Abstract
Aims
The recent trend toward the use of natural functional and medical supplements has motivated the focus on the search and revival of traditional medicinal plant applications for many years. As a valuable dietary crop, okra fruit (Abelmoschus esculentus (L.) Moench) has been used for thousands of years as a medicinal food. This clinical trial aimed to assess the efficacy and safety of the okra pod capsule as an adjuvant treatment in controlling type 2 diabetes mellitus and provide clinical trial-based evidence about its anti-inflammatory effects.
Methods
A total of 100 type II diabetic patients, aged between 40 and 60 years, were randomly assigned into two groups of okra and placebo. The first group was administered 1000 mg of powdered okra fruit three times a day for 3 months, while the other group received a placebo capsule with the same dosage. Both groups continued the standard antidiabetic therapy (consisting of metformin and gliclazide, as well as a nutritional regimen). At the start and three months later, various factors were measured, including FBG, insulin, HbA1c, cholesterol, triglycerides, HDL, LDL, CRP, liver and renal function tests, blood pressure, and BMI changes.
Results
According to the results, patients who received okra treatment exhibited a significant decrease in FBG, HbA1c, total cholesterol, and triglyceride levels when compared to both the baseline and the placebo group. Patients in the okra group have lower levels of hs-CRP compared with the placebo group after 3 months of treatment. No liver, kidney, and blood pressure or other side effects were observed in the groups associated with okra treatment.
Conclusions
The present study demonstrated that adjunctive consumption of okra, in type 2 diabetic patients with 1000 mg three times a day for three months, improves lipid profile, glycemic control, and chronic inflammation without any tangible adverse effects.
Clinical Trial Registry: IRCT.Ir (IRCT20120112008712N2). https://www.irct.ir/trial/42042.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a highly prevalent metabolic disorder worldwide which affects 9.3% of 20 to 79-year-old adults (463 million) in 2019 and the latest estimates from the International Diabetes Federation (IDF) indicate that this figure may increase to 10.9% (equivalent to 700 million individuals) by 2045 [1]. The global prevalence of type 2 diabetes (T2D) displaying a rapid upsurge and it is accounting for the majority of diabetes cases, ranging from 90 to 95% [2]. Despite recent advances in medical care, diabetes continues to be one of the life-threatening diseases of the industrial era and accounts for 11% of annual deaths and imposes €626 billion directly on the healthcare systems alone [3]. The onset of T2DM is characterized by insulin resistance and inflammation, a condition that often arises in the context of elevated fat mass and the accumulation of by-products stemming from lipid metabolism [4]. Oxidative stress promotes the secretion of pro-inflammatory cytokines such as interleukin-6 (IL-6) and C-reactive protein (CRP). These cytokines may develop insulin resistance and beta-cell dysfunction, which are the primary defects in T2DM. Moreover, inflammation can lead to endothelial dysfunction, which contributes to the development of macro- and micro-vascular complications such as cardiovascular disease, retinopathy, nephropathy, and neuropathy [5, 6].
Most conventional chemical anti-diabetic medicines have limited efficacy and are associated with many serious adverse effects that physicians and patients are not satisfied with the control of the disease, along with the high costs of treatment that have led to a growing tendency to herbal therapeutics [7]. None of the common anti-diabetic medicines have a significant lipid-lowering effect. Plants are a rich source of bioactive compounds such as flavonoids, and antioxidants several studies have outlined their efficacy and safety in the management of blood glucose and lipid levels in diabetes [8]. From this point of view, numerous studies have demonstrated the efficacy of consuming various natural plants and dietary compounds in reducing glucose and lipid levels while simultaneously exhibiting antioxidant effects [9]. Medicinal plants and natural compounds have some strengths and beneficial points compared to conventional medications. The affordability, low incidence of adverse effects, and enhanced patient compliance with herbal remedies contribute to the aforementioned advantages. Consequently, it is crucial to gain a thorough understanding of the advantageous effects and underlying mechanisms of action exhibited by these antioxidant-rich dietary compounds, such as okra, in diabetic patients [10]. Based on the connection between chronic inflammation and the emergence of chronic illnesses like diabetes, utilizing antioxidants along with glycemic and lipid-lowering agents presents an appealing approach to treating diabetic complications. This strategy aims to postpone or even reverse the progression of the condition. Nowadays, therapeutic demand is shifting toward low-complication treatments, such as herbal therapy, which is one of the most frequently requested medications for the treatment of various ailments such as metabolic syndrome [11].
Abelmoschus esculentus L (also known as Okra, Lady's finger, gumbo, bamieh, kacang, bendi, dharos, and qiu kui) is an annual plant from the Malvaceae family. It is widely consumed as a vegetable and has a long history of traditional medicinal use. Okra shows potential benefits in managing diabetes. Its bioactive components, such as polyphenols and flavonoids (quercetin, kaempferol, isorhamnetin), possess antioxidant and anti-inflammatory properties. Okra also contains natural polysaccharides like mucilage, pectin, xylans, xyloglucans, and celluloses [12, 13]. Both mucilage and pectin are believed to contribute to the potential anti-diabetic effects of okra. Mucilage regulates blood sugar levels by slowing down glucose absorption, while pectin improves insulin sensitivity and glucose metabolism [14]. In addition, Okra is also rich in folic acid, carotenoids, thiamine, riboflavin, niacin, ascorbic acid, oxalic acid, and amino acids [15].
Okra has a diverse range of traditional and modern medicinal uses, including anti-diabetic, digestive system support, appetizer, aphrodisiac, relief for chronic joint pains and inflammation, neuroprotective properties, antimicrobial activity, treatment for respiratory conditions such as cough and asthma, and potential anti-cancer effects [15,16,17]. Moreover, in Persian culture, okra is consumed as a decoction made by boiling okra pods in water and consumed as a tea. It can also be consumed as fresh juice or added to soups and stews [18]. Modern pharmacological studies have shown promising properties of okra in managing diabetes [19,20,21]. Up to date, several pre-clinical animal studies have examined the effects of Abelmoschus esculentus L (okra) on obese and diabetic animals. Even so, the outcomes of these studies remain inconclusive and inconsistent [10]. Nevertheless, recent clinical trials have demonstrated the favorable impact of okra treatment on glycemic control in individuals with pre-diabetes and type 2 diabetes. These studies provide evidence of significant reductions in fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) levels, indicating the positive effects of okra in managing glycemic factors [10, 21,22,23]. However, although some clinical trials have been conducted recently, there is still a lack of sufficient evidence-based efficacy and safety studies in humans [14].
This double-blind, placebo-controlled, randomized clinical trial aimed to assess the effects of 3000 mg of okra capsules (taken three times per day) on glycemic control, lipid profile, and hs‐CRP levels in T2DM patients. The potential relationship between okra and the immune system, particularly its ability to inhibit inflammatory reactions in humans, has not been studied yet and remains a mystery. To our knowledge, this is the first randomized clinical trial to investigate and extensively discuss the anti-inflammatory effects of okra, as well as its impact on glycemic control, lipid lowering, and safety in individuals with type 2 diabetes over a 12-week intervention period. This study is unique in terms of the sub-chronic intervention (3 months), the dosage (3000 mg/day), the form of drug consumption, and the adequate sample size (100 patients).
Methods
Ethics
The Ethics Committee of Maragheh University of Medical Sciences, Maragheh, Iran, approved the protocol of this study, under the case number, IR.MARAGHEHPHC.REC.1398. 025. The Ethics Committee of Zanjan University of Medical Sciences approved the protocol of this study, under the case number, IR.ZUMS.REC.1399. 240. The study protocols are available on the Iranian Ministry of Health website under the code: IRCT20120112008712N2. Written informed consent was obtained from all study subjects after explaining the study protocol.
Plant material
Fresh fruits of Abelmoschus esculentus (okra) were procured from a local fruit and vegetable store in Zanjan (Iran). A voucher specimen was preserved at the Herbarium of the Faculty of Pharmacy, Zanjan University of the Medical Sciences, Iran (Voucher Number: ZUMS-1703).
The whole fruits were washed with tap water and dried under shade and grinded to powder by a metallic laboratory mill. The final powder was sieved through sixteen number mesh and was scaled and filled into capsules (500 mg). The placebos were made from microcrystalline cellulose, and they were of the same color and size and labeled in similar containers to okra capsules.
Participants
The trial was utilized using a double-blind, parallel-group, placebo-controlled design and was conducted between October 2019 and September 2021 at the Diabetes Clinics of Maragheh University of Medical Sciences, Iran. All patients underwent an initial screening assessment, which included a medical history review, physical examination, measurement of vital signs, electrocardiogram, and laboratory parameter testing, before beginning the study. The American Diabetes Association criteria were used to diagnose diabetes, fasting plasma glucose (FPG) 126 mg/dl or higher, or oral glucose tolerance test (OGTT) 200 mg/dl or higher, or glycated hemoglobin (HbA1c) between 7.0% (53 mmol/mol) and 10.0% (86 mmol/mol) for more than three months [24]. Patients were recruited to the study if they met the inclusion criteria as follows: aged 40 to 65 years old who received oral hypoglycemic agents for diabetes, with a body mass index (BMI) less than 30 kg/m2, and a history of disease between 1 and 5 years. Exclusion criteria were possessed of any additional disorders such as chronic liver or kidney disease, cardiovascular or pulmonary disease, thyroid disorders, malignancy or chronic inflammatory disease, or if they were pregnant or lactating, smokers or alcohol consumers or had any surgery during the last 6 months. Outpatient follow-up appointments were scheduled on the treatment initiation day and subsequently at weeks 4, 8, and 12. Safety was evaluated through a comprehensive analysis of adverse events (AEs), encompassing treatment-emergent events, instances of symptomatic hypoglycemia, and clinical laboratory data. Serum biochemistry laboratory tests were conducted to assess liver function, creatinine levels, and blood urea nitrogen (BUN) levels.
Study design
Eligible volunteers with T2DM were recruited for a 3-month randomized and double-blind clinical trial. The sample size for the study was determined specifically for the variable of fasting plasma glucose (FPG), which served as one of the primary endpoint measurements. This determination was based on the findings from our pilot study, which was conducted in a comparable setting and involved a similar population of patients recently diagnosed with T2DM. The sample size was calculated based on a confidence interval of 95%, type 1 error (α) = 0.05, and type 2 error (β) = 0.2 with a power of 80% and attrition rate = 20%. To calculate the sample size, we utilized the following formula:
X1 = 173 (mean FPG in the Okra group), X2 = 184 (mean FPG in the placebo group), SD1 = 19 (Standard Deviation in the Okra group), SD1 = 31 (Standard Deviation in the placebo group).
According to the formula, this study would need 84 T2DM patients. A total of 100 patients were assigned to each group based on the inclusion criteria with regard to the attrition rate of 15%.
Randomization was performed by using a computer-generated random number sequence (RAS/Version 1.0.0 software) through four blocks into the two groups. The process of randomization and allocation of participants was blinded from the researchers and participants until when the final data were analyzed. There were letters A, B, C, or D on the containers, where A and B were placebos and C and D were Okra capsules. The patients and the physicians were blinded to the content of the containers.
Intervention and follow-up
All patients were randomly allocated to one of two research groups: received daily six capsules containing 500 mg of powdered okra fruit three times (two capsules 30 min before each major meal) with a glass of water and those in the control group received the same number of capsules per day containing 500‐mg toasted flour for the duration of the 12 weeks. The appearance of the placebo capsules (including color and packaging) was similar to that of the okra group. During the trial, all patients were asked not to modify their oral hypoglycemic medications, usual diet, or daily physical habits, nor to take any supplements. Every week, telephone interviews were conducted to follow up the participants. Compliance with supplements was assessed based on how many capsules remained unused after each week.
Data collection
After overnight fasting, a 10-ml blood sample was drawn from all participants before and after the 3-month intervention period. Fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (Chol), high-density lipoprotein (HDL), low-density lipoprotein (LDL), serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), alkaline phosphatase (ALP) blood urea nitrogen (BUN), and serum creatinine (Cr) were measured with the commercially available kit (Pars Azmoon Co., Tehran, Iran) using an autoanalyzer (Hitachi 902 Analyzer). The enzyme-linked immunosorbent assay (Elisa) commercial kit (Monobind, California, USA) was used to measure the fasting insulin concentration. We used insulin and fasting plasma glucose (FPG) levels to determine the homeostasis model assessment-insulin resistance (HOMA-IR). Glycated hemoglobin (HbA1c) was calculated in the whole blood sample using DS5 chromatography. Enzyme-linked immunosorbent assay kit (Monobind, USA) was utilized to determine the concentrations of high-sensitivity C-reactive protein (hs-CRP). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were double-checked after 15 min of seated rest using a conventional sphygmomanometer (ALPK2, Zhejiang, China). The average of the two measurements was considered. All measurements were conducted at the baseline and after three months of the study in both groups.
Statistical analysis
Statistical analysis was performed using SPSS software (v25.0; SPSS Inc., Chicago, IL). To evaluate the normal distribution of variables, the Kolmogorov–Smirnov test was applied. Differences between baseline demographics and clinical characteristics, presented as mean ± standard deviation, parametric variables were compared using the analysis of variance and the student t-test. Analysis of variance was used for other clinical parameters. A result of p < 0.05 was considered statistically significant.
Results
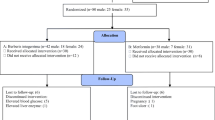
Figure 1 illustrates the participants' enrollment in the study, and the reasons for exclusion from the trial and the final number of patients concluded in the statistical analysis. As shown in the flow diagram, a total of 138 patients were screened for eligibility from July 2019, through September 2020. Of these, 38 patients were excluded (not meeting inclusion criteria [n = 21], declined to participate [n = 7] and for other reasons [n = 10]), and eventually, 100 diabetic volunteers were randomized to the okra or the placebo groups in the 3-month run-in phase trial (Fig. 1). Six patients left the study after randomization because they had undergone surgery (n = 1), unwilling to cooperate (n = 3), traffic accident (n = 1), or were switched to insulin therapy (n = 1). The patients' intentions to treatment were in the acceptable range (over 80% compliance). Hence, 94 patients were included in compliance with the treatment statistical analysis (48 in the okra group and 46 in the placebo groups). The baseline demographic characteristics of the participants in both groups are outlined in Table 1. The mean age, male and female proportion, duration of diabetes, body weight, and BMI are shown in Table 1. There were no significant differences in regard to the demographic characteristics between the two groups at the baseline (p > 0.05). In addition, oral anti-hyperglycemic medications consumption patterns showed no significant difference between both groups (Table 2).
As shown in Table 3, the level of FPG before and after 3 months of treatment with A. esculentus was 171.95 ± 29.0 vs. 134.29 ± 21.85 (p = 0.0001), showing a significant reduction in comparison with the placebo group. In the placebo group, after 90 days of intervention, the changes in glycemic parameters were not statistically considerable. The mean hemoglobin A1c level at baseline was 8.41 ± 1.32 and after the intervention period, reduced to 7.63 ± 1.18 in the okra group, and in the control group, the baseline value was 8.40 ± 0.99 and 8.20 ± 2.14 at the end of the study, which was statistically considerable (p = 0.013). Data obtained from the current study demonstrated that there was a significant reduction in sera triglycerides, fasting insulin (p = 0.01), and HOMA-IR after intervention (p < 0.0001). In the okra group, at baseline, serum total cholesterol, triglycerides, HDL, and LDL were 195.2 ± 42.49, 184.9 ± 45.01, 46.45 ± 8.61, and 117.31 ± 36.41, respectively. Daily consumption of A. esculentus capsules significantly reduced total cholesterol and triglycerides to 184.4 ± 47.17 and 159.38 ± 55.39, respectively. The okra group in comparison with the placebo group demonstrated remarkable changes in lipid profile factors such as TC, TG, and LDL (p = 0.011, p = 0.01, and p = 0.04, respectively). Despite the rise in LDL levels in placebo patients, okra consumption led to a reduction of LDL levels to 112.6 ± 40.58. Moreover, it increased HDL to 47.63 ± 9.72. Whereas, in the control group, at the baseline and end of the observation period, total cholesterol was 189.9 ± 38.26 vs. 201 ± 37.39 (p = 0.202). In the okra and placebo group, the level of hepatic enzymes included alanine aminotransferase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) and did not change statistically after treatment. In addition, at day 90 of okra consumption serum high-sensitivity CRP (HSCRP) level decreased to 7.25 ± 3.36 (p = 0.001). Meanwhile, in the placebo group, a significant rise to 8.6 ± 5.48 in CRP factor is considered at the end of the observation period.
After 3-month treatment, there was no statistical difference in the systolic and diastolic blood pressure between the okra and control groups. The renal biochemical parameters, Cr and BUN, did not show any statistically significant difference between the okra and placebo groups after 90 days of treatment. Also, there were no significant changes in Cr and BUN in the intervention group as compared to the baseline.
Safety
Participants did not complain of any important side effects. However, two cases of cramps in the first few days of the study were reported in the treatment group. Okra was safe and well-tolerated in this study.
Discussion
Despite recent advances in antidiabetic medication and the high variety of antidiabetic drugs available for the treatment of T2DM, currently available therapeutics may not be successful for all patients, necessitating the use of additional therapies or chronic insulin doses, both of which have serious side effects. It is crucial to look for adjuvant and complementary therapies to achieve satisfying goals in the treatment of diabetic patients.
The Abelmoschus esculentus L (okra) pods have been used for the treatment of diabetes mellitus in Persian traditional medicine; furthermore, recent clinical studies confirmed its antidiabetic effect [21, 22]. However, to our knowledge, its anti-inflammatory and safety properties are not well defined in adequately long and powered trials. In the current study, we evaluated the effects of okra supplementation on glycemic parameters, lipid profiles, and CRP as an inflammatory biomarker among patients with T2DM. Based on the results of this trial, daily consumption of okra in doses of 3000 mg for 3 months had favorable effects in modulating the level of FPG, HbA1C, fasting insulin levels, HOMA-IR, and triglycerides, LDL as well as total cholesterol level in T2DM patients. There was a considerable reduction in the hs-CRP level as an inflammatory biomarker in the okra group. Meanwhile, okra consumption did not have a significant impact on HDL, BMI, systolic and diastolic blood pressure, and liver and kidney function tests at the end of the intervention.
In accordance with our findings, previous empirical studies have suggested a positive correlation between the consumption of okra and the favorable effects on the metabolic parameters related to glucose and lipid regulation among type 2 diabetic patients. A recent randomized clinical study of 25 T2DM patients who received a 10 g okra powder mixture in their main meals for 8 weeks had improved lipid and blood sugar levels (FPG) in comparison with the control group without a significant effect on HbA1c [21]. In another trial conducted on 120 T2DM patients, okra pod supplement administration with a dose of 4 g/day for 2 months reduced FPG, BS (blood Sugar), and HbA1c, while the lipid profile of the intervention group was not statistically remarkable in comparison with the placebo group [22]. Another RCT conducted by Khodija et al. in 2020 aimed to compare the impact of steamed and boiled okra on fasting blood glucose levels in 40 patients with T2DM and hypercholesterolemia showed that consumption of 40 g of okra for 2 weeks significantly decreases FBG [23]. Another RCT conducted by Sarbini et al. in 2019, involving 22 individuals with T2DM, showed that the intake of 50 mg of okra extract twice daily for 2 months resulted in a significant reduction in fasting blood sugar levels [25].
As we know, 1% improvement in HbA1C level may lead to about a 21% decrease in unwanted outcomes of diabetes [26, 27]. HbA1C mean value decreased by 0.94% in the okra group after a three-month intervention, but only a 0.14% reduction was observed in the placebo group by the end of the trial, indicating that the intervention with okra supplements would be successful in preventing adverse diabetes outcomes. RCTs suggest a potential benefit of Okra on blood sugar control. In addition, our findings are compatible with the results presented by Saatchi et al., where they observed a significant decrease in HbA1C levels (0.9%) among T2DM patients who were administered 1000 mg of okra whole fruit capsules orally for 2 months [22]. The efficacy of okra as an anti-hyperglycemic treatment has been established, potentially offering protection to individuals with prediabetes and type 2 diabetes (T2D) from secondary complications like atherosclerosis and cardiovascular diseases (CVDs). Additionally, preclinical research indicates that okra may mitigate hyperglycemia and oxidative stress, further suggesting its potential to safeguard against diabetes-related complications. But additional research is necessary to ascertain if the hypoglycemic effects persist over time, particularly in patients with a long disease progression.
The precise mechanism of okra in regulating glycemic status-relevant markers has remained largely unknown. Hypoglycemic effects of okra may arise from multiple pathways. These pathways include increasing glycogen synthesis, delaying glucose absorption in the intestines, enhancing glucose adsorption capacity, and supporting the regeneration of pancreatic islets [10, 28, 29]. It has been suggested that the bioactive components of okra may play a crucial role in glycemic control. For example, okra polysaccharides may ameliorate insulin sensitivity and glucose tolerance in patients with T2DM by enhancing insulin signaling, triggering glucose uptake by peripheral tissue such as skeletal muscle, relieving mitochondrial dysfunction and decreasing oxidative stress, and reducing glucose resistance [30]. Type 2 diabetes is often associated with a higher rate of glucose absorption, leading to glucose intolerance. The causes of this phenomenon are likely to be complex and involve various factors that affect the processes associated with glucose absorption. The interventions that modify the absorptive processes has demonstrated effectiveness in the treatment of type 2 diabetes [29]. Okra reduces the absorption of carbohydrates in the intestine, leading to higher levels of glycolysis and glucose utilization in hepatocytes by activating insulin-sensitive glucose transporters and preventing its release into circulation from the liver [10, 31]. Furthermore, summarizing the antidiabetic activity of okra, it can be attributed to its bioactive components, which may play a role in inhibiting apoptosis of pancreatic islet cells, suppressing α-amylase and α-glucosidase activity, exhibiting anti-inflammatory properties, activating the cAMP-PKA pathway, enhancing insulin secretion, and potentially affecting pathways independent of insulin such as reducing glucose uptake and downregulating glycogen phosphorylase mRNA expression [21, 32].
Dyslipidemia, as a common disorder in T2DM patients, is strongly linked to the progression of atherosclerosis and increased mortality rates among individuals with type 2 diabetes (T2D) [33]. While medications like statins or fibrates are widely prescribed and have demonstrated effectiveness in managing dyslipidemia, they often come with frequent adverse effects. Furthermore, despite the availability of these medications, the mortality rate associated with T2DM complications such as myocardial infarction (MI) or stroke continues to rise steadily [10, 34, 35]. The lipid-lowering effect of okra has been confirmed through numerous in vivo studies. Additionally, okra contains other dietary phytochemical combinations that contribute to its potential effectiveness as a natural alternative to conventional chemical drugs especially in patients who do not tolerate common chemical medications or do not respond well to modern therapeutics.
A recent randomized controlled trial (RCT) conducted by Moradi et al., suggested that the consumption of okra blended in main meals for 8 weeks led to a significant improvement in triglyceride (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels [21]. Similarly, Ngoc et al. [36] reported that okra, given at doses of 30 g/kg body weight, reduced TC and TG levels in mice with hyperlipidemia. Another study demonstrated that the ethanol extract of okra, containing flavonoids such as isoquercitrin and quercetin 3-O-gentiobioside, improved serum lipid profiles in diet-induced obese mice [31]. In another study, streptozotocin-induced diabetic rats were given 200 mg/kg/day of okra powder for 30 days. Their research revealed that the lipid profile of okra-treated rats improved after the intervention period [37]. The lipid-lowering effect of okra can be attributed to various mechanisms. These include a reduction in lipid peroxidation by binding with bile acids, prevention of bile acid reabsorption, and inhibition of liver cholesterol biosynthesis [36]. Okra's flavonoid content has been found to suppress the expression of the nuclear receptor transcription factor PPARγ, which plays a significant role in regulating lipid and glucose homeostasis [31]. Furthermore, the active components in okra have been observed to decrease reactive oxygen species (ROS) and malondialdehyde (MDA) levels by enhancing the activity of key enzymes in the enzymatic antioxidant system, namely superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT). These mechanisms collectively contribute to the lipid management effects of okra [38].
The pathogenesis of T2DM is strongly linked to chronic inflammation and oxidative stress. Hyperglycemia triggers various inflammatory pathways in the body. Moreover, hyperglycemia can induce oxidative stress, which occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body's antioxidant defenses. Excess glucose in the bloodstream can increase ROS production, leading to cellular damage and inflammation. Elevated levels of pro-inflammatory cytokines, such as IL1, IL6, TNF-alpha, and hs-CRP, are associated with this condition. Furthermore, inflammation also plays a crucial role in the development of atherosclerotic lesions [39]. Studies have shown that elevated levels of CRP are associated with an increased risk of developing cardiovascular disease in T2DM patients [40]. CRP has been shown to impair insulin signaling and glucose uptake, leading to insulin resistance, which is a hallmark of type 2 diabetes. In addition, CRP is elevated in individuals with T2DM, particularly in those with poor glycemic control. Elevated CRP levels have also been associated with an increased risk of complications in individuals with T2DM, including cardiovascular disease and diabetic nephropathy [41]. Thus, C-reactive protein (CRP) could be considered an indicator of systemic inflammation and cardiovascular consequences of T2DM progress [42]. However, the in vivo immunomodulatory effect of okra has not yet been documented. Some recent in vitro studies demonstrated the potential anti-inflammatory effects of okra [43,44,45]. To the best of the authors' knowledge, the current investigation is the first evaluation of the anti-inflammatory effects of chronic consumption of okra powder capsules in type 2 diabetic patients in a double-blind randomized clinical trial. Based on our findings, sera hs-CRP levels in T2DM patients significantly reduced after receiving okra for 90 days. Additionally, we discovered that the sera hs-CRP levels in the okra group had decreased by a mean of 18% from the initial levels. The main phenolic compounds found in okra fruit are quercetin and isoquercitrin derivatives, rutin, catechin, and protocatechuic derivatives, the most abundant of them is quercetin-3-O-gentiobioside. Quercetin-3-O-gentiobioside is the major contributor to antioxidant capacity and also has an inhibitory effect on digestive enzymes such as lipase, α -glucosidase, and α-amylase [46]. As quercetin is the primary constituent of okra flavonoids, it is hypothesized that the anti-inflammatory property of okra extract is attributed to the presence of this compound [43]. Through a reduction in CRP in vivo, quercetin poses a key role in the anti-inflammatory effects of okra in metabolic and hepatic disorders [47]. It has been demonstrated that okra infusion water lowers CRP in streptozosin-induced diabetes in vivo [48]. Okra's ability to stop the release of these inflammatory mediators suggests that it may have additional uses as an anti-inflammatory supplement.
Conclusion
Okra is a highly nutritious vegetable that offers a wide range of essential nutrients required by the human body, including protein, dietary fiber, unsaturated fatty acids, minerals, and vitamins. Furthermore, scientific evidence supports various health benefits associated with okra. For instance, extracts from okra have demonstrated the ability to enhance human immunity, counteract oxidative stress, and mitigate damage caused by external factors. Okra has also shown potential in alleviating chronic conditions like diabetes and hyperlipidemia, as well as inhibiting inflammatory cascades. Consequently, okra holds significant application value and promising prospects for further development. In summary, this study demonstrated that okra consumption for 90 days has beneficial effects on the modulation of blood glucose and lipids levels, serum insulin, insulin resistance, and inflammation as well as, elevating HDL-C concentrations in patients with T2DM. Chronic consumption of okra seems to be safe in humans and has several therapeutic benefits, and hasn't been associated with any unwanted outcomes. However, the lack of clinical trials evaluating the effects of okra on inflammatory mediators as well as the clinical outcomes of inflammation-related diseases is a major limitation and further research should focus on adding sufficient power to the results of this study. Although this study is a double-blind randomized clinical trial with sufficient sample size, it is recommended that further large-scale trials (at least one year) with run-in phase period, and possibly non-inferiority, head-to-head comparison trials versus available medications to confirm the beneficial effects of okra as an adjunctive therapy for T2DM patients.
Change history
26 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00592-023-02170-4
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Serum aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- BUN:
-
Blood urea nitrogen
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- ELISA:
-
Enzyme-linked immunosorbent assay
- FPG:
-
Fasting plasma glucose
- HbA1c :
-
Hemoglobin A1c
- HDL:
-
High-density lipoprotein
- HOMA-IR:
-
Homeostasis model assessment-insulin resistance
- IDF:
-
International Diabetes Federation
- LDL:
-
Low-density lipoprotein
- MetS:
-
Metabolic syndrome
- OGTT:
-
Oral glucose tolerance test
- T2DM:
-
Type 2 diabetes melitus
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- WHO:
-
World Health Organization
References
Saeedi P, Petersohn I, Salpea P, et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. J Diabetes Res 157: 107843. https://doi.org/10.1016/j.diabres.2019.107843
Tavakoly R, Maracy MR, Karimifar M, Entezari MH (2018) Does fenugreek (Trigonella foenum-graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial. Eur J Integr Med 18:13–17
Goldenberg JZ, Day A, Brinkworth GD, et al. (2021) Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 372
Wu H, Ballantyne CM (2020) Metabolic inflammation and insulin resistance in obesity. Circ Res 126(11):1549–1564
Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM (2018) Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother 106:381–389. https://doi.org/10.1016/j.biopha.2018.06.148
Gora IM, Ciechanowska A, Ladyzynski P (2021) NLRP3 inflammasome at the interface of inflammation, endothelial dysfunction, and type 2 diabetes. Cells 10(2):314
Asadi A, Shidfar F, Safari M, et al (2019) Efficacy of Melissa officinalis L. (lemon balm) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a randomized, double‐blind, clinical trial. Phytother Res 33(3):651–659. https://doi.org/10.1002/ptr.6254
Momtaz S, Salek-Maghsoudi A, Abdolghaffari AH et al (2019) Polyphenols targeting diabetes via the AMP-activated protein kinase pathway; future approach to drug discovery. Crit Rev Clin Lab Sci 56(7):472–492
Unuofin JO, Lebelo SL (2020) Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: an updated review. Oxidative medicine and cellular longevity 2020
Mokgalaboni K, Lebelo SL, Modjadji P, Ghaffary S (2023) Okra ameliorates hyperglycaemia in pre-diabetic and type 2 diabetic patients: a systematic review and meta-analysis of the clinical evidence. Front Pharmacol 14
Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R (2018) The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol 55:341–353
Peter EL, Nagendrappa PB, Ajayi CO, Sesaazi CD (2021) Total polyphenols and antihyperglycemic activity of aqueous fruits extract of Abelmoschus esculentus: modeling and optimization of extraction conditions. PLoS ONE 16(4):e0250405
Sereno AB, Pinto CD, Andrade FA, et al (2022) Effects of okra (Abelmoschus esculentus (L.) Moench) on glycemic markers in animal models of diabetes: a systematic review. J Ethnopharmacol 115544. https://doi.org/10.1016/j.jep.2022.115544
Elkhalifa AEO, Alshammari E, Adnan M et al (2021) Okra (Abelmoschus esculentus) as a potential dietary medicine with nutraceutical importance for sustainable health applications. Molecules 26(3):696. https://doi.org/10.3390/molecules26030696
Esmaeilzadeh D, Razavi BM, Hosseinzadeh H (2020) Effect of Abelmoschus esculentus (okra) on metabolic syndrome: a review. Phytother Res 34(9):2192–2202. https://doi.org/10.1002/ptr.6679
Islam MT (2019) Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): a literature-based review. Phytother Res 33(1):72–80
Lee T, Joo N (2021) Anti-inflammatory and antioxidant properties of ethanol extracts of raw, blanched, steamed, and sous-vide cooked okra (Abelmoschus esculentus L.) in LPS or H2O2-treated RAW264. 7 cells. Appl Sci 11(5): 2432
Lim T, Lim T (2012) Abelmoschus esculentus. Edible Medicinal Non Med Plants: Volume 3, Fruits, 160–167
Huang C-N, Wang C-J, Lin C-L, Lin H-T, Peng C-H (2017) The nutraceutical benefits of subfractions of Abelmoschus esculentus in treating type 2 diabetes mellitus. PloS one 12(12): e0189065. https://doi.org/10.1371/journal.pone.0189065
Peng C-H, Lin H-C, Lin C-L, Wang C-J, Huang C-N (2019) Abelmoschus esculentus subfractions improved nephropathy with regulating dipeptidyl peptidase-4 and type 1 glucagon-like peptide receptor in type 2 diabetic rats. J Food Drug Anal 27(1):135–144. https://doi.org/10.1016/j.jfda.2018.07.004
Moradi A, Tarrahi MJ, Ghasempour S, Shafiepour M, Clark CC, Safavi SM (2020) The effect of okra (Abelmoschus esculentus) on lipid profiles and glycemic indices in type 2 diabetic adults: randomized double blinded trials. Phytother Res 34(12):3325–3332. https://doi.org/10.1002/ptr.6782
Saatchi A, Aghamohammadzadeh N, Beheshtirouy S, Javadzadeh Y, Afshar FH, Ghaffary S (2022) Anti-hyperglycemic effect of Abelmoschus culentesus (Okra) on patients with diabetes type 2: a randomized clinical trial. Phytother Res 36(4):1644–1651. https://doi.org/10.1002/ptr.7341
Khodija U, Wiboworini B, Kartikasari L (2020) Comparing the effect of steamed and boiled okra (Abelmoschus esculentus) on fasting blood glucose among type 2 diabetes mellitus patients with hypercholesterolemia. Int J Nutr Sci 5(2):65–71
Association AD (2021) 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 44(Supplement 1): S15-S33
Sarbini D, Huriyati E, Sadewa H, Wahyuningsih MSH (2019) The effect of rosella (Hibiscus sabdariffa linn) on insulin resistance in patients with type 2 diabetes mellitus: a randomized clinical trial
Zakerkish M, Jenabi M, Zaeemzadeh N, Hemmati AA, Neisi N (2019) The effect of Iranian propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-43838-8
Group UPDS (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352(9131):837–853
Yaradua I, Ibrahim M, Matazu K et al (2017) Antidiabetic activity of Abelmoschus esculentus (Ex-Maradi Okra) fruit in alloxan-induced diabetic rats. Niger J Biochem Mol Biol 32:44–52
Wu T, Rayner CK, Jones KL, Xie C, Marathe C, Horowitz M (2020) Role of intestinal glucose absorption in glucose tolerance. Curr Opin Pharmacol 55:116–124
Dantas TL, Alonso Buriti FC, Florentino ER (2021) Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants 10(8):1683. https://doi.org/10.3390/plants10081683
Fan S, Zhang Y, Sun Q et al (2014) Extract of okra lowers blood glucose and serum lipids in high-fat diet-induced obese C57BL/6 mice. J Nutr Biochem 25(7):702–709
Nikpayam O, Safaei E, Bahreini N, Saghafi-Asl M (2021) The effects of okra (Abelmoschus esculentus L.) products on glycemic control and lipid profile: a comprehensive systematic review. J Funct Foods 87: 104795. https://doi.org/10.1016/j.jff.2021.104795
Wengrofsky P, Lee J, Makaryus AN (2019) Dyslipidemia and its role in the pathogenesis of atherosclerotic cardiovascular disease: implications for evaluation and targets for treatment of dyslipidemia based on recent guidelines. In: Dyslipidemia. IntechOpen
Dludla PV, Nkambule BB, Nyambuya TM et al (2022) Vitamin C intake potentially lowers total cholesterol to improve endothelial function in diabetic patients at increased risk of cardiovascular disease: a systematic review of randomized controlled trials. Front Nutr 9:2524
Garjani A, Andalib S, Ziaee M, Maleki-Dizaji N (2008) Biphasic effects of atorvastatin on inflammation. Pak J Pharm Sci 21(2):125–130
Ngoc TH, Ngoc QN, Tran A, Phung NV (2008) Hypolipidemic effect of extracts from Abelmoschus esculentus L. (Malvaceae) on tyloxapol-induced hyperlipidemia in mice. Mahidol Univ J Pharm Sci 35(1–4): 42–46
Majd NE, Tabandeh MR, Shahriari A, Soleimani Z (2018) Okra (Abelmoscus esculentus) improved islets structure, and down-regulated PPARs gene expression in pancreas of high-fat diet and streptozotocin-induced diabetic rats. Cell J (Yakhteh) 20(1):31. https://doi.org/10.22074/cellj.2018.4819
Wu J, Shi S, Wang H, Wang S (2016) Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: a review. Carbohydr Polym 144:474–494
Ziaee M, Mashayekhi S, Ghaffari S, Mahmoudi J, Sarbakhsh P, Garjani A (2019) Predictive value of endocan based on TIMI risk score on major adverse cardiovascular events after acute coronary syndrome. Angiology 70(10):952–959
Kanmani S, Kwon M, Shin M-K, Kim MK (2019) Association of C-reactive protein with risk of developing type 2 diabetes mellitus, and role of obesity and hypertension: a large population-based Korean cohort study. Sci Rep 9(1):4573
Bilgin S, Kurtkulagi O, Tel BMA et al (2021) Does C-reactive protein to serum albumin ratio correlate with diabEtic nephropathy in patients with Type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes 15(6):1071–1074
Mahdavi AM, Javadivala Z, Ahmadian E (2022) Effects of Okra (Abelmoschus esculentus L.) on inflammatory mediators: a systematic review of preclinical studies. Food Funct
Liu Y, Qi J, Luo J et al (2021) Okra in food field: nutritional value, health benefits and effects of processing methods on quality. Food Rev Intl 37(1):67–90
Xiong B, Zhang W, Wu Z et al (2021) Preparation, characterization, antioxidant and anti-inflammatory activities of acid-soluble pectin from okra (Abelmoschus esculentus L.). Int J Biol Macromol 181:824–834
Liu Y, Ye Y, Hu X, Wang J (2021) Structural characterization and anti-inflammatory activity of a polysaccharide from the lignified okra. Carbohydr Polym 265:118081
Nasrollahi Z, ShahaniPour K, Monajemi R, Ahadi AM (2022) Abelmoschus esculentus (L.) Moench improved blood glucose, lipid, and down‐regulated PPAR‐α, PTP1B genes expression in diabetic rats. J Food Biochem 46(7):e14097
Li Y (2017) Hypoglycemic effect of okra extract on type 2 diabetic mice and correlation with TNF-α and IDE. Chin Trad Herbal Drugs, 3131–3137
Tyagita N, Utami K, Zulkarnain F et al (2019) Okra infusion water improving stress oxidative and inflammatory markers on hyperglycemic rats. Bangladesh J Med Sci 18(4):748–752. https://doi.org/10.3329/bjms.v18i4.42879
Acknowledgements
This research was financially supported by a Grant from the Research Vice-Chancellor of Maragheh University of Medical Sciences, Maragheh, Iran (Grant No. 68504). The authors also would like to sincerely appreciate the vice president of research of the Faculty of Pharmacy, Zanjan University of Medical Sciences for support.
Author information
Authors and Affiliations
Contributions
M.Z. and M.T. contributed to conceptualization; M.Z, M.T, and A.B. helped in methodology; Z.M. and A.B. contributed to formal analysis; M.S. and M.T. helped in preparing of dosage forms; S.P, H. GM., A.A., and S.M. contributed to patient visiting; M.Z, A.B., and M.T helped in writing—original draft preparation; M.Z, M.T., S.P, and H. GM. contributed to writing—review and editing; M.Z. helped in supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests for this manuscript.
Ethical approval
This study was approved by the Ethics of Committees of Zanjan University of Medical Sciences and in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for this retrospective study.
Additional information
Managed by Antonio Secchi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tavakolizadeh, M., Peyrovi, S., Ghasemi-Moghaddam, H. et al. Clinical efficacy and safety of okra (Abelmoschus esculentus (L.) Moench) in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Acta Diabetol 60, 1685–1695 (2023). https://doi.org/10.1007/s00592-023-02149-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02149-1