rate of ulcer healing and reducing the time-to-healing- However, despite the scarce number of trials prevented any reliable conclusion for any of the explored dressing, some local treatment seemed to show better profiles, such as sucrose-octasulfate, honey dressing, hyaluronic acid, and hydrogel. Hydrocolloid/hydrofibers, hemoglobin spray, protease-modulating matrix, and silver dressings have been less studied and/or did not report any significant advantages over the SoC in term of ulcer healing and/or time-to-healing.
Sucrose-octasulfate has been recently assessed in a relatively large-scale, high-quality trial [7], investigating the effect of sucrose octasulfate dressing, also known as technology lipido-colloid with nano-oligosaccharide factor The rationale of this dressing consists in the inhibition of matrix metalloproteinase (MMPs), which are over produced at the wound bed level, leading to abnormal tissue breakdown and prolonged healing time. The Explorer study [7] showed significant beneficial effects in the treatment of noninfected, neuroischemic, hard-to-heal DFUs on several outcomes, such as healing rate and time-to-healing. Despite, similar biological actions, protease-modulating matrix dressings did not report any advantages over the SoC. However, this dressing has been evaluated in one single, low-quality, short-term trial, preventing therefore any reliable conclusion.
Hydrogels have several remarkable properties, including moisturization, permeability, and the ability to promote autolytic debridement, which could explain the favorable results obtained in the present meta-analysis. Hydrogels, in fact, reproduce the natural environment of the extracellular matrix, providing an ideal setting for cell proliferation [33].
Honey dressings has emerged as a promising therapeutic strategy for treating DFU due to their antibacterial properties and physical–chemical characteristics. In fact, honey exhibits antibacterial activity by releasing hydrogen peroxide and lowering pH wound levels and promotes granulation tissue formation due to high sugar and phenolic contents [29]. Despite similar antibacterial effects, silver dressings did not show any significant beneficial effects on ulcer healing and time-to-healing. These neutral results are somewhat surprising, since in other different settings, such as that of leg ulcers, silver dressings showed favorable effects in term of ulcer healing and time-to-healing [34]. Once again, such neutral results can be due to the paucity of included trials, rather than an ineffective action of these dressings. Similar considerations can be made for other local treatments, such as hydrocolloids/hydrofibers and hemoglobin spray reporting conflicting [21, 22] or neutral [24] results.
Several limitations should be acknowledged and considered in interpreting the obtained results. The quality of a meta-analysis always depends on the quality of included parent studies. The present meta-analysis, unfortunately, included several low-quality, short-term and not adequately sized trials possibly introducing biases and affecting the overall results. The majority of the included studies were open-label and randomization and allocation procedures not always well described. The heterogeneity of inclusion and exclusion criteria, study procedures, and definition of endpoints could have further contributed to introduce another bias (inconsistency bias). However, despite these possible sources of heterogeneity, for the primary outcome, we did not detect any relevant heterogeneity, which was low-moderate. This allows us to consider the obtained results reliable. Another limitation is represented by the adopted selective and restrictive inclusion and exclusion criteria (i.e., only randomized studies, with a minimum duration of at least 12 weeks, and including only diabetic foot ulcers) which allowed us to include a relatively scarce number of trials. This could have reduced the overall heterogeneity, but, at the same time, limited the statistical power for some of the included outcomes, possibly underestimating the effects of some active local dressings on DFU prognosis. Moreover, the included trials enrolled both plantar and nonplantar DFU, which could have different time-to-healing and benefit differently from pressure relief systems. A further limitation is represented by the relatively short duration of the included studies. Only three studies fulfilled the inclusion criteria reported in our previous methodological paper (i.e., studies with a duration of at least 6 moths [9]). The results obtained are therefore coming from post-hoc analyses. These limitations suggest caution in interpreting the overall results of the present meta-analysis and highlight the urgent need for higher quality studies with longer follow-up, enrolling larger cohorts of patients, and taking into account also economic aspects.
In conclusion, advanced wound dressings, particularly sucrose-octasulfate, hydrogels, hyaluronic acid, and honey dressings, can actively promote wound healing and shortening time-to-healing in patients with non-infected, predominantly neuropathic DFU together with SoC (including a multidisciplinary assessment [5], plantar pressure relief [35], and local debridement [36]).
References
Aronson R, Chu L, Joseph N, Brown R (2021) Prevalence and risk evaluation of diabetic complications of the foot among adults with Type 1 and Type 2 diabetes in a large Canadian population (PEDAL Study). Can J Diabetes 45(7):588–593 (in Eng). https://doi.org/10.1016/j.jcjd.2020.11.011
Morbach S, Furchert H, Gröblinghoff U et al (2012) Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care 35(10):2021–2027 (in Eng). https://doi.org/10.2337/dc12-0200
Elgzyri T, Larsson J, Nyberg P, Thörne J, Eriksson KF, Apelqvist J (2014) Early revascularization after admittance to a diabetic foot center affects the healing probability of ischemic foot ulcer in patients with diabetes. Eur J Vasc Endovasc Surg 48(4):440–446 (in Eng). https://doi.org/10.1016/j.ejvs.2014.06.041
Tan JS, Friedman NM, Hazelton-Miller C, Flanagan JP, File TM Jr (1996) Can aggressive treatment of diabetic foot infections reduce the need for above-ankle amputation? Clin Infect Dis 23(2):286–291 (in Eng). https://doi.org/10.1093/clinids/23.2.286
Meloni M, Giurato L, Monge L et al (2024) Effect of a multidisciplinary team approach in patients with diabetic foot ulcers on major adverse limb events (MALEs): systematic review and meta-analysis for the development of the Italian guidelines for the treatment of diabetic foot syndrome. Acta Diabetol (in Eng). https://doi.org/10.1007/s00592-024-02246-9
Bolton L (2022) Diabetic foot ulcer: treatment challenges. Wounds 34(6):175–177 (in Eng). https://doi.org/10.25270/wnds/2022.175177.
Edmonds M, Lázaro-Martínez JL, Alfayate-García JM et al (2018) Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol 6(3):186–196 (in Eng). https://doi.org/10.1016/s2213-8587(17)30438-2
Martí-Carvajal AJ, Gluud C, Nicola S et al (2015) Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev 2015(10):Cd008548 (in Eng). https://doi.org/10.1002/14651858.CD008548.pub2.
Monami M, Scatena A, Miranda C et al (2023) Development of the Italian clinical practice guidelines for the treatment of diabetic foot syndrome: design and methodological aspects. Acta Diabetol 60(11):1449–1469 (in Eng). https://doi.org/10.1007/s00592-023-02150-8
Guyatt GH, Oxman AD, Santesso N et al (2013) GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol 66(2):158–72 (in Eng). https://doi.org/10.1016/j.jclinepi.2012.01.012.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928 (in Eng). https://doi.org/10.1136/bmj.d5928
Imran M, Hussain MB, Baig M (2015) A randomized, controlled clinical trial of honey-impregnated dressing for treating diabetic foot ulcer. J Coll Phys Surg Pak 25(10):721–725 (in Eng). https://doi.org/10.2015/jcpsp.721725
Kamaratos AV, Tzirogiannis KN, Iraklianou SA, Panoutsopoulos GI, Kanellos IE, Melidonis AI (2014) Manuka honey-impregnated dressings in the treatment of neuropathic diabetic foot ulcers. Int Wound J 11(3):259–263 (in Eng). https://doi.org/10.1111/j.1742-481X.2012.01082.x
Siavash M, Shokri S, Haghighi S, Shahtalebi MA, Farajzadehgan Z (2015) The efficacy of topical royal jelly on healing of diabetic foot ulcers: a double-blind placebo-controlled clinical trial. Int Wound J 12(2):137–142 (in Eng). https://doi.org/10.1111/iwj.12063
Essa MS, Ahmad KS, Zayed ME, Ibrahim SG (2023) Comparative study between silver nanoparticles dressing (SilvrSTAT Gel) and conventional dressing in diabetic foot ulcer healing: a prospective randomized study. Int J Low Extrem Wounds 22(1):48–55 (in Eng). https://doi.org/10.1177/1534734620988217
Lafontaine N, Jolley J, Kyi M et al (2023) Prospective randomised placebo-controlled trial assessing the efficacy of silver dressings to enhance healing of acute diabetes-related foot ulcers. Diabetologia 66(4):768–776 (in Eng). https://doi.org/10.1007/s00125-022-05855-7
Viswanathan V, Kesavan R, Kavitha KV, Kumpatla S (2011) A pilot study on the effects of a polyherbal formulation cream on diabetic foot ulcers. Indian J Med Res 134(2):168–73 (in Eng). (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3181016/pdf/IJMR-134-168.pdf).
Barbosa MG, Carvalho VF, Paggiaro AO (2022) Hydrogel enriched with sodium alginate and vitamins A and E for diabetic foot ulcer: a randomized controlled trial. Wounds 34(9):229–235 (in Eng). https://doi.org/10.25270/wnds/20103.
Djavid GE, Tabaie SM, Tajali SB et al (2020) Application of a collagen matrix dressing on a neuropathic diabetic foot ulcer: a randomised control trial. J Wound Care 29(Sup3):S13–S18 (in Eng). https://doi.org/10.12968/jowc.2020.29.Sup3.S13.
Jeffcoate WJ, Price PE et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technol Assess 2009;13(54):1–86, iii–iv (in Eng). https://doi.org/10.3310/hta13540.
Piaggesi A, Baccetti F, Rizzo L, Romanelli M, Navalesi R, Benzi L (2001) Sodium carboxyl-methyl-cellulose dressings in the management of deep ulcerations of diabetic foot. Diabet Med 18(4):320–324 (in Eng). https://doi.org/10.1046/j.1464-5491.2001.00466.x
Tonaco LAB, Gomes FL, Velasquez-Melendez G, Lopes MTP, Salas CE (2018) The proteolytic fraction from latex of vasconcellea cundinamarcensis (P1G10) enhances wound healing of diabetic foot ulcers: a double-blind randomized pilot study. Adv Ther 35(4):494–502 (in Eng). https://doi.org/10.1007/s12325-018-0684-2
Jonker L, Smith D, Mark E, Thornthwaite S, Gunn C, Fisher S (2021) A pragmatic, single-center, prospective, randomized controlled trial of adjunct hemoglobin-mediated granulox topical oxygen therapy twice weekly for foot ulcers. J Am Podiatr Med Assoc 2021;111(5) (in Eng). https://doi.org/10.7547/19-189.
Lee M, Han SH, Choi WJ, Chung KH, Lee JW (2016) Hyaluronic acid dressing (Healoderm) in the treatment of diabetic foot ulcer: a prospective, randomized, placebo-controlled, single-center study. Wound Repair Regen 24(3):581–588 (in Eng). https://doi.org/10.1111/wrr.12428
Veves A, Sheehan P, Pham HT (2002) A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 137(7):822–827 (in Eng). https://doi.org/10.1001/archsurg.137.7.822
Edmonds ME, Bodansky HJ, Boulton AJM et al (2018) Multicenter, randomized controlled, observer-blinded study of a nitric oxide generating treatment in foot ulcers of patients with diabetes-ProNOx1 study. Wound Repair Regen 26(2):228–237 (in Eng). https://doi.org/10.1111/wrr.12630
Armstrong DG, Tan TW, Boulton AJM, Bus SA (2023) Diabetic foot ulcers: a review. JAMA 330(1):62–75 (in Eng). https://doi.org/10.1001/jama.2023.10578
Yildiz Karadeniz E, Kaplan SE (2023) Use of honey in diabetic foot ulcer: Systematic review and meta-analysis. J Tissue Viability 32(2):270–278 (in Eng). https://doi.org/10.1016/j.jtv.2023.03.002
Dissemond J, Böttrich JG, Braunwarth H, Hilt J, Wilken P, Münter KC (2017) Evidence for silver in wound care—meta-analysis of clinical studies from 2000–2015. J Dtsch Dermatol Ges 15(5):524–535 (in Eng). https://doi.org/10.1111/ddg.13233
Elraiyah T, Tsapas A, Prutsky G et al (2016) A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. J Vasc Surg 63(2 Suppl):46S-58S.e1–2 (in Eng). https://doi.org/10.1016/j.jvs.2015.10.007.
Wang Y, Chao N, Yin D (2022) A net meta-analysis of the effectiveness of different types of dressings in the treatment of diabetic foot. Comput Math Methods Med 2022:4915402 (in Eng). https://doi.org/10.1155/2022/4915402
Dumville JC, Deshpande S, O'Meara S, Speak K (2013) Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2013(8):Cd009099 (in Eng). https://doi.org/10.1002/14651858.CD009099.pub3.
Zhang C, Zhang S, Wu B, Zou K, Chen H (2023) Efficacy of different types of dressings on pressure injuries: systematic review and network meta-analysis. Nurs Open 10(9):5857–5867 (in Eng). https://doi.org/10.1002/nop2.1867
Gauna C, Romeo F, Scatena A et al (2024) Offloading systems for the treatment of neuropathic foot ulcers in patients with diabetes mellitus: a meta-analysis of randomized controlled trials for the development of the Italian guidelines for the treatment of diabetic foot syndrome. Acta Diabetol (in Eng). https://doi.org/10.1007/s00592-024-02262-9
Chen P, Vilorio NC, Dhatariya K et al (2024) Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev 40(3):e3644 (in Eng). https://doi.org/10.1002/dmrr.3644
for the Panel of the Italian Guidelines for the Treatment of Diabetic Foot Syndrome and on behalf of SID and AMD
The Panel of the Italian Guidelines for the treatment of Diabetic Foot Syndrome is composed by: Andrea Bernetti, Corrado Bordieri, Cristina Cappella; Alessandro De Cassai, Marco Falcone, Mauro Gargiulo; Valentina Lorenzoni; Gerardo Medea, Cesare Miranda, Matteo Monami, Luca Monge, Alessia Scatena, Germano Scevola; Eugenio Stabile; Laura Stefanon, Rodolfo Tramonta, Cristiana Vermigli, Antonio Volpe, Luigi Uccioli
Funding
This research was performed as a part of the institutional activity of the unit with no specific funding. All expenses including salaries of the investigators were covered by public research funds assigned to the unit.
Author information
Authors and Affiliations
Consortia
Contributions
All the authors approved the final version of this manuscript. Dr. Alessia Scatena is the person who takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. MM and BR were involved in each of the following points: 1. Design, 2. Data Collection, 3. Analysis, 4. Writing manuscript. AS and LM were involved in each of the following points: 1. Analysis, 2. Writing manuscript. CM, LU, MG, and CV were involved in each of the following points: 1. Data Collection, 2. Manuscript revision.
Corresponding author
Ethics declarations
Conflicts of interest
MM received speaking fees from Zuccato S.r.l. and Athena. The other authors do not have any direct relevant conflicts of interest to disclose.
Research involving human participants and/or animals
This article contains no studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent is needed.
Additional information
Managed by Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Monami, M., Ragghianti, B., Scatena, A. et al. Effectiveness of different advanced wound dressings versus standard of care for the management of diabetic foot ulcers: a meta-analysis of randomized controlled trials for the development of the Italian guidelines for the treatment of diabetic foot syndrome. Acta Diabetol (2024). https://doi.org/10.1007/s00592-024-02320-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00592-024-02320-2