Abstract
Aim
Even though the association between diabetes and periodontitis is taken for granted, results on this association are conflicting within the literature. This systematic review assessed whether poorly controlled diabetes was associated with periodontitis onset or progression.
Methods
Electronic searches were performed in PubMed, Scopus and Embase databases. Hand search was carried out in the reference list of all articles included. Gray literature was investigated with a Google Scholar search. Prospective longitudinal studies on the association between diabetes and periodontitis were considered for this review. Studies should have presented at least two measurements of periodontal conditions over time. Data on study design, crude and adjusted estimates were collected. We used meta-analysis to estimate the pooled effect of hyperglycemia in people with diabetes on periodontitis onset or progression. Meta-regression and subgroup analyses were employed to investigate potential sources of heterogeneity between studies.
Results
Thirteen studies matched the inclusion criteria, comprising 49,262 individuals, including 3197 diagnosed with diabetes. Meta-analyses of adjusted estimates showed that diabetes increased the risk of incidence or progression of periodontitis by 86% (RR 1.86 [95% CI 1.3–2.8]). However, there is scarce information on the association between diabetes and periodontal destruction.
Conclusions
This study provides evidence that diabetes is associated with increased risk of periodontitis onset and progression in adults. Upcoming prospective longitudinal studies ought to overcome methodological caveats identified in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus encompasses a set of metabolic disorders characterized by defects in insulin action, secretion or both causing a hyperglycemic state [1]. Diabetes mellitus is a public health problem with epidemic proportions intimately related to the rising number of overweight and obese individuals, and the current projection is over 640 million people with diabetes by 2040 [2]. Considering the current numbers and the projections [2], it is estimated that 9 million people develop diabetes each year and that up to 80% of the population with diabetes die because of its consequences [3, 4]. Secondary complications of uncontrolled glucose in individuals with diabetes comprise nephropathy, retinopathy with possible blindness [5], neuropathy, macro- and microvascular diseases [6, 7] and delayed tissue healing [8].
Periodontitis is characterized by the inflammatory destruction of the tooth-supporting tissues, including cement, periodontal ligament and alveolar bone, and has been listed as the sixth major complication of individuals with diabetes [9]. Even though periodontal inflammation is supposed to be triggered by bacteria, most of the damage is a consequence of a disproportionate and unbalanced host response to the biofilm presence in connection with the inability of the host to resolve the inflammatory process [10, 11]. Consequently, periodontitis can lead to tooth loss, loss of chewing ability, poor nutrition and poorer quality of life [12]. In the overall population, severe periodontitis is the sixth most prevalent chronic disease, with rising incidence ratios due to increase in life expectancy and more teeth retained across the lifespan [13].
It is biologically plausible to link diabetes with periodontitis onset and progression. There are several reasonable mechanisms explaining their connection. For example, chronic immune system activation with increased levels of circulating leukocytes and pro-inflammatory markers has been reported in people with inadequately controlled diabetes [14]. This sustained systemic low-grade inflammation could promote alterations in the physiology of the periodontium causing its breakdown [15]. Furthermore, hyperglycemia seems to alter systemic and gingival microvasculature, leading to increased inflammation of the periodontal tissues [16].
Even though several cross-sectional studies have reported an association between diabetes and periodontitis, longitudinal prospective studies with adequate design and data to infer temporal and causal relationships are scarce. Most of the methodological limitations arise from short follow-up period to allow disease occurrence, too small sample size, dropouts, and the use of convenience samples [17]. A previous systematic review, mostly based on cross-sectional studies, demonstrated a higher prevalence of periodontitis in subjects with diagnosis of diabetes [18]. Evidence from cross-sectional studies hinders the analysis of causal relationships, which is possible using prospective longitudinal studies. However, since several papers with longitudinal prospective design were published after the aforementioned systematic review, the consistency of the prospective evidence has not been appraised and summarized. Additionally, the magnitude of the effect of poorly controlled diabetes on periodontitis incidence or progression was never measured. This study aimed to systematically appraise the evidence originated from prospective longitudinal studies investigating the relationship between diabetes mellitus and periodontitis.
Materials and methods
This study was conducted following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [19]. This review was registered with the Joanna Briggs Institute database [20].
Review question
Does inadequately controlled diabetes mellitus increase the risk of periodontitis onset and progression?
Eligibility criteria
Prospective longitudinal studies investigating the association between diabetes and periodontitis were selected if they presented at least two measurements of periodontal conditions over time (e.g. attachment level—AL, periodontal probing depth—PPD or alveolar bone height—ABL). Data on periodontitis onset or progression were collected as designated by the authors. Reports on periodontal healing after periodontal treatment (initial and supportive therapy) were excluded. In addition, literature reviews, case–control studies, cross-sectional studies, retrospective longitudinal studies, case reports, comments or conference abstracts were also eliminated. In the case of multiple publications of the same population, the report with the longest follow-up was included.
Search strategy
Two independent investigators (GGN and FRML) searched in PubMed, Embase and Scopus databases for prospective studies reporting associations between diabetes and periodontitis. The initial search was elaborated on PubMed using the strategy: ((((((((“Periodontal Diseases”[Mesh]) OR “Periodontal Diseases”[all]) OR “Chronic Periodontitis”[Mesh]) OR “Chronic Periodontitis”[all]) OR “Periodontitis”[Mesh]) OR “Periodontitis”[all])) AND ((((((((((“Diabetes Mellitus”[Mesh]) OR “Diabetes Mellitus”[all]) OR “Insulin Resistance”[Mesh]) OR “Insulin Resistance”[all]) OR “Glucose Intolerance”[Mesh]) OR “Glucose Intolerance”[all]) OR “Metabolic Control”[all]) OR “Impaired Glycaemia”[all]) OR “Glycated Hemoglobin”[all]) OR “Glycated Haemoglobin”[all])). We added the following specific filter for restricting the search to longitudinal prospective studies: (“Cohort Studies”[all]) OR “Longitudinal Studies”[all]) OR “Longitudinal”[all]) OR “Cohort”[all]) OR “Follow-up Studies”[all]) OR “Follow-up”[all]) OR “Prospective Studies”[all]) OR “Prospective”[all]). The search included articles published up to and including May 2017 without date or language restriction.
Study selection
We combined the results and excluded duplicates using the EndNote X8.01 software (Thomson Reuters, New York, NY, USA). According to the eligibility criteria, titles and abstracts were assessed independently by two authors (GGN and FRML), then the lists were compared, and disagreements were solved by consensus. The same two reviewers assessed the full-text of studies with potential to be included in the review followed by comparison of their lists, and disagreement was resolved by discussion. References of the remaining manuscripts were hand-searched for additional articles. Gray literature was examined by inspecting the first 200 items of a Google Scholar search.
Data extraction and quality assessment
Information extracted from the studies included: author and year, sample size and main characteristics, geographic location of the study, follow-up period, definition and criteria used to evaluate periodontitis and diabetes, and criteria used to evaluate changes in periodontal condition. We also collected data on the analytical approach employed, crude and adjusted results, and confounders included in the analysis. Data were independently extracted by the same two reviewers and compared, and in case of disagreement, discussions were held to reach a consensus. When more than one category of periodontitis or diabetes was reported, only the most extreme category of comparison was included in the meta-analysis. Manuscript quality was independently assessed using the nine-star Newcastle–Ottawa scale (NOS) for cohort studies [21]. Lists were compared and a consensus reached.
Data synthesis and analysis
Statistical analyses were performed in Stata 14.2 (StataCorp, College Station, TX, USA). Fixed- and random-effects models were fitted to the data to obtain a combined relative risk estimate. In the presence of significant or considerable heterogeneity (I2 > 50% or Chi-square P value < 0.05) [22], the random-effect model was preferred since it considers both between-study and within-study variability. Estimates reported in odds ratio (OR) were converted into relative risk (RR) when possible [23]. In the absence of RR or OR measures, when the study presented the necessary data or the authors provided the data after contact, RR estimates were calculated. Separate models were used for analyzing crude and adjusted results, but only the pooled model of adjusted results was further analyzed. Studies with both estimates were included in both models.
Meta-regression including subgroup analyses were performed to investigate potential sources of between-study variability. For analysis, the characteristics of the studies were combined accordingly: socioeconomic status of the country where the study was conducted (high-income/low-middle-income) [24]; geographic location (Americas/Europe/Asia-Oceania); sample size (< 500/≥ 500 people); follow-up period (< 5 years/≥ 5 years); criteria for diabetes diagnosis (fasting plasma glucose—FPG/HbA1c or HOMA/self-reported); periodontal examination protocol (full mouth/partial mouth or index teeth/radiographic assessment/self-reported); criteria for periodontal diagnosis (AL/PPD/ABL); number of sites/teeth used to evaluate incidence or progression of periodontitis—extent (1/≥ 2); amount of periodontal destruction used to evaluate incidence or progression of periodontitis—severity (1–2 mm/≥ 3 mm). One at a time, each study characteristic was entered as covariate in the meta-regression model. Thus, the adjusted R2 of each bivariate model was taken as the percentage of heterogeneity explained by the covariate. Subgroups analysis was performed according to the study characteristics that explained some heterogeneity. Small-study effect was tested using the Egger test and contour-enhanced funnel-plot. This plot details statistical significance on a funnel-plot, indicating the level of significance of each estimate pooled in the meta-analysis. Sensitivity analysis was performed by omitting one estimate at a time from the meta-estimate model.
Results
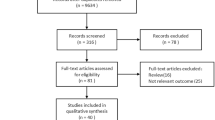
Altogether, 1787 studies were identified, and after removal of duplicates and title/abstract screening, the full-text document of 23 studies was obtained and assessed. Thirteen studies matched the inclusion criteria, comprising 49,262 participants, including 3197 diagnosed with diabetes and/or metabolic syndrome with a diabetes component (Fig. 1). Main reasons for study exclusion after full-text assessment are shown in Appendix 1. Data for inclusion in meta-analysis were available only for six studies.
Follow-up mean was approximately 4.8 years, ranging from 8 months to 20 years, and five studies presented follow-up shorter than 4 years [25,26,27,28,29]. Two studies [30, 31] were conducted in middle-income countries, while the remaining in high-income countries. The majority of the studies used the absence of diabetes mellitus diagnostic group as reference, while in two studies the reference category was presenting controlled diabetes [28, 30], because the sample comprised diabetic adults only. Diabetes mellitus presence was clinically determined except in one study [32], in which self-reported information was used. Among the clinical measures, HbA1c was used in seven studies [25, 27, 28, 30, 33,34,35] and FPG in two studies [36, 37], and three investigations just reported that the participants were referred from a diabetes service with previous diagnostic of diabetes mellitus [26, 29, 31]. Self-reported questionnaire [32] and detection of proximal bone loss in radiographs [35] were used in one study each to determine the periodontitis presence. Among the eleven studies with clinical examination, the whole mouth was preferred except for two studies where index teeth were chosen [34, 37]. A combination of AL and PPD was used in four studies [25, 29, 31, 33], AL in two studies [26, 27], PPD only in one study [30] and development of a Community Periodontal Index (CPI) score ≥ 3 in four [28, 34, 36, 37]. A large variation in the number of variables chosen for analysis adjustment was observed, with four studies not presenting adjusted estimates [26, 29,30,31]. The key characteristics of the studies included in the systematic review are shown in Table 1.
Using crude data from meta-analysis, diabetic subjects present a 70% higher incidence or progression risk of periodontitis than non-diabetics (RR 1.70; 95% CI 1.3–2.3). Even though heterogeneity between studies was high (I2 89.7%; Chi square < 0.001), after adjustment estimates showed that diabetes increased the risk of incidence or progression of periodontitis by 86% (RR 1.86 [95% CI 1.3–2.8]) (Fig. 2).
The relatively small number of studies with sufficient data to be included in meta-regression analysis precluded the exploration of multiple variables as possible sources of heterogeneity. The criteria used for periodontitis and diabetes assessment and the sample size explained 22.7, 12.8 and 25.2% of the variability between studies, respectively (Table 2). Appendix 2 displays the subgroup analysis considering the criteria used for periodontitis assessment. Appendix 3 displays the quality assessment of each study according to the NOS scale for cohort studies. Scores were shown according to each of the three domains of the instrument (selection, comparability and outcome).
The small number of studies included in the meta-analysis precluded also the statistical and the visual assessments for small-study effect (Appendix 4). The Egger’s test has low statistical power when sample size is lower than 20 studies [38]; additionally, the interpretation of a funnel-plot with a small number of studies could misrepresent the actual findings. The removal of any study from meta-regression did not affect the pooled RR according to the sensitivity analysis (Fig. 3).
Discussion
All prospective studies included in this systematic review reported positive associations between high levels of glucose and periodontitis onset and progression. Our data showed an 86% increase in the incidence or progression risk of having periodontitis among inadequately controlled diabetes compared with non-diabetics or well-controlled diabetics. The assumption that diabetes mellitus is a risk factor for periodontitis has largely been based on results from cross-sectional and animal studies, with scarce longitudinal prospective data. The uniqueness of this review was the idea to pool together diabetes effect on periodontitis using only prospective longitudinal studies to assert the temporality of the effects. Despite the significant association obtained in the meta-analysis, this estimate should be cautiously interpreted, due to methodological aspects of the studies included in the review.
Our results demonstrated that some methodological aspects have a direct influence on this association. Results of the meta-regression and subgroup analyses revealed that the sample size explained approximately 25% of the variability between studies (Table 2). As corroborated by previous studies, small sample size may overestimate the association between presumed exposure and outcome [39, 40]. In addition, one may also speculate whether the sample size may be a proxy of the convenience of the sample. Indeed, among the studies included in our review, we could observe that most of the small studies did not present a representative sample (e.g., sample chosen according to the researcher interest). Therefore, the evidence on the association between diabetes and periodontitis originated from large population-based studies with representative samples is scant.
Furthermore, the use of self-reported information about having been told previously to have had diabetes or to use or have used medication to control diabetes resulted in lower RR compared to results from blood tests (HbA1c or FPG), explaining approximately 13% of the heterogeneity between studies (Table 2). Despite the applicability of self-reported information on the assessment of general health conditions, this is a common source of variability in meta-analysis [41]. However, the omission of such an estimate from the pooled analysis would not nullify our results (Fig. 3).
One point that should be critically examined is the criteria defined by the authors to determine the presence of periodontitis. In the meta-regression analysis, it explained 22.7% of the heterogeneity between studies (Table 2). Even though clinical attachment level is the gold-standard measurement to detect periodontitis progression, half of the studies included in the meta-analysis used the Community Periodontal Index, which is based on the pocket probing depth, a poor proxy of changes in attachment level [42]. Despite the widespread use of CPI, it presents serious limitations on the diagnosis and monitoring of periodontitis [43]. Additionally, pocket probing depth can either under- or overestimate attachment loss depending on the background features of the study group under investigation [44]. Information on clinical attachment level is preferred, because it reflects cumulative periodontal destruction [45]. However, the only study using clinical attachment level to assess periodontitis was not able to find an association between diabetes and periodontal destruction. Therefore, it remains unclear whether diabetes is associated with long-term periodontal destruction.
Other concerns of this study should be also examined. Firstly, even though the role of diabetes in the risk of periodontitis is frequently taken for granted in the literature, we could find modest evidence on the topic. Furthermore, there is scarce evidence on the association between diabetes and incident cases of periodontitis, and most of the few studies investigating the incidence of periodontitis have used pocket probing depth (i.e., CPI) to define this disease. It is not possible to guarantee that all data in the literature regarding the association between diabetes and periodontitis were included in this review, since glucose levels might have been used as a covariate for analysis adjustment in studies not captured with our search strategy. Nevertheless, it is expected that the comprehensive search in three broad databases plus Google Scholar identified most of the prospective longitudinal studies available, if not all. Another concern is the pooling of results from studies with different methodological characteristics. Risk factor studies, most of the time, intend to address questions that cannot be answered with randomized studies, simply because they focus on exposures that cannot be controlled, and due to ethical consideration, participants cannot be exposed to harmful risk factors or just followed in the expectancy to disease to occur [46]. Well-conducted meta-analysis of observational studies is a valid method for assessing data, since it helps to identify reasons for variability in results among the studies and to identify areas in need of further exploration [46]. Moreover, a previous publication demonstrated that meta-analyses of observational studies usually present effect estimates comparable to those arising from randomized controlled trials meta-analysis [47]. Considering this information, we decided to supplement our appraisal with a meta-analysis.
Bearing in mind the high heterogeneity observed in meta-analysis of observational studies, we conducted meta-regression and subgroup analyses to identify the potential sources of variability between studies. This approach allowed us to identify differences in the estimates originating from studies using different criteria for periodontitis risk and progression, for instance, so as the need for more studies on the topic using clinical attachment level to diagnose and monitor periodontitis. Finally, since diabetes and periodontitis share common risk factors, and detrimental conditions usually coexist, it is not possible to rule out the residual effect of those conditions associated with both diabetes and periodontitis [48]. Even though eight studies in this review have adjusted their analysis for potential confounders, only three of them included at least age, sex, socioeconomic status, smoking and a weight-related variable in the analysis [49]. Additionally, one should bear in mind that despite the useful applicability of analytical adjustment, it might not be enough. Let us examine the case of smoking, for example. Even though some studies have presented results adjusted for smoking, one should remember the limitations of measuring the dimension of exposure to tobacco smoking. First, its assessment relies mainly on self-reported information of the current smoking status and not on smoking over time. Second, there is a social undesirability in reporting unhealthy conditions, and regardless of the truthfulness in reporting, the inclusion of a dichotomous variable in the analytical models does not rule out the effect of such a condition.
We should also examine the strengths of this study. The included prospective longitudinal studies represent the best sources of evidence available for determining the strength and the temporality of effects of the relationship between poorly controlled diabetes and periodontitis as well as the best attempt to identify sources of bias. A strong point of this review is the combined sample size of approximately 49,262 people including 3197 diagnosed with diabetes mellitus. Furthermore, in the meta-analysis, five out of the six included manuscripts received between six and eight points in the Newcastle–Ottawa quality assessment scale [21], and none could be classified as of high risk of bias. This way, data combination from these studies into a meta-analysis results in a more trustful estimate of the association between diabetes and periodontitis than the estimate of any of these studies alone [50].
It is biologically plausible to link diabetes with periodontitis onset and progression. Polymorphonuclear leukocytes (PMNs) permanence in tissues after an insult results in extensive tissue damage, frequently related to faster progression of chronic inflammatory diseases in general [51]. The formation of advanced glycation end products (AGEs) is a well-documented consequence of chronic hyperglycemia [8]. PMNs, monocytes and macrophages express the AGE receptor and produce excessive superoxide, interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, resulting in tissue destruction [52, 53]. The elevated prolonged inflammation process induces high proportions of apoptotic cells, resulting in periodontitis [54]. Moreover, since fibroblasts go into apoptosis by the action of AGEs and pro-inflammatory cytokines, periodontal tissues healing is compromised [52]. At the same time, inadequately controlled diabetes mellitus is known by the high formation of reactive oxygen species (ROS), even in unstimulated cells, which may directly injure vital structures, the cell membrane, and cause cell necrosis or apoptosis in both connective and bone tissues [52, 53]. This hyper-responsive phenotype of diabetic inflammatory cells can be reversed with pharmacological AGE receptor blockage or by the reduction of the number of AGE receptor ligands through hyperglycemia control [55]. Additionally, diabetes may cause microvascular pathological alterations in the gingiva, which in turn lead to increased periodontal inflammation. Thus, it is suggested that uncontrolled hyperglycemia may explain exacerbated gingival hemorrhaging [16].
Persistent inflammatory conditions associated with diabetes mellitus significantly boost the pro-inflammatory activity and infiltration of PMNs into infected sites [51], and it is hypothesized whether the same happens in periodontitis sites [56]. The exchange of PMNs from animals with systemic inflammation to healthy ones, and vice versa, demonstrated that PMNs and tissue environment are altered during chronic inflammation [51]. It is suggested that when individuals with systemic inflammation are exposed de novo to a bacterial infection, the immune response seems even more exaggerated [51]. Thus, it is possible to extrapolate that recurrent exposure of uncontrolled diabetes mellitus individuals to periodontopathogenic bacteria will lead to or accelerate the destruction of periodontal tissues.
Taken together, our findings show that diabetes is associated with increased risk of periodontitis onset and progression. Even though the role of diabetes in the risk of periodontitis is taken for granted in the literature, there are several ways in which the fundament of this knowledge can improve. Upcoming prospective longitudinal studies ought to overcome methodological caveats identified in our review, like scarce information on periodontal destruction and small sample sizes. Furthermore, future studies should estimate the cluster effect of common risk factors to determine their combined effect with blood glucose level on periodontitis onset and progression.
References
American Diabetes Association (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl 1):S81–S90. https://doi.org/10.2337/dc14-S081
Internation Diabetes Federation (2015) International Diabetes Federation Diabetes Atlas, 7th edn, vol 144. http://www.diabetesatlas.org/. Accessed 14 Jun 2017
Fox CS, Coady S, Sorlie PD et al (2007) Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 115(12):1544–1550. https://doi.org/10.1161/CIRCULATIONAHA.106.658948
Smith NL, Barzilay JI, Kronmal R et al (2006) New-onset diabetes and risk of all-cause and cardiovascular mortality: the Cardiovascular Health Study. Diabetes Care 29(9):2012–2017. https://doi.org/10.2337/dc06-0574
Lu J, Hou X, Zhang L et al (2015) Association between body mass index and diabetic retinopathy in Chinese patients with type 2 diabetes. Acta Diabetol 52(4):701–708. https://doi.org/10.1007/s00592-014-0711-y
Tasci I, Basgoz BB, Saglam K (2016) Glycemic control and the risk of microvascular complications in people with diabetes mellitus. Acta Diabetol 53(1):129–130. https://doi.org/10.1007/s00592-015-0778-0
Tadic M, Cuspidi C, Vukomanovic V et al (2016) The influence of type 2 diabetes and arterial hypertension on right ventricular layer-specific mechanics. Acta Diabetol 53(5):791–797. https://doi.org/10.1007/s00592-016-0874-9
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070. https://doi.org/10.1161/CIRCRESAHA.110.223545
Löe H (1993) Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16(1):329–334
Van Dyke TE (2014) Commentary: periodontitis is characterized by an immuno-inflammatory host-mediated destruction of bone and connective tissues that support the teeth. J Periodontol 85(4):509–511. https://doi.org/10.1902/jop.2014.130701
Hyvarinen K, Salminen A, Salomaa V, Pussinen PJ (2015) Systemic exposure to a common periodontal pathogen and missing teeth are associated with metabolic syndrome. Acta Diabetol 52(1):179–182. https://doi.org/10.1007/s00592-014-0586-y
Brennan DS, Spencer AJ, Roberts-Thomson KF (2007) Quality of life and disability weights associated with periodontal disease. J Dent Res 86(8):713–717. https://doi.org/10.1177/154405910708600805
Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W (2014) Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res 93(11):1045–1053. https://doi.org/10.1177/0022034514552491
Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD (2013) Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Invest 4(5):502–509. https://doi.org/10.1111/jdi.12088
Pink C, Kocher T, Meisel P et al (2015) Longitudinal effects of systemic inflammation markers on periodontitis. J Clin Periodontol 42(11):988–997. https://doi.org/10.1111/jcpe.12473
Hujoel PP, Stott-Miller M (2011) Retinal and gingival hemorrhaging and chronic hyperglycemia. Diabetes Care 34(1):181–183. https://doi.org/10.2337/dc10-0901
Vandenbroucke JP, von Elm E, Altman DG et al (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 18(6):805–835. https://doi.org/10.1097/EDE.0b013e3181577511
Chavarry NG, Vettore MV, Sansone C, Sheiham A (2009) The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent 7(2):107–127
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
The Joanna Briggs Institute (2017) Registered topics in the JBI database. http://joannabriggs.org/research/registered_titles.aspx. Accessed 08 Oct 2017
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2014) The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf. Accessed 08 June 2017
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Zhang J, Yu KF (1998) What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280(19):1690–1691
World Bank Group (2017) Country and lending groups. http://data.worldbank.org/about/country-and-lending-groups. Accessed 08 June 2017
Bandyopadhyay D, Marlow NM, Fernandes JK, Leite RS (2010) Periodontal disease progression and glycaemic control among Gullah African Americans with type-2 diabetes. J Clin Periodontol 37(6):501–509. https://doi.org/10.1111/j.1600-051X.2010.01564.x
Cohen DW, Friedman LA, Shapiro J, Kyle GC, Franklin S (1970) Diabetes mellitus and periodontal disease: two-year longitudinal observations. I. J Periodontol 41(12):709–712. https://doi.org/10.1902/jop.1970.41.12.709
Iwasaki M, Sato M, Minagawa K, Manz MC, Yoshihara A, Miyazaki H (2015) Longitudinal relationship between metabolic syndrome and periodontal disease among Japanese adults aged ≥70 years: the Niigata Study. J Periodontol 86(4):491–498. https://doi.org/10.1902/jop.2015.140398
Karikoski A, Murtomaa H (2003) Periodontal treatment needs in a follow-up study among adults with diabetes in Finland. Acta Odontol Scand 61(1):6–10
Sbordone L, Ramaglia L, Barone A, Ciaglia RN, Iacono VJ (1998) Periodontal status and subgingival microbiota of insulin-dependent juvenile diabetics: a 3-year longitudinal study. J Periodontol 69(2):120–128. https://doi.org/10.1902/jop.1998.69.2.120
Novaes AB Jr., Silva MAP, Batista EL Jr, Dos Anjos BA, Novaes AB, Pereira ALA (1997) Manifestations of insulin-dependent diabetes mellitus in the periodontium of young Brazilian patients. A 10-year follow-up study. J Periodontol 68(4):328–334
Firatli E (1997) The relationship between clinical periodontal status and insulin-dependent diabetes mellitus. Results after 5 years. J Periodontol 68(2):136–140. https://doi.org/10.1902/jop.1997.68.2.136
Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ (2012) Type 2 diabetes mellitus and 20 year incidence of periodontitis and tooth loss. Diabetes Res Clin Pract 98(3):494–500. https://doi.org/10.1016/j.diabres.2012.09.039
Demmer RT, Kerner W, Holtfreter B et al (2012) The influence of type 1 and type 2 diabetes on periodontal disease progression: prospective results from the Study of Health in Pomerania (SHIP). Diabetes Care 35(10):2036–2042. https://doi.org/10.2337/dc11-2453
Morita I, Inagaki K, Nakamura F et al (2012) Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res 91(2):161–166. https://doi.org/10.1177/0022034511431583
Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M (1998) Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol 3(1):30–39. https://doi.org/10.1902/annals.1998.3.1.30
Chiu SYH, Lai H, Yen AMF, Fann JCY, Chen LS, Chen HH (2015) Temporal sequence of the bidirectional relationship between hyperglycemia and periodontal disease: a community-based study of 5885 Taiwanese aged 35–44 years (KCIS No. 32). Acta Diabetol 52(1):123–131. https://doi.org/10.1007/s00592-014-0612-0
Lee KS, Kim EK, Kim JW et al (2014) The relationship between metabolic conditions and prevalence of periodontal disease in rural Korean elderly. Arch Gerontol Geriatr 58(1):125–129. https://doi.org/10.1016/j.archger.2013.08.011
Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53(11):1119–1129
Zhang Z, Xu X, Ni H (2013) Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care 17(1):R2. https://doi.org/10.1186/cc11919
Dechartres A, Trinquart L, Boutron I, Ravaud P (2013) Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 346:f2304. https://doi.org/10.1136/bmj.f2304
Nascimento GG, Leite FR, Conceicao DA, Ferrua CP, Singh A, Demarco FF (2016) Is there a relationship between obesity and tooth loss and edentulism? A systematic review and meta-analysis. Obes Rev 17(7):587–598. https://doi.org/10.1111/obr.12418
Michalowicz BS, Hodges JS, Pihlstrom BL (2013) Is change in probing depth a reliable predictor of change in clinical attachment loss? J Am Dent Assoc 144(2):171–178
Baelum V, Manji F, Wanzala P, Fejerskov O (1995) Relationship between CPITN and periodontal attachment loss findings in an adult population. J Clin Periodontol 22(2):146–152
Agerholm DM, Ashley FP (1996) Clinical assessment of periodontitis in young adults—evaluation of probing depth and partial recording methods. Commun Dent Oral Epidemiol 24(1):56–61
Mdala I, Olsen I, Haffajee AD, Socransky SS, Thoresen M, de Blasio BF (2014) Comparing clinical attachment level and pocket depth for predicting periodontal disease progression in healthy sites of patients with chronic periodontitis using multi-state Markov models. J Clin Periodontol 41(9):837–845. https://doi.org/10.1111/jcpe.12278
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Shrier I, Boivin JF, Steele RJ et al (2007) Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol 166(10):1203–1209. https://doi.org/10.1093/aje/kwm189
Nascimento GG, Peres MA, Mittinty MN et al (2017) Diet-induced overweight and obesity and periodontitis risk: an application of the parametric G-formula in the 1982 Pelotas Birth Cohort. Am J Epidemiol 185(6):442–451. https://doi.org/10.1093/aje/kww187
Leung MY, Carlsson NP, Colditz GA, Chang SH (2017) The burden of obesity on diabetes in the United States: Medical Expenditure Panel Survey, 2008 to 2012. Value Health 20(1):77–84. https://doi.org/10.1016/j.jval.2016.08.735
Pogue J, Yusuf S (1998) Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet 351(9095):47–52. https://doi.org/10.1016/S0140-6736(97)08461-4
Bian Z, Guo Y, Ha B, Zen K, Liu Y (2012) Regulation of the inflammatory response: enhancing neutrophil infiltration under chronic inflammatory conditions. J Immunol 188(2):844–853. https://doi.org/10.4049/jimmunol.1101736
Chapple IL, Matthews JB (2000) The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 43:160–232. https://doi.org/10.1111/j.1600-0757.2006.00178.x
Nassar H, Kantarci A, van Dyke TE (2000) Diabetic periodontitis: a model for activated innate immunity and impaired resolution of inflammation. Periodontology 2000 43:233–244. https://doi.org/10.1111/j.1600-0757.2006.00168.x
Graves DT, Liu R, Alikhani M, Al-Mashat H, Trackman PC (2006) Diabetes-enhanced inflammation and apoptosis—impact on periodontal pathology. J Dent Res 85(1):15–21
Ding Y, Kantarci A, Hasturk H, Trackman PC, Malabanan A, Van Dyke TE (2007) Activation of RAGE induces elevated O2-generation by mononuclear phagocytes in diabetes. J Leukoc Biol 81(2):520–527. https://doi.org/10.1189/jlb.0406262
Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, Van Dyke TE (2006) Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol 177(10):7250–7256
Seppälä B, Seppälä M, Ainamo J (1993) A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol 20(3):161–165. https://doi.org/10.1111/j.1600-051X.1993.tb00338.x
Seppälä B, Ainamo J (1994) A site-by-site follow-up study on the effect of controlled versus poorly controlled insulin-dependent diabetes mellitus. J Clin Periodontol 21(3):161–165. https://doi.org/10.1111/j.1600-051X.1994.tb00297.x
Novaes AB Jr, Gutierrez FG, Novaes AB (1996) Periodontal disease progression in type II non-insulin-dependent diabetes mellitus patients (NIDDM). Part I-Probing pocket depth and clinical attachment. Braz Dent J 7(2):65–73
Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ (1998) Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol 69(1):76–83. https://doi.org/10.1902/jop.1998.69.1.76
Thomson WM, Slade GD, Beck JD, Elter JR, Spencer AJ, Chalmers JM (2004) Incidence of periodontal attachment loss over 5 years among older South Australians. J Clin Periodontol 31(2):119–125. https://doi.org/10.1111/j.0303-6979.2004.00460.x
Morita I, Okamoto Y, Yoshii S, Nakagaki H, Mizuno K, Sheiham A, Sabbah W (2011) Five-year incidence of periodontal disease is related to body mass index. J Dent Res 90(2):199–202. https://doi.org/10.1177/0022034510382548
Timonen P, Saxlin T, Knuuttila M, Suominen AL, Jula A, Tervonen T, Ylöstalo P (2013) Role of insulin sensitivity and beta cell function in the development of periodontal disease in adults without diabetes. J Clin Periodontol 40(12):1079–1086. https://doi.org/10.1111/jcpe.12162
Knight ET, Leichter JW, Tawse-Smith A, Thomson WM (2015) Quantifying the association between self-reported diabetes and periodontitis in the New Zealand population. J Periodontol 86(8):945–954. https://doi.org/10.1902/jop.2015.150048
Chang JS, Tsai CR, Chen LT, Shan YS (2016) Investigating the association between periodontal disease and risk of pancreatic cancer. Pancreas 45(1):134–141. https://doi.org/10.1097/MPA.0000000000000419
Shearer DM, Thomson WM, Broadbent JM, Mann J, Poulton R (2017) Periodontitis is not associated with metabolic risk during the fourth decade of life. J Clin Periodontol 44(1):22–30. https://doi.org/10.1111/jcpe.12641
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Nascimento, G.G., Leite, F.R.M., Vestergaard, P. et al. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol 55, 653–667 (2018). https://doi.org/10.1007/s00592-018-1120-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1120-4