Abstract
Aims
To conduct a meta-analysis of head-to-head trials comparing aerobic exercise training of different intensities on glycemic control in type 2 diabetes.
Methods
Databases, including MEDLINE and EMBASE, were searched up to January 2016. Randomized trials of at least 12 weeks in duration that compared two exercise interventions of different intensities were identified. Two reviewers independently extracted data from eligible trials. Using fixed effect model, weighted mean differences (WMD) between different exercise intensities were calculated for changes in glycated hemoglobin (HbA1c) and secondary outcomes, such as fasting glucose and fasting insulin.
Results
Eight studies with a total of 235 participants were eligible. The exercise interventions lasted from 12 weeks to 6 months. The prescribed exercise intensities varied among studies. Four studies utilized vigorous exercise intensities for short durations by performing interval training. Overall, higher-intensity exercise resulted in a greater reduction in HbA1c compared to lower-intensity exercise (WMD = −0.22 %; 95 % confidence interval [−0.38, −0.06]; or −2.4 mmol/mol [−4.15, −0.66], I 2 = 0). Adherence to exercise and proportion of dropouts did not differ within trials. No adverse events were reported in these small trials with selected inclusion criteria.
Conclusions
Although our meta-analysis had a limited sample size, increasing exercise intensity safely accentuated reductions in HbA1c in some people with type 2 diabetes. Different approaches have been used to increase exercise intensity (i.e., some used interval training, whereas others used higher-intensity continuous exercise). However, at this time, it is unclear which form, if any, leads to the most favorable results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effects of exercise on glycemic control have been extensively documented by several meta-analyses [1–3]. These studies have contributed to evidence-based recommendations for physical activity/exercise in people with type 2 diabetes [4, 5]. While there is strong evidence to support that exercise interventions improve glycemic control compared to a sedentary control group or standard care, comparatively little information is available to examine which frequency, intensity, time, or type of exercise is best.
Subsequent systematic reviews and meta-analyses have shed light on this issue by synthesizing the results from head-to-head trials directly comparing aerobic versus resistance exercise training [6, 7]. These reviews suggest that aerobic exercise training leads to slightly greater improvements in glycated hemoglobin (HbA1c) compared to resistance exercise (i.e., statistically significant reductions of approximately 0.2 % points in HbA1c [6, 7]) and that combined aerobic and resistance exercise training leads to greater improvements than either alone [7].
In recent years, there has been much interest on the role of exercise intensity. Indeed, the American College of Sports Medicine (ACSM) ranked “high-intensity interval training” or HIIT as its top fitness trend in its Worldwide Survey of Fitness Trends for 2014 [8]. While it is relatively well established that higher-intensity exercise can lead to greater improvements in some indicators of fitness, even in patients with cardio-metabolic diseases [9], it is unclear whether this type of exercise leads to greater improvements in glycemic control. Part of the controversy in regard to the effect of exercise intensity is that previous systematic reviews, including our own, have relied on meta-regression analyses which examined association between exercise intensity and changes in Hb1Ac [10, 11]. Meta-regression is susceptible to being influenced by important confounders since participants are not randomly assigned to exercise groups of different intensities.
Therefore, the primary objective of the current study was to systematically review and, if appropriate, perform a meta-analysis of randomized trials directly comparing exercise interventions of different intensities on HbA1c in type 2 diabetes. Adverse events, exercise adherence, proportion of dropouts, as well as other indicators or glycemic control or insulin sensitivity that were measured in more than two studies were included as secondary outcomes.
Methods
Literature search
A literature search was performed in the second week of January 2016 in seven databases (MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, CINAHL, Sport Discus, Scopus, and Web of Science Core collection). No restrictions were applied in terms of language or date of publication. The keywords and controlled vocabulary (if available, e.g., MeSH terms) related to type 2 diabetes, exercise, and intensities were searched. An example of a complete search is available in the supplementary material. In addition, the reference lists of eligible articles were searched for potential studies.
Study selection
Two authors (Y.L, K.F) independently reviewed the title and abstracts of all records identified by the literature search. Records deemed potentially eligible by either reviewer were reviewed in full text by both reviewers. Disagreements regarding eligibility of articles were resolved by consensus with a third reviewer (N.G.B.). The eligibility of the studies was formulated according to the following PICOS criteria:
-
Population Adults with type 2 diabetes.
-
Intervention Structured aerobic exercise interventions were defined in terms of frequency, intensity, type, and time. Aerobic exercise, sometimes known as cardiorespiratory exercise, was defined in accordance with the American College of Sports Medicine (ACSM) as “regular, purposeful exercise involving major muscle groups and is continuous and rhythmic in nature” [12]. Only interventions lasting 12 weeks or longer were eligible. Exercise adherence was required to be monitored by direct supervision or objective measures such as heart rate monitors or accelerometers. It was recognized that some forms of interval exercise may include anaerobic energy production. These were included in the review since most of the energy during repeated high-intensity intervals alternating with recovery intervals would be produced aerobically.
-
Comparison and co-interventions Only studies comparing higher- versus lower-intensity aerobic exercise interventions were eligible. Many types of comparisons were eligible, for example high versus moderate intensity, or moderate versus low intensity. Exercise groups did not have to be matched in terms of energy expenditure or workload. If co-interventions were present (e.g., dietary recommendations), they had to be equivalent in both groups.
-
Outcome Eligible studies were required to report our primary outcome of HbA1c before and after the interventions. Secondary outcomes were not required for eligibility.
-
Study design Randomized studies which assigned participants to at least two different exercise intensity groups were included. Randomization to a control group was not necessary.
Data extraction and quality assessment
Characteristics of the aerobic training interventions were extracted for each study, including exercise frequency, intensity, time, and type. The intensity reported in each training protocol was categorized as vigorous or high- (≥64 % VO2max), moderate- (46–63 % VO2max), and light (≤45 % VO2max)-intensity trainings based on the definition by ACSM [12]. Heart rate and heart rate reserve equivalents are also provided by the ACSM [12]. Baseline characteristics such as age, body mass index, and duration of diabetes of the participants were extracted. We attempted to resolve ambiguities regarding the exercise protocols or results by contacting authors.
The means and standard deviations (SD) were extracted for baseline, post-training, and the absolute change from baseline. Several strategies were used if the change-from-baseline SD was not provided. First, we contacted the authors to obtain the missing information. Second, change-from-baseline SD was estimated from p values or 95 % confidence intervals (95 % CI) according to the methods described in the Cochrane Handbook (Section 7.7.3.3) [13]. Third, the correlation coefficient between pre- and post-training values was calculated in the studies that reported the baseline, post-training, and change means and SD, and then the mean correlation coefficient from these studies was used to impute the missing change-from-baseline SD (described in the Cochrane Handbook, Section 16.1.3.2 [13]). For the study by Chao et al. [14], the reported change-from-baseline SD in fasting glucose or HbA1c was much smaller compared to other studies in our review. This was unexpected given that the within-group changes were larger in this study. Estimation of the SD from the p value suggested that standard error for the change and not SD may have been presented in the article.
In addition to HbA1c, other outcomes related to glycemic control or insulin sensitivity were analyzed if they were available from more than two studies. These outcomes included fasting glucose, fasting insulin, and insulin resistance estimated by the homeostasis model assessment of insulin resistance (HOMA-IR). Adherence to the exercise intervention, expressed as the proportion of sessions attended, as well the proportion of dropouts and number of adverse events, was also extracted.
Risk of bias
Risk of bias was assessed according to the criteria developed from Section 8.5.a in the Cochrane Handbook (The Cochrane Collaboration’s tool for assessing risk of bias) [13]: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment for the primary outcome, (5) incomplete outcome data, (6) selective reporting, and (7) other sources of bias. The risk of bias for these seven categories was coded as “low,” “high,” or “unsure” by two authors. Publication bias was examined by visually inspecting funnel plots that plotted the mean difference on the x-axis and the standard error of the mean difference on the y-axis. The inclusion of non-directly supervised training designs may increase the risk of certain bias and compromise internal validity even when other measures such as heart rate monitors were used to calculate the achieved training intensity. Sensitivity analyses were performed to determine whether exclusion of the non-directly supervised interventions affected the results.
Statistical analysis
Statistical analyses were performed with Review Manager Software (RevMan 5.1, Cochrane Collaboration, Copenhagen, Denmark). The within-person change-from-baseline mean and SD were entered for each exercise intensity group to calculate the mean difference between groups and its 95 % CI for each study. For HbA1c and glucose, weighted mean differences (WMD) were obtained using a fixed effect model. All HbA1c data were entered as percentages for the analyses. The WMD were also presented in mmol/mol by multiplying the percentage values by 10.929. For insulin and HOMA-IR, some studies had much greater values than others at baseline (reflecting large differences in insulin resistance, different assay methods, or different calculations). Since such differences had a large impact on the change-from-baseline SD and therefore the weight attributed to each study, standardized mean differences (SMD) were used for these outcomes.
Heterogeneity was estimated with the χ 2 test of heterogeneity. The percent of total variability attributable to heterogeneity (i.e., not to chance) was expressed as the I-squared (I 2). Significant χ 2 tests for heterogeneity or I 2 above 40 % were considered indicative of heterogeneity. Several strategies were determined a priori to address heterogeneity, including subgroup analyses, sensitivity analyses, or random effects model if appropriate. Subgroups analyses were also performed in the absence of heterogeneity and were defined according to the exercise intensity and the continuous or intermittent nature of the exercise prescription.
Results
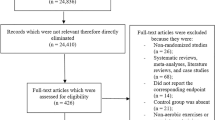
The literature review retrieved 2358 articles after duplicates were removed, see Fig. 1 for a summary of the study selection flow diagram. After reviewing abstracts and titles, 2315 articles were excluded. The most common reasons for exclusion were inappropriate population, comparing aerobic exercise training with control rather than comparing different exercise intensities, the presence of different co-interventions, and illegible study design (e.g., not randomized trial). The full texts of remaining 43 articles were assessed for eligibility, of which eight were included for the qualitative description and quantitative analyses.
The reasons for exclusion at the full-text stage are described in Fig. 1. Nine records were excluded because the interventions were shorter than 12 weeks and could therefore underestimate the effect on HbA1c. Eight records (six abstracts [15–20] and two articles [21, 22]) were potentially eligible but were excluded due to insufficient data on the outcomes of interest or missing information regarding the exercise intervention. We attempted to contact authors for additional information before excluding these articles. Seven articles were excluded because we were unable to differentiate between participants with and without type 2 diabetes. For example, the article by Hamed et al. [23] was excluded because we were unsure if results included only participants with type 2 diabetes or also those with impaired fasting glucose. The article stated that: “They had type II diabetes according to Oral Glucose Tolerance Test (i.e., impaired fasting glucose: >100–125 mg/dl—2 h postprandial: 140–199 mg/dl)”. Two articles were excluded because moderate-intensity exercise interventions were either not supervised [24] or because accelerometer suggested a greater increase in “physical activity-associated energy expenditure above moderate activity level” in the moderate-intensity group [25].
Study description
Details of the study characteristics are listed in Table 1. A total of 235 subjects participated in the eight studies. The age of the participants ranged from adolescents [26] to older adults of 55–75 years of age [27]. Comparisons among exercise interventions were categorized as:
-
high-intensity interval training versus moderate-intensity continuous training (HIIT vs. MICT) [27–29],
-
high-intensity interval training versus low-intensity continuous training (HIIT vs. LICT) [26],
-
High-intensity continuous training versus moderate-intensity continuous training (HICT vs. MICT) [14, 30, 31],
-
moderate-intensity continuous training versus low-intensity continuous training (MICT vs. LICT) [32].
All of the studies, except for the one study comparing MICT versus LICT [32], developed their exercise interventions to match the exercise energy expenditure or exercise volume between the higher- versus lower-intensity groups. This implies that the higher-intensity groups exercised for shorter durations [14, 30] than their lower-intensity counterparts or had similar exercise duration but longer duration recovery intervals between their higher-intensity intervals in the HIIT group (see Table 1). The MICT versus LICT comparison [32] was not matched for energy expenditure but for exercise duration. Therefore, the higher-intensity subgroup in this study had greater energy expenditure than their lower-intensity counterparts.
The length of aerobic exercise training programs ranged from 12 weeks to 6 months. Most studies had direct supervision of exercise training with the exception of two studies that used accelerometers [28] or heart rate monitors [32] to assess compliance with the exercise duration and intensity.
Glucose-lowering medication use was available in four studies (see supplementary material for details). Metformin was the most common medication. It was prescribed in 72 and 71 % of higher- and lower-intensity exercise participants, respectively.
Exercise training intensity and HbA1c
A total of 233 participants from eight studies reported changes in HbA1c (Fig. 2). There was an overall greater reduction in HbA1c with higher-intensity training compared to lower-intensity training (WMD = −0.22 % [−0.38, −0.06], or −2.4 mmol/mol [−4.2, −0.7], p = 0.007, I 2 = 0 %). Sensitivity analyses which excluded the two studies that lacked direct supervision of exercise training did not meaningfully affect the results (WMD: −0.21 % [−0.39, −0.03], or −2.3 mmol/mol [−4.3, −0.3], p = 0.02, I 2 = 0 %, n = 196).
Effect of higher- versus lower-intensity exercise on glycated hemoglobin (HbA1c). WMD weighted mean difference, SD standard deviation, 95 % CI 95 % confidence interval, HIIT high-intensity interval training, MICT moderate-intensity continuous training, LICT low-intensity continuous training, HICT high-intensity continuous training, Hetero heterogeneity
Of the subgroups examined, only the HIIT versus MICT comparison, which included three studies, showed a significantly greater reduction in the higher-intensity group (WMD: −0.23 % [−0.43, −0.02], or −2.5 mmol/mol [−4.7, −0.2], p = 0.03, I 2 = 0, n = 66).
Exercise training intensity and fasting glucose
A total of 234 participants from eight studies reported fasting glucose (Fig. 3). There was no difference between higher versus lower exercise intensity (WMD: −0.04 [−0.33, 0.25], p = 0.79, I 2 = 8 %). None of the subgroup or sensitivity analyses identified significant effects of exercise intensity.
Effect of higher- versus lower-intensity exercise on fasting glucose. WMD weighted mean difference, SD standard deviation, 95 % CI 95 % confidence interval, HIIT high-intensity interval training, MICT moderate-intensity continuous training, LICT low-intensity continuous training, HICT high-intensity continuous training, Hetero heterogeneity
Exercise training intensity and fasting insulin or HOMA-IR
A total of 159 participants from five studies reported fasting insulin (Fig. 4). Overall, there was no significant effect of exercise intensity on fasting insulin (SMD: −0.26 [−0.57, 0.06], p = 0.11, I 2 = 0 %). A total of 186 participants from six studies reported insulin resistance as measured by HOMA-IR. HOMA-IR was not significantly affected by exercise intensity (SMD: −0.22 [−0.51, 0.07], p = 0.13, I 2 = 0 %, Fig. 5). None of the subgroup or sensitivity analyses identified significant effects of exercise intensity.
Effect of higher- versus lower-intensity exercise on fasting insulin. SMD standardized mean difference, SD standard deviation, 95 % CI 95 % confidence interval, HIIT high-intensity interval training, MICT moderate-intensity continuous training, LICT low-intensity continuous training, HICT high-intensity continuous training, Hetero heterogeneity
Effect of higher- versus lower-intensity exercise on HOMA-IR. SMD standardized mean difference, SD standard deviation, 95 % CI 95 % confidence interval, HIIT high-intensity interval training, MICT moderate-intensity continuous training, LICT low-intensity continuous training, HICT high-intensity continuous training, Hetero heterogeneity
Dropouts, exercise adherence, and adverse events
The number of participants who dropped out before completing the exercise training was reported in 5 of 8 trials. One study reported no dropouts, and three studies each had ≤10 % dropout per group, see Table 1. The study by Hansen et al. had the longest training interventions included in our review (6 months) and reported highest dropout rates of 32 and 20 % for the HICT and MICT, respectively (i.e., 8/25 vs. 5/25). Overall, the odds ratio for dropout was not different between the higher- versus lower-intensity exercise training (OR 1.25 [0.5, 3.15], p = 0.64, I 2 = 0 %). Mean adherence to the exercise intervention was reported in 4 of 8 trials and was above 85 % for each of their eight training groups. Another trial considered participants as dropout if they did not reach 80 % adherence (note that there was one dropout per each group of 14 participants [29]). There was no difference in adherence between higher- versus lower-intensity exercise (SMD: −0.13 [−0.55, 0.28], p = 0.53, I 2 = 0 %). Only one study reported adverse events [28]. Other studies did not report any adverse events, but the reasons for dropouts were often not provided. See Table 1 details.
Risk of bias
According to our inclusion criteria, all trials were described as randomized. In addition, all trials were evaluated to have low risk of bias for “blinding of outcome assessment,” “incomplete outcome data,” “selective reporting,” and “other sources of bias.” Trials generally received a rating of unclear risk for “allocation concealment” since insufficient information was present to permit judgment. A high risk of bias resulted from the inability to blind participants to their group allocation. Funnel plots did not suggest asymmetry for any of the outcomes except HbA1c. The asymmetry was caused by the study by Lee et al. [26] which showed the most beneficial effect of higher-intensity exercise with a small sample size (n = 10 per group) and a higher estimated change-from-baseline SD. While asymmetrical funnel plots can suggest publication bias, it is also possible that the asymmetry was caused by the fact that this study was the only one conducted in adolescents and the only one comparing activity characterized as high versus low intensity. Removing this study from the overall analysis did not affect the HbA1c results (WMD: −0.21 [−0.37, −0.05], or −2.3 mmol/mol [−4.0, −0.5], p = 0.009, I 2 = 0 %). Funnel plots as well as the risk of bias summary figures are provided in the supplementary material.
Discussion
Our meta-analysis suggests greater reduction in HbA1c with higher-intensity exercise compared to lower-intensity exercise in type 2 diabetes. Our findings are important given the existing inconsistent findings from meta-regression analyses examining this issue [10, 11]. There are, however, important limitations to meta-regression analysis since the relationship is examined across trials and does not have the benefit of randomization to each intervention [33]. Although the reduction in HbA1c may be considered small in absolute terms (−0.22 % points or −2.4 mmol/mol), it is important to consider that the mean effect of exercise on HbA1c is thought to be about −0.65 % points or −7.1 mmol/mol [1–3]. In relative terms, a 33 % improvement in the effect of exercise on HbA1c without increasing the duration of exercise (and perhaps reducing it) may be perceived as clinically relevant to some patients and practitioners.
Despite leading to greater improvements in HbA1c, higher-intensity exercise training did not cause reductions in fasting glucose compared to lower-intensity exercise. Previous meta-analysis has found reductions in fasting glucose with regular exercise training compared to control or baseline levels [34]. However, we could not confirm these findings in another meta-analysis of mostly shorter-term exercise studies measuring fasting glucose with continuous glucose monitoring [35]. Although speculative, it is possible that greater weight/fat loss or a negative energy balance may be required for exercise to achieve improvements in fasting glucose. Interestingly, in our current meta-analysis, the greatest effect of higher-intensity exercise on fasting glucose was seen in the study that did not match energy expenditure between groups. In this study by Krause et al. [32], the difference in the change-from-baseline fasting glucose was −1.1 mmol/L (95 % CI −2.24, 0.04) in favor of the higher-intensity exercise group, which also showed greater reductions in percent body fat [32].
The prescription of higher-intensity exercise was not associated with lower adherence, a greater number of dropouts or more adverse events. However, it is important to consider that the total sample size was small and that the exercise interventions were of relatively short duration and mostly supervised. In addition, the participants enrolled in these studies are likely not representative of all people with diabetes. These studies sometimes included younger participants with short durations of diabetes. In addition, some studies had detailed pre-exercise inclusion criteria that included a baseline exercise stress test (e.g., [27, 29],). Higher-intensity exercise may not be appropriate for all people with type 2 diabetes.
In 2015 alone, there have been at least two other systematic reviews on related topics [36, 37]. These reviews were limited to high-intensity interval training (HIIT) and did not included studies comparing continuous exercise of different intensities. The systematic review by Curry et al. identified six studies on the effect of HIIT in type 2 diabetes, but only included two of the longer-term studies from our review (i.e., Mitranun [29] and Terada [27]) and did not perform any meta-analysis. The very comprehensive review by Jelleyman et al. [36] had broader inclusion criteria than ours. For example, it was not limited to type 2 diabetes and included HIIT study of at least 2 weeks in duration. They performed many analyses, including head-to-head comparison between HIIT and continuous training (similar to our MICT). Their analysis on HbA1c identified a similar, but not statistically significant WMD of −0.18 % [−0.36, 0.03] compared to our subgroup analyses showing a significant difference between HIIT and MICT (WMD = −0.23 % [−0.43, −0.02]). The similar findings are not surprising since both reviews were based on the studies from Karstoft et al. [28], Terada et al. [27], and Mitranun et al. [29]. However, the review by Jelleyman also included the study by Hollekim-Strand [24], which we excluded because the continuous exercise group performed home-based exercise, whereas only the HIIT group was supervised. This likely only had a small effect since the mean difference in that study was estimated at −0.20 % [−0.60, 0.20]. Another small difference is that we obtained the within-group change-from-baseline mean and SD data directly from the authors of the study by Karstoft et al. [28]. The data in their article show that HbA1c stayed at 6.6 % from pre- to post-intervention (mean change entered as 0.0 %) in the lower-intensity group. However, the more precise change data that we obtained showed a change of 0.075 ± 0.336 % (entered as 0.1 ± 0.3 % in our review).
As opposed to our findings, the overall analysis by Jelleyman observed greater improvement in insulin resistance with HIIT versus MICT. However, as in our analyses, the difference did not reach statistical significance when they considered the subgroup with metabolic syndrome or type 2 diabetes. Given the higher variability associated with measures such as fasting insulin and HOMA, the absence of a significant difference could be due to lower statistical power.
Our meta-analysis builds on the one by Jelleyman [36] by expanding the comparisons to include higher- versus lower-intensity continuous exercise. While this is a novel contribution of our analyses, it was also one of the more difficult aspects to interpret. In our overall analyses, the higher-intensity groups showed a greater reduction in HbA1c. However, only the subgroup analyses comparing HIIT versus MICT, representing 57.7 % of the total weight, showed a significant difference. Even though the test for subgroup differences did not reach statistical significance (χ 2 = 1.71, p = 0.64, I 2 = 0 %), one of the main criticisms of meta-analyses is combining results from different types of interventions (i.e., “combine apples with oranges” [13]). Looking specifically at the HICT versus MICT or MICT versus LICT comparisons, the differences did not reach statistical significance. When dealing with small mean differences such as these, it is possible that the absence of a significant effect in some subgroups is due to low statistical power.
It is noteworthy that our a priori inclusion criteria led us to having to make a difficult decision regarding one of the largest trials comparing HICT versus MICT. As part of the Italian Diabetes and Exercise Study (IDES), the article by Balducci et al. [38] reported comparisons between different aerobic exercise intensities, with 152 and 136 participants completing HICT and MICT, respectively. HICT performed exercise at 70 % of predicted VO2max, whereas the MICT completed an equal volume at 55 % VO2max (see Table 2). This study did not meet our inclusion criteria because it included a co-intervention that was different in both groups. The HICT group also performed resistance training at 80 % of predicted 1-repetition maximum, whereas the MICT group also trained at 60 % of 1-repetition maximum. Consequently, it would be impossible to tell whether greater improvements in the HICT would be due to the higher-intensity aerobic or resistance training (or both). However, it is encouraging that the mean decrease in HbA1c between the higher- versus lower-intensity groups (reported as −0.17 % [−0.44, 0.10]) [38] was in line with our results. Had we included this study in our meta-analysis, the overall results (WMD: −0.21 % [−0.36, −0.07], or −2.3 mmol/mol [−3.9, −0.8], p = 0.004, χ 2 = 2.71, p = 0.95, I 2 = 0 %) and those from the HICT versus MICT training subgroup (WMD: −0.16 % [−0.38, 0.05], or −1.7 mmol/mol [−4.2, 0.5], p = 0.13, Hetero.: χ 2 = 0.78, p = 0.86, I 2 = 0 %) would have been very similar.
The main limitations of our study included low sample size and the inclusion of studies with different exercise interventions or populations. Despite these limitations, the changes in HbA1c were consistent among all of our comparisons. In this rapidly growing area of research, our search identified several recent abstracts for which we were unable to obtain sufficient data. It is likely that many of these abstracts (including the one from Panday et al. [20], n = 76) will soon be available as full articles and add to this rapidly growing literature.
Although most trials attempted to match the workload or exercise energy expenditure between the higher and lower exercise intensity groups, we cannot be certain that energy expenditure was in fact the same in both groups. For example, higher-intensity exercise normally has greater excess post-exercise energy expenditure. Another challenge that we faced was that most trials did not report the change-from-baseline SD. We are grateful to the authors that provided these data [28, 30], but in other cases we had to estimate these SD from p values or the responses from other similar trials in the review. We encourage future trials to report changes from baseline.
In conclusion, our meta-analysis found a small but greater improvement in HbA1c following higher-intensity compared to lower-intensity aerobic exercise in type 2 diabetes. Subgroup analyses provided stronger support for high-intensity interval training than for continuous exercise of higher intensity, but studies with larger samples sizes are needed in this area. Also noteworthy for practitioners and people with type 2 diabetes, higher-intensity exercise can require less time to reach the same energy expenditure and did not result in poorer adherence, greater dropouts, or more adverse events in the select participants from randomized trials.
References
Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ (2001) Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. J Am Med Assoc: JAMA 286:1218–1227
Umpierre D, Ribeiro PA, Kramer CK et al (2011) Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA, J Am Med Assoc 305:1790–1799
Thomas DE, Elliott EJ, Naughton GA (2006) Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev 3:CD002968
Colberg SR, Albright AL, Blissmer BJ et al (2010) Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42:2282–2303
Canadian Diabetes Association Clinical Practice Guidelines Expert C, Sigal RJ, Armstrong MJ, Colby P et al (2013) Physical activity and diabetes. Can J Diabetes 37(1):S40–S44
Yang Z, Scott CA, Mao C, Tang J, Farmer AJ (2014) Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med 44:487–499
Schwingshackl L, Missbach B, Dias S, Konig J, Hoffmann G (2014) Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia 57:1789–1797
Thompson W (2013) Now trending: worldwide survey of fitness trends for 2014. ACSM’S Health Fit J 17:10–20
Weston KS, Wisloff U, Coombes JS (2014) High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48:1227–1234
Boulé NG, Kenny GP, Haddad E, Wells GA, Sigal RJ (2003) Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 46:1071–1081
Umpierre D, Ribeiro PA, Schaan BD, Ribeiro JP (2013) Volume of supervised exercise training impacts glycaemic control in patients with type 2 diabetes: a systematic review with meta-regression analysis. Diabetologia 56:242–251
Garber CE, Blissmer B, Deschenes MR et al (2011) American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43:1334–1359
The Cochrane Collaboration (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. In: Higgins JPT, Green S (eds). http://handbook.cochrane.org. Accessed 28 Oct 2015
Chao M, Liang F, Wang Z, Wang L, Ouyang G, Wang XD (2015) Effects of aerobic exercises in different intensities to the physiological indicators of type 2 diabetes patients. Chin J Rehabil Med 30:883–887
Sgadari A, Broccatelli M, De Bellonia S, Bernabei R (2014) Hi-intensity interval training vs. constant load training: the effects on body composition and endothelial function in patients with type 2 diabetes mellitus. Med Sci Sports Exerc 46:166
Han K, Koo B, Kim H et al (2008) Moderate intensity exercise was more effective in body fat loss, but vigorous exercise improved the insulin resistance in overweight type 2 diabetes mellitus. Diabetologia 51:S269
Han KA, Min KW, Koo BK, Pak KS, Kim HJ, Kim EJ (2009) Effects of moderate versus vigorous intensity exercise training on inflammatory markers, endothelial function, and insulin resistance in the patients with type 2 diabetes: longitudinal study. Diabetes 58:1080
Marianna B, Sgadari A, De Bellonia S, Bernabei R (2014) Hi-intensity interval training and constant load training induce similar muscle force changes in patients with type 2 diabetes mellitus. Med Sci Sports Exerc 46:167
Yoon BK, Park SJ, Cha HR (2012) The effect of different exercise intensities on blood profiles and body composition for type II diabetes. Med Sci Sports Exerc 44:502
Pandey AK, Clarus S, Poirier P (2015) The comprative effect of burst exercise vs. sustained exercise on the cardiometabolic status of newly diagnosed diabetic patients. Can J Cardiol 31:S207
Yan H, Prista A, Ranadive SM et al (2014) Effect of aerobic training on glucose control and blood pressure in T2DDM East African males. ISRN Endocrinol 2014:864897
Jung JY, Han KA, Ahn HJ et al (2012) Effects of aerobic exercise intensity on abdominal and thigh adipose tissue and skeletal muscle attenuation in overweight women with type 2 diabetes mellitus. Diabetes Metab J 36:211–221
Hamed NS, Raoof NALA (2014) Effect of high intensity interval training on diabetic obese women with polyneuropathy: a randomized controlled clinical trial. Phys Ther Rehabil 1:1–8
Hollekim-Strand SM, Bjorgaas MR, Albrektsen G, Tjonna AE, Wisloff U, Ingul CB (2014) High-intensity interval exercise effectively improves cardiac function in patients with type 2 diabetes mellitus and diastolic dysfunction: a randomized controlled trial. J Am Coll Cardiol 64:1758–1760
Ku YH, Koo BK, Ahn HJ et al (2009) Effects of aerobic exercise intensity on insulin resistance in patients with type 2 diabetes mellitus. Korean Diabetes J 33:401–411
Lee SS, Yoo JH, So YS (2015) Effect of the low- versus high-intensity exercise training on endoplasmic reticulum stress and GLP-1 in adolescents with type 2 diabetes mellitus. J Phys Ther Sci 27:3063–3068
Terada T, Friesen A, Chahal BS, Bell GJ, McCargar LJ, Boule NG (2013) Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res Clin Pract 99:120–129
Karstoft K, Winding K, Knudsen SH et al (2013) The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 36:228–236
Mitranun W, Deerochanawong C, Tanaka H, Suksom D (2014) Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand J Med Sci Sports 24:e69–e76
Hansen D, Dendale P, Jonkers RA et al (2009) Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia 52:1789–1797
Li J, Zhang W, Guo Q et al (2012) Duration of exercise as a key determinant of improvement in insulin sensitivity in type 2 diabetes patients. Tohoku J Exp Med 227:289–296
Krause M, Rodrigues-Krause J, O’Hagan C et al (2014) The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur J Appl Physiol 114:251–260
Thompson SG, Higgins JPT (2002) How should meta-regression analyses be undertaken and interpreted? Stat Med 21:1559–1573
Snowling NJ, Hopkins WG (2006) Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 29:2518–2527
MacLeod SF, Terada T, Chahal BS, Boule NG (2013) Exercise lowers postprandial glucose but not fasting glucose in type 2 diabetes: a meta-analysis of studies using continuous glucose monitoring. Diabetes Metab Res Rev 29:593–603
Jelleyman C, Yates T, O’Donovan G et al (2015) The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev 16:942–961
Curry M, Mehta SP, Chaffin JC, Duran E, Washington B, Bose SS (2015) The effect of low-volume, high-intensity interval training on blood glucose markers, anthropometric measurements, and cardiorespiratory fitness in patients with type 2 diabetes. Crit Rev Phys Rehabil Med 27:19–35
Balducci S, Zanuso S, Cardelli P et al (2012) Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES). PLoS ONE 7:e49297
Acknowledgments
The authors would like to thank Drs. Hansen, Manders, and Karstoft for sharing change-from-baseline standard deviations from their studies. We would also like to thank Linda Slater for performing the computerized literature searches. This study was supported by the University of Alberta, Faculty of Physical Education and Recreation, Physical Activity and Diabetes Laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Terada and Dr. Boulé have received funding and equipment from Medtronic of Canada for projects unrelated to this article. Dr. Terada and Dr. Boulé are the authors of one of the articles included in this meta-analysis.
Ethical standard
All procedures performed in the study by Terada et al. were in accordance with the ethical standards of the University of Alberta Research Ethics Board and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors, with the exception of the eligible and previously published study by Terada et al. [27].
Informed consent
All of the eligible articles included in the meta-analysis stated that they had obtained informed consent from participants.
Additional information
Managed by Massimo Porta.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liubaoerjijin, Y., Terada, T., Fletcher, K. et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol 53, 769–781 (2016). https://doi.org/10.1007/s00592-016-0870-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0870-0