Abstract
Aims
Oxidative stress plays a pivotal role in the pathogenesis of type 2 diabetes (T2D). In vitro and animal studies have shown that resveratrol exerts an antioxidant effect, but clinical trials addressing this effect in patients with T2D are limited. The aim of this study was to determine whether resveratrol supplementation affects oxidative stress markers in a randomized, placebo-controlled, double-blind clinical trial.
Methods
A total of 48 patients with T2D randomly were assigned to receive 800 mg/day resveratrol or placebo for 2 months. Plasma total antioxidant capacity, malondialdehyde concentration, protein carbonyl and total thiol contents, intracellular superoxide anion (O2−·) and hydrogen peroxide (H2O2) in PBMCs, the expression of genes involved in oxidative stress responses (Nrf2, SOD, Cat, HO-1, RAGE, NOS) in PBMCs, and metabolic and anthropometric parameters were measured at the baseline and at the trial end.
Results
Compared with the placebo group, resveratrol reduced plasma protein carbonyl content and PBMCs O2−· level and significantly increased plasma total antioxidant capacity and total thiol content. Furthermore, the expression of Nrf2 and SOD was significantly increased after resveratrol consumption. Resveratrol had no significant effects on the metabolic and anthropometric parameters except for a significant reduction in weight, BMI, and blood pressure levels. Resveratrol was well tolerated, and no serious adverse event was occurred.
Conclusions
Our study demonstrated that 8 weeks of supplementation with 800 mg/day resveratrol has an antioxidant effect in the blood and PBMCs of patients with T2D.
Clinical Trial Registry number and website IRCT registration number: IRCT2015072523336N1 and http://en.search.irct.ir/view/24752.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes (T2D) is one of the most common metabolic disorders that is now considered as a major public healthcare problem all around the world [1]. According to the World Health Organization (WHO) reports, more than 382 million people worldwide are suffering from diabetes and this number will reach 438 million in 2030 [2]. Increasing evidence has implicated that oxidative stress plays a pivotal role in the development and progression of diabetes and its complications [3]. Oxidative stress is defined as an event resulting from the imbalance between the oxidant and antioxidant substances [4]. Several clinical and experimental studies indicated that an increased oxidative stress in diabetic patients most likely results from overproduction of mitochondrial reactive oxygen species (ROS) stimulated by hyperglycemia [5]. Excessive ROS accumulation in diabetes may induce the oxidative modification of cellular macromolecules such as lipids, proteins, and nucleic acids. In this regard, measurements of the levels of malondialdehyde (MDA), protein carbonyl and sulfhydryl contents, total antioxidant capacity (TAC), and ROS have been proposed as the potential biomarkers for oxidative stress [6]. There is also convincing experimental and clinical evidence that the disruption of natural antioxidant defense system might lead to oxidative stress [7, 8]. Antioxidant defense system consists of a series of specific enzymes, metal binding proteins, and a number of low molecular weight antioxidants [9]. Under oxidative stress conditions, the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), a master modulator of the antioxidant response, regulates the expression of a significant number of antioxidant genes including catalase (Cat), glutathione peroxidase, glutathione reductase, glutathione-S-transferase, superoxide dismutase (SOD), and heme oxygenase-1 (HO-1) [10, 11]. Given the role of oxidative stress in the development of chronic diseases such as diabetes, the use of antioxidants provides an attractive therapeutic strategy for the delay or reversal of diabetic complications. In this context, natural products are documented to reduce oxidative stress and are acknowledged as the antioxidant interventions.
Resveratrol (trans-3,5,40-trihydroxystilbene) is a natural polyphenol synthesized by several plant species including grapes, peanuts, and berries [12]. Resveratrol was shown to prevent and treat chronic conditions such as neurodegenerative disorders, cardiovascular disorders, cancer, diabetes, and metabolic diseases [13]. The evidence has demonstrated that the beneficial effect of resveratrol on health is mediated through its antioxidant and anti-inflammatory properties, cardioprotective, and neuroprotective activities [9, 13]. Resveratrol reduces oxidative stress via increase in TAC, inhibition of lipid peroxidation, and reducing ROS formation [14]. In addition, free radical scavenging is one of the biological activities that have been ascribed to resveratrol [12]. In addition to in vitro and animal studies, randomized clinical trial studies have shown a protective effect of resveratrol against several diseases mediated by oxidative stress [15, 16]. However, the effects of resveratrol supplementation on the levels of oxidative stress markers in T2D are not completely elucidated. Given the key role of oxidative stress in T2D pathogenesis and because of the antioxidant property of resveratrol, we in the present study assumed that resveratrol supplementation might be effective in decreasing oxidative stress in patients with T2D. Thus, we designed this placebo-controlled, double-blind, randomized clinical trial to investigate the effects of 800 mg/day dose of resveratrol supplementation for 8 weeks on oxidative stress indices in plasma and peripheral blood mononuclear cells (PBMCs) of patients with T2D. We also aimed to evaluate the effect of resveratrol supplementation on metabolic and anthropometric parameters.
Materials and methods
Study design and participants
Study population comprised of forty-eight patients with T2D, aged 30–70 years. These patients participated in a randomized, placebo-controlled, double-blind clinical trial in the Endocrine and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
The main inclusion criteria were patients with known T2D, patients on diet and/or hypoglycemic agents other than insulin, and having no allergy to grapes, green tea, and peanuts. None of the patients were on anti-inflammatory supplementation. Patients were requested not to take any antioxidant supplements during the trial. Exclusion criteria were patients with type 1 diabetes, pregnant women, lactating mothers, patients with severe heart disease, hepatic disease, and renal impairment, and current participation in any weight loss program or planning to enroll in any such program during the study. Participants were randomly allocated into two treatment groups to take either resveratrol or placebo.
Informed consent and ethics committee approval
The present study was approved by the Ethics Committee of Tehran University of Medical Sciences. The research was recorded in the Iranian Web site for registration of clinical trials (http://www.irct.ir: IRCT2015072523336N1). All participants gave written informed consent prior to entering the study.
Randomization
Stratified randomization was done using computer-generated random numbers. Eligible participants were randomized to receive daily capsules of placebo or resveratrol in a double-blind fashion. Both the investigator and patients were blinded from the time of randomization until analysis was completed.
Outcomes
The primary aim of the study was to assess the differences in changes of the levels of several oxidative stress markers such as ROS levels in PBMCs, TAC, MDA, total thiol and carbonyl contents of plasma and gene expression of SOD, Cat, Nrf2, HO-1, nitric oxide synthases (NOS), and receptor of advanced glycation end product (RAGE) from baseline to the end of the trial. Secondary outcomes were defined by the differences in changes of the laboratory parameters including fasting blood sugar (FBS), glycated hemoglobin (HbA1c), insulin, homeostasis model assessment of insulin resistance (HOMA-IR), total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triacylglycerol (TG), urea, uric acid, creatinine, total protein, serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), and anthropometric variables such as weight, body mass index (BMI) and waist circumference.
Intervention
The subjects were prescribed two dietary supplements a day (twice a day of 400 mg of resveratrol or twice a day of 400 mg of placebo). The selected dose of resveratrol was almost similar to resveratrol supplementation doses in Movahed et al. [17] and Thazhath et al. [18] studies. The appearance of the placebo capsules in color, shape, size and packaging was identical to the resveratrol capsules. Resveratrol (trans-resveratrol, 99% purified) and placebo (microcellulose) were obtained from Mega resveratrol, UK. All patients were allowed to continue their existing antidiabetic medications throughout the study. The patients also instructed to abstain from food and beverages containing substantial amounts of resveratrol. They were also advised not to take any other food supplements. Each participant was asked to complete a questionnaire during the study to monitor their health status and the use of drugs or supplements.
Treatment adherence
The researcher monitored the adherence to the study protocol by weekly phone calls. Subjects were questioned at each phone call regarding new symptoms and/or health changes since the prior interview. In addition, participants were asked to return unused capsules at the end of the trial. In order to assess the compliance, the remaining supplements were counted and subtracted from the amount of supplements provided to the participants.
Physical measurements
Systolic and diastolic blood pressures were assessed twice on the right arm after a 10-min rest in the sitting position, by a standard mercury sphygmomanometer. Also anthropometric parameters were measured at the baseline and after 2 months of follow-up. Weight was measured by a Seca scale (Germany) to the nearest of 0.1 kg, and height was measured using a stadiometer to the nearest of 0.1 cm. Waist and hip circumferences were measured at the narrowest level over light clothing by a plastic tape meter to the nearest 0.1 cm. BMI was calculated as the ratio of the current body weight to height2 (kg/m2).
Assessment of biochemical variables and oxidative stress biomarkers
After an overnight fast, venous blood samples were taken at the beginning and at the end of the study. The blood was immediately centrifuged at 3000×g for 5 min, and plasma was kept frozen at − 80 °C until assayed. Biochemical variables in serum were determined using enzymatic methods by BIOLIS 24i Premium Autoanalyser (Tokyo Boeki Machinery Ltd., Japan). The biochemical tests included FBS, total cholesterol, LDL-C, HDL-C, TG, urea, uric acid, creatinine, total protein, SGOT, SGPT, and high-sensitivity C-reactive protein (hsCRP). HbA1c was assessed with HPLC method by Tosoh G8 instrument (South San Francisco, CA). Insulin was quantified by Insulin AccuBind ELISA Kit (Monobind Inc, California, USA).
Plasma MDA level was measured as the thiobarbituric acid reactive substance (TBARS) using 1,1,3,3-tetramethoxypropane as a standard [19]. Plasma TAC was measured on the basis of the ferric reducing ability of plasma. Protein carbonyl content in plasma was assessed using 2,4-dinitrophenylhydrazine assay with slight modifications. Total thiol content was measured using spectrophotometric method [19].
PBMCs isolation
PBMCs were isolated immediately after blood collection from 10 ml heparinized whole blood by the use of Ficoll–Hypaque density gradient centrifugation. After washing with PBS, isolated PBMCs were counted and viability test was performed with trypan blue staining. For real-time PCR assay and flow cytometry analysis, PBMCs were aliquoted into separate sterile 2-ml Eppendorf tubes.
Measurement of intracellular ROS
Intracellular superoxide anion (O2−·) and hydrogen peroxide (H2O2) levels were assessed by flow cytometry on freshly isolated PBMCs using dihydroethidium (DHE) and 2′,7′′-dichloro-dihydro-fluorescin diacetate (DCFH-DA) probes, respectively. Briefly, ~ 1 × 106 PBMCs were washed twice with PBS and centrifuged at 1500 rpm for 10 min. The cells were then resuspended in PBS and were incubated with 10 μM DCFH-DA and 1.25 µM DHE for 40 and 20 min in dark at room temperature, respectively [20]. All emitted fluorescent signals from stained PBMCs were detected using the Becton Dickinson FACSCalibur flow cytometer. Green fluorescence emission for DCFH-DA (between 500 and 530 nm) and red fluorescence emission for DHE (between 590 and 700 nm) were evaluated in the FL-1 and FL-2 channels, respectively. Exclusion of apoptotic and dead cells was evaluated using YO-PRO1 and propidium iodide (PI) counterstains. Output data were analyzed with FlowJo software, and the results were reported as mean fluorescence intensity (MFI). Fluorescent data of all events were collected until 10,000 gated PBMCs were examined.
Quantitative real-time PCR
Total RNA from PBMCs was extracted using the GeneAll Hybrid-R RNA purification kit (GeneAll Biotechnology Co., Seoul, South Korea). RNA was reverse-transcribed into cDNA with RevertAid RT Reverse Transcription Kit (Thermo Fisher, USA). Real-time quantitative PCR (qPCR) was run on Corbett Rotor Gene 6000 Light Cycler (Qiagen, Hilden, Germany). Changes in the expression of selected genes were validated in duplicate using SYBR Green RealQ Plus 2× Master Mix Green (Ampliqon). Results were normalized to β-actin. The delta–delta Ct method was used to calculate the relative expression. Primer sequences used in this study are shown in Table 1 of “Supplementary file.”
Sample size calculation and statistical analyses
Sample size calculation was based on changes in the mean value of the total antioxidant capacity of plasma as a primary outcome. To detect a 25% change in the primary outcome at a two-sided 0.05 significance level with a power of 0.8, nineteen participants were required in each group. In addition, because we anticipated a 25% dropout rate, a final sample size of 24 patients per group was planned for requirement in this study.
All analyses were performed with SPSS 22.0 (SPSS Inc., Chicago, USA). Qualitative data are shown as proportions. Quantitative data are expressed as the mean value ± SD or as the median and the 25th to 75th interquartile range. For RT-PCR results, the statistical analyses were applied to the normalized ∆Ct values (Ct target − Ct β actin). Assumptions of the normality and equal variances were checked to perform the appropriate statistical test. Variables with skewed distribution were logarithmically transformed prior to analysis. Baseline data were analyzed to determine the possible significant starting intergroup differences. A paired t test and independent t-test were used for numerical normally distributed data. The Wilcoxon signed-rank test and Mann–Whitney U test were used for nonparametric distributions. To account for the differences between the arms of the analyzed variables at enrollment, comparison of changes from baseline between the resveratrol and placebo arms was made by one-way analysis of covariance (ANCOVA), to adjust for baseline imbalances of the analyzed endpoints. The covariates used for adjustment were age, duration of diabetes, age at the onset of diabetes, BMI, cholesterol, and type of drugs consumption. Values of P < 0.05 were considered statistically significant.
Results
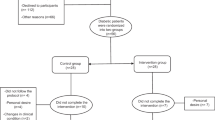
The flow diagram of the trial is shown in Fig. 1. Forty-six subjects completed the study. At the baseline, forty-eight participants were randomly assigned to the resveratrol group (n = 23) and placebo group (n = 25). Of 48 participants, two from the placebo arm were dropped out.
Compliance, tolerability, and safety
Compliance assessment was based on pill counts. A compliance audit of returned capsules indicated 94% compliance in resveratrol group and 96% in placebo group. During the treatment period, resveratrol was well tolerated and no patient reported any significant adverse effect.
Baseline characteristics
Baseline characteristics of the participants are detailed in Table 1. The baseline characteristics revealed that there was no significant difference among the variables between two groups except for total cholesterol and LDL-C. Total cholesterol and LDL-C levels were significantly higher in the resveratrol group compared to the placebo group. The differences in the levels of LDL-C and TC between resveratrol and placebo groups might be due to taking different lipid-lowering drugs by patients.
Effects of resveratrol on oxidative stress parameters
Differences between two groups in assessed oxidative stress parameters at the end of the trial are shown in Table 2. We first investigated the intracellular superoxide anion (O2−·) and hydrogen peroxide (H2O2) levels in PBMCs using flow cytometry. The results revealed that production of hydrogen peroxide (H2O2) did not significantly differ between resveratrol and placebo groups, whereas patients undertaking resveratrol had a significantly lower intracellular superoxide anion (O2−·) production after receiving resveratrol for 2 months.
We further analyzed the changes in the transcript levels of Nrf2, HO-1, NOS, RAGE, Cat and SOD in PBMCs. Transcription of HO-1, NOS, RAGE and Cat did not change with resveratrol supplementation, whereas Nrf2 and SOD expressions significantly increased in resveratrol group (P = 0.047 and P = 0.005, respectively). Plasma protein carbonyl content was significantly decreased in resveratrol group compared with the placebo group (P = 0.007). Furthermore, total thiol content was significantly increased after 2 months of resveratrol supplementation. Our finding also showed a significant increase in TAC as measured by FRAP assay in resveratrol group. The results demonstrated that MDA did not differ between two groups.
Effects of resveratrol on metabolic and anthropometric parameters
Table 3 shows the biochemical and anthropometric parameters of the subjects at the baseline and after 2 months of resveratrol supplementation or placebo. There were no significant differences in the mean plasma glucose and HbA1c levels between two groups. Changes in serum insulin and HOMA-IR levels were not significantly different between two groups. No significant differences in TG, total cholesterol, LDL-C, HDL-C, SGOT, SGPT, urea, uric acid, creatinine, and total protein levels were observed between resveratrol and placebo groups.
We also analyzed the effect of resveratrol supplementation on anthropometric parameters. There was a significant decrease in weight (− 1.18 ± 2.77 vs. 0.22 ± 2.05 kg, P = 0.006) and BMI (− 0.68 ± 1.05 vs. 0.07 ± 0.74 kg/m2, P = 0.006) in resveratrol group compared to placebo treatment. No significant changes were observed for waist and hip circumferences and waist-to-hip ratio after resveratrol consumption. Resveratrol supplementation led to a significant decrease in systolic (− 10.2 ± 8.5 vs. − 1.3 ± 10.8 mmHg, P = 0.002) and diastolic (− 7.3 ± 6.8 vs. 1.1 ± 9.0 mmHg, P = 0.000) blood pressures compared to placebo treatment (Table 3).
Discussion
There is now convincing evidence that oxidative stress plays an important role in the etiology and/or progression of a number of human diseases [21]. Based on the data from in vitro and in vivo studies, resveratrol exerts the antioxidant effects via modulation of key players involved in the oxidative stress [22, 23]. However, clinical trials addressing the antioxidant effects of resveratrol are still limited. A number of clinical trials have demonstrated the antioxidant effects of resveratrol in different pathological conditions [24, 25]. The present study adds to the knowledge about the antioxidant effects of resveratrol supplementation in patients with T2D.
In the present study we collected the data from a set of oxidative stress markers. Enhanced ROS production has been implicated in the pathogenesis of several metabolic diseases including chronic liver disease, atherosclerosis, and diabetes [26]. We assessed the level of total antioxidant status and total thiol content representing the capacity of plasma factors to counteract oxidative stress. In addition, we evaluated plasma protein carbonyl content and MDA levels as convenient and simple surrogate markers of protein and lipid oxidation, respectively [7]. To investigate more specifically the relationship between resveratrol consumption and oxidative stress, we assessed the expression level of key genes involved in oxidative stress. This set of markers has allowed us to better understand the possible effect of resveratrol on oxidative stress in patients with T2D. The data of this study demonstrated that resveratrol supplementation in patients with T2D for 8 weeks reduces plasma and PBMCs oxidative stress markers. More specifically, our double-blind, randomized study showed (a) a significant increase in TAC and protein thiol content of plasma, (b) a significant decrease in plasma protein carbonyl content, (c) a statistically significant reduction in intracellular superoxide anion (O2−·) production in PBMCs, and (d) a significant increase in the expression of Nrf2 and SOD in PBMCs of T2D patients following resveratrol consumption. These findings suggest that resveratrol supplementation is capable of reducing oxidative stress in patients with T2D.
The present findings appear to be in accordance with the results of several studies suggesting an antioxidant role for resveratrol. For instance, Carrizzo et al. [27] indicated that resveratrol supplementation in patients with hypertension and dyslipidemia enhanced the expression of mitochondrial SOD by the Nrf2-dependent mechanism. This finding in humans was in agreement with the experimental models showing that resveratrol was able to increase SOD expression in the animals and cell lines [28]. Consistent with our results, a randomized clinical trial conducted by Ghanim et al. [29] reported that resveratrol supplementation (40 mg/day) for 6 weeks decreased ROS level in mononuclear cells of 20 healthy adults. De Groote et al. [30] displayed that 150 mg/day resveratrol supplementation for 28 days significantly increased reduced glutathione (GSH) level in adult obese subjects. Buonocore et al. [31] showed that 60-day resveratrol consumption had a significant positive effect on oxidative biomarkers such as TAC. A study by Losa et al. [32] showed that PBMCs treatment with resveratrol increases the antioxidant capacity by increasing the amount of intracellular glutathione. Result of a randomized clinical trial on 50 healthy adult smokers showed that 30-day resveratrol supplementation had a significant effect on TAC [33]. Recently, Imamura et al. [34] reported that 100 mg resveratrol for 12 weeks in 50 patients with T2D led to a decrease in serum level of diacron reactive oxygen metabolites, an oxidative stress marker. Furthermore, serum SOD and TAC were increased, whereas serum MDA was decreased in patients with ulcerative colitis that received either 500 mg/day resveratrol capsules or the same amount of placebo for 6 weeks [35]. Taken together, the data from our study and the others provide the evidence that the findings are consistent and resveratrol has an antioxidant effect in the blood and PBMCs of patients with T2D.
The results of the present study did not support the hypothesis that resveratrol might have a beneficial effect on the level of biochemical parameters in patients with T2D. Several studies evaluated the levels of biochemical parameters after resveratrol supplementation [17, 36, 37]. Hausenblas et al. [38] in a meta-analysis of data from six unique datasets, examining a total of 196 T2D patients (104 resveratrol; 92 control/placebo), demonstrated a statistically significant effect of resveratrol supplementation on systolic blood pressure, HbA1c, and creatinine, but not for fasting glucose, HOMA-IR, diastolic blood pressure, insulin, TG, LDL-C, and HDL-C. Sahebkar et al. [39] in a meta-analysis revealed that resveratrol supplementation did not alter plasma levels of total cholesterol, LDL-C, HDL-C, TG, and glucose. A recent meta-analysis also demonstrated that resveratrol supplementation did not change blood glucose, insulin, TG, and LDL levels in patients with non-alcoholic fatty liver (NAFLD) disease [40]. Our data revealed that weight and BMI tended to decrease upon resveratrol treatment. In agreement with these findings, Mendez-del Villa et al. [41] found that administration of 500 mg resveratrol three times per day for 90 days significantly decreased weight, BMI, fat mass, and waist circumference in 24 patients with metabolic syndrome. In addition, Faghihzadeh et al. [42] reported improvements in weight, BMI, waist circumference of 50 overweight (NAFLD) patients treated with 500 mg/day of resveratrol or placebo for 12 weeks. Cell culture and animal studies have suggested that the anti-adipogenic effect of resveratrol is mediated through suppressing the feeding center [43], inhibiting adipogenesis [43], increasing fatty acid oxidation [44], inducing the formation of the brown-like adipocyte in white adipose tissue [45], increasing apoptosis in mature adipocytes [46], inhibition of de novo lipogenesis and adipose tissue fatty acid uptake [47], and increasing brown adipose tissue thermogenesis and, consequently, the associated energy dissipation [48].
In contrast to our findings, Christenson et al. [49] in a systematic review of eight randomized clinical trials including 208 participants demonstrated that there were no significant differences in weight and BMI between resveratrol and placebo groups. Furthermore, Elgebaly et al. [40] in a recent systematic review of four randomized clinical trials suggested that resveratrol supplementation does not affect the levels of weight and BMI in NAFLD patients. Taken together, these data suggest that the current evidence is insufficient to support the efficacy of resveratrol supplementation in management of diabetes-related metabolic traits. Most of the studies were criticized because of concerns due to small sample size, short follow-up, and lack of adjustments for appropriate covariates in the statistical analyses [50]. Thus, further larger and longer duration trials are required to study the relationship between resveratrol consumption and T2D.
Given the strong link between the excess body fat and worsened glycemic control, our findings of reduced body weight and no changes of the HbA1c level upon resveratrol supplementation are somehow unexpected. No significant effect of resveratrol consumption on the level of HbA1c (despite a reduction in body weight in resveratrol group) might be attributed to the short-term follow-up of this study. It was reported that 3–24 months of follow-up of weight loss is required to observe a significant effect on HbA1c [51]. Furthermore, the data from previous trials regarding the effect of weight reduction on HbA1c revealed that HbA1c-lowering is greater in populations with poor glycemic control than in well-controlled populations with the same degree of weight loss [51]. In the present study, all subjects recruited were patients with well glycemic control.
In addition, the current study suggests that consumption of resveratrol had a favorable effect on systolic and diastolic blood pressures. Consistent with our results, a recent meta-analysis of six randomized clinical trials investigating the effect of resveratrol on blood pressure reported that high dose of resveratrol (≥ 150 mg/day) significantly reduced blood pressure [8]. The improvement in blood pressure following resveratrol consumption may result from its ability to activate adenosine monophosphate-activated protein kinase (AMPK), which directly phosphorylates eNOS leading to increasing NO production [52]. Furthermore, resveratrol has been shown to act as an endothelin (ET)-1 antagonist and downregulates the concentration of angiotensin (Ang) II, two substances that have been found to play roles in the development of hypertension [52].
The current study had a few limitations. The limitation of our study is a relatively short-term follow-up period. The sample size of this study was also relatively small. Future studies with longer duration and larger sample sizes are needed to confirm our findings.
In conclusion, our study demonstrated that 8 weeks supplementation with 800 mg/day resveratrol decreases markers of oxidative stress in the plasma and PBMCs of patients with T2D. However, resveratrol does not improve the metabolic patterns of T2D patients except in the case of obesity-related parameters such as weight, BMI and blood pressure. Despite the obvious usefulness and advantages of resveratrol as an antioxidant, more studies with longer durations and different dosages of supplementation are needed to evaluate the efficacy of this polyphenol in the management of T2D.
Change history
16 June 2018
Oxidative stress plays a pivotal role in the pathogenesis of type 2 diabetes (T2D). In vitro and animal studies have shown that resveratrol exerts an antioxidant effect, but clinical trials addressing this effect in patients with T2D are limited. The aim of this study was to determine whether resveratrol supplementation affects oxidative stress markers in a randomized, placebo-controlled, double-blind clinical trial.
Abbreviations
- BMI:
-
Body mass index
- Cat:
-
Catalase
- DCFH-DA:
-
Dichloro-dihydro-fluorescein diacetate
- DHE:
-
Dihydroethidium
- FBS:
-
Fasting blood sugar
- FRAP:
-
Ferric reducing ability of plasma
- HDL-C:
-
High-density lipoprotein cholesterol
- HO-1:
-
Heme oxygenase 1
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- hsCRP:
-
High-sensitivity C-reactive protein
- LDL-C:
-
Low-density lipoprotein cholesterol
- MDA:
-
Malondialdehyde
- NOS:
-
Nitric oxide synthases
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- PBMCs:
-
Peripheral blood mononuclear cells
- RAGE:
-
Receptor of advanced glycation end product
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TAC:
-
Total antioxidant capacity (TAC)
- T2D:
-
Type 2 diabetes
- WC:
-
Waist circumference
References
Brasnyó P, Sümegi B, Winkler G, Wittmann I (2013) Resveratrol and oxidative stress in diabetes mellitus. In: Preedy V (ed) Diabetes: oxidative stress and dietary antioxidants. Elsevier Inc., Amsterdam
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103(2):137–149. https://doi.org/10.1016/j.diabres.2013.11.002
Ilechukwu C, Ebenebe U, Ubajaka C, Ilika A, Emelumadu O, Nwabueze S (2014) The role of oxidative stress in diabetes mellitus: a 24-year review. Afrimedic J 5(1):1–7
Francisqueti FV, Chiaverini LC, Santos KC et al (1992) The role of oxidative stress on the pathophysiology of metabolic syndrome. Rev Assoc Med Bras 63(1):85–91. https://doi.org/10.1590/1806-9282.63.01.85
Folli F, Corradi D, Fanti P et al (2011) The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro-and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev 7(5):313–324
Tiwari BK, Pandey KB, Abidi A, Rizvi SI (2013) Markers of oxidative stress during diabetes mellitus. J Biomark, Art id 378790
Martín-Gallán P, Carrascosa A, Gussinyé M, Domínguez C (2003) Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med 34(12):1563–1574. https://doi.org/10.1016/s0891-5849(03)00185-0
Timmers S, de Ligt M, Phielix E et al (2016) Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: a randomized controlled trial. Diabetes Care 39(12):2211–2217
Gawlik K, Naskalski J, Fedak D et al. (2015) Markers of antioxidant defense in patients with type 2 diabetes. Oxid Med Cell Longev 2016:2352361
Javkhedkar AA, Quiroz Y, Rodriguez-Iturbe B et al (2015) Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 308(10):R840–R846. https://doi.org/10.1152/ajpregu.00308.2014
Jimenez-Osorio AS, Picazo A, Gonzalez-Reyes S, Barrera-Oviedo D, Rodriguez-Arellano ME, Pedraza-Chaverri J (2014) Nrf2 and redox status in prediabetic and diabetic patients. Int J Mol Sci 15(11):20290–20305. https://doi.org/10.3390/ijms151120290
Gerszon J, Rodacka A, Puchała M (2014) Antioxidant properties of resveratrol and its protective effects in neurodegenerative diseases. Adv Cell Biol. https://doi.org/10.2478/acb-2014-0006
Smoliga JM, Baur JA, Hausenblas HA (2011) Resveratrol and health—a comprehensive review of human clinical trials. Mol Nutr Food Res 55(8):1129–1141. https://doi.org/10.1002/mnfr.201100143
Gülçin İ (2010) Antioxidant properties of resveratrol: a structure—activity insight. Innov Food Sci Emerg Technol 11(1):210–218. https://doi.org/10.1016/j.ifset.2009.07.002
de la Lastra CA, Villegas I (2007) Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Portland Press Limited, London
Wood LG, Wark PA, Garg ML (2010) Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal 13(10):1535–1548
Movahed A, Nabipour I, Lieben Louis X et al (2013) Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid Based Complement Altern Med eCAM 2013:851267. https://doi.org/10.1155/2013/851267
Thazhath SS, Wu T, Bound MJ et al (2016) Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: a randomized controlled trial. Am J Clin Nutr 103(1):66–70
Chakraborty A, Chowdhury S, Bhattacharyya M (2011) Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract 93(1):56–62
Ghorbani M, Vatannejad A, Khodadadi I, Amiri I, Tavilani H (2016) Protective effects of glutathione supplementation against oxidative stress during cryopreservation of human spermatozoa. Cryoletters 37(1):34–40
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329(1):23–38
Schmatz R, Perreira LB, Stefanello N et al (2012) Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie 94(2):374–383. https://doi.org/10.1016/j.biochi.2011.08.005
Soufi FG, Mohammad-nejad D, Ahmadieh H (2012) Resveratrol improves diabetic retinopathy possibly through oxidative stress–nuclear factor κB—apoptosis pathway. Pharmacol Rep 64(6):1505–1514
Mecocci P, Polidori MC (1822) Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim Biophys Acta 5:631–638. https://doi.org/10.1016/j.bbadis.2011.10.006
Ndiaye M, Philippe C, Mukhtar H, Ahmad N (2011) The grape antioxidant resveratrol for skin disorders: promise, prospects, and challenges. Arch Biochem Biophys 508(2):164–170. https://doi.org/10.1016/j.abb.2010.12.030
Shradha B, Sisodia S (2010) Diabetes, dyslipidemia, antioxidant and status of oxidative stress. Intern J Res Ayurveda Pharm 1(1):33–42
Carrizzo A, Forte M, Damato A et al (2013) Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem Toxicol 61:215–226. https://doi.org/10.1016/j.fct.2013.07.021
Leonard SS, Xia C, Jiang B-H et al (2003) Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun 309(4):1017–1026
Ghanim H, Sia CL, Abuaysheh S et al (2010) An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab 95(9):E1–E8. https://doi.org/10.1210/jc.2010-0482
De Groote D, Van Belleghem K, Deviere J, Van Brussel W, Mukaneza A, Amininejad L (2012) Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann Nutr Metab 61(1):15–24. https://doi.org/10.1159/000338634
Buonocore D, Lazzeretti A, Tocabens P et al (2012) Resveratrol-procyanidin blend: nutraceutical and antiaging efficacy evaluated in a placebocontrolled, double-blind study. Clin Cosmet Investig Dermatol 5:159–165. https://doi.org/10.2147/CCID.S36102
Losa G (2003) Resveratrol modulates apoptosis and oxidation in human blood mononuclear cells. Eur J Clin Investig 33(9):818–823
Bo S, Ciccone G, Castiglione A et al (2013) Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem 20(10):1323–1331
Imamura H, Yamaguchi T, Nagayama D, Saiki A, Shirai K, Tatsuno I (2017) Resveratrol ameliorates arterial stiffness assessed by cardio-ankle vascular index in patients with type 2 diabetes mellitus. Int Heart J 58(4):577–583
Samsamikor M, Daryani NE, Asl PR, Hekmatdoost A (2016) Resveratrol supplementation and oxidative/anti-oxidative status in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res 47(4):304–309
Bhatt JK, Nanjan MJ (2013) Resveratrol supplementation in patients with type 2 diabetes mellitus: a prospective, open label, randomized controlled trial. Int Res J Pharm 4(8):245–249. https://doi.org/10.7897/2230-8407.04849
Brasnyo P, Molnar GA, Mohas M et al (2011) Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 106(3):383–389. https://doi.org/10.1017/S0007114511000316
Hausenblas HA, Schoulda JA, Smoliga JM (2015) Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—systematic review and meta-analysis. Mol Nutr Food Res 59(1):147–159. https://doi.org/10.1002/mnfr.201400173
Sahebkar A (2013) Effects of resveratrol supplementation on plasma lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev 71(12):822–835. https://doi.org/10.1111/nure.12081
Elgebaly A, Radwan IA, AboElnas MM et al (2017) Resveratrol supplementation in patients with non-alcoholic fatty liver disease: systematic review and meta-analysis. J Gastrointest Liver Dis 26(1):59–67. https://doi.org/10.15403/jgld.2014.1121.261.ely
Méndez-del Villar M, González-Ortiz M, Martínez-Abundis E, Pérez-Rubio KG, Lizárraga-Valdez R (2014) Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord 12(10):497–501
Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A (2014) Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res 34(10):837–843. https://doi.org/10.1016/j.nutres.2014.09.005
Kim S, Jin Y, Choi Y, Park T (2011) Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 81(11):1343–1351
Lasa A, Churruca I, Eseberri I, Andrés-Lacueva C, Portillo MP (2012) Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol Nutr Food Res 56(10):1559–1568
Wang S, Liang X, Yang Q et al (2015) Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int J Obes 39(6):967–976
Yang JY, Della-Fera MA, Rayalam S et al (2008) Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci 82(19–20):1032–1039. https://doi.org/10.1016/j.lfs.2008.03.003
Hu P, Zhao L, Chen J (2015) Physiologically achievable doses of resveratrol enhance 3T3-L1 adipocyte differentiation. Eur J Nutr 54(4):569–579. https://doi.org/10.1007/s00394-014-0738-4
Lagouge M, Argmann C, Gerhart-Hines Z et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127(6):1109–1122. https://doi.org/10.1016/j.cell.2006.11.013
Christenson J, Whitby SJ, Mellor D et al (2016) The effects of resveratrol supplementation in overweight and obese humans: a systematic review of randomized trials. Metab Syndr Relat Disord 14(7):323–333. https://doi.org/10.1089/met.2016.0035
Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomas-Barberan FA, Teresa Garcia-Conesa M, Carlos Espin J (2013) Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des 19(34):6064–6093
Gummesson A, Nyman E, Knutsson M, Karpefors M (2017) Effect of weight reduction on glycated haemoglobin in weight loss trials in patients with type 2 diabetes. Diabetes Obes Metab 19(9):1295–1305. https://doi.org/10.1111/dom.12971
Liu Z, Song Y, Zhang X et al (2005) Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin Exp Pharm Physiol 32(12):1049–1054
Acknowledgements
We greatly appreciate the assistance provided by the staff of the Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences. We also thank all volunteers for their participation in the study. This work was financially supported by grants from the Deputy of Research, Tehran University of Medical Sciences (Grant 94-01-30-28313), and from the Diabetes Research Center, Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences (Grant 1393-04-97-1879).
Author information
Authors and Affiliations
Contributions
SE conducted the research, analyzed the data or performed statistical analysis, and wrote the paper. HK conducted the research. ENE helped in patients recruitment. RM designed the research, analyzed the data or performed statistical analysis, and had primary responsibility for final content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Ethical standard statement
The present study was approved by the Ethics Committee of Tehran University of Medical Sciences. The research was recorded in the Iranian Web site for registration of clinical trials (http://www.irct.ir: IRCT2015072523336N1).
Informed consent
All participants gave written informed consent prior to entering the study.
Additional information
Managed by Massimo Porta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seyyedebrahimi, S., Khodabandehloo, H., Nasli Esfahani, E. et al. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol 55, 341–353 (2018). https://doi.org/10.1007/s00592-017-1098-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-1098-3