Abstract
Background
Resistance and aerobic exercises are both recommended as effective treatments for people with type 2 diabetes. However, the optimum type of exercise for the disease remains to be determined to inform clinical decision-making and facilitate personalized exercise prescription.
Objectives
Our objective was to investigate whether resistance exercise is comparable to aerobic exercise in terms of effectiveness and safety in people with type 2 diabetes.
Data sources
PubMed, EMBASE, CENTRAL, CINAHL, and SPORTdiscus were systematically searched up to March 2013. The reference lists of eligible studies and relevant reviews were also checked.
Study Selection
We used the following criteria to select studies for inclusion in the review: (i) the study was a randomized controlled trial; (ii) the participants were people with type 2 diabetes aged 18 years or more; (iii) the trial compared resistance exercise with aerobic exercise for a duration of at least 8 weeks, with pre-determined frequency, intensity, and duration; and (iv) the trial provided relevant data on at least one of the following: glycaemic control, blood lipids, anthropometric measures, blood pressure, fitness, health status, and adverse events.
Study Appraisal and Synthesis Methods
The assessment of study quality was based on the Cochrane Risk of Bias tool. For effectiveness measures, differences (resistance group minus aerobic group) in the changes from baseline with the two exercises were combined, using a random-effects model wherever possible. For adverse events, the relative risks (resistance group vs. aerobic group) were combined.
Results
Twelve trials (n = 626) were included. Following the exercise interventions, there was a greater reduction of glycosylated hemoglobin with aerobic exercise than with resistance exercise (difference 0.18 % (1.97 mmol/mol), 95 % confidence interval (CI) 0.01, 0.36). This difference became non-significant with sensitivity analysis (p = 0.14). The differences in changes from baseline were also statistically significant for body mass index (difference 0.22, 95 % CI 0.06, 0.39), peak oxygen consumption (difference −1.84 mL/kg/min, 95 % CI −3.07, −0.62), and maximum heart rate (difference 3.44 beats per minute, 95 % CI 2.49, 4.39). Relative risks for adverse events (all) and serious adverse events were 1.17 (95 % CI 0.77, 1.79) and 0.89 (95 % CI 0.18, 4.39), respectively.
Limitations
Most included trials were short term (8 weeks to 6 months), and seven had important methodological limitations. Additionally, the meta-analyses for some of the secondary outcomes had a small number of participants or substantial statistical heterogeneity.
Conclusions
Although differences in some diabetic control and physical fitness measures between resistance exercise and aerobic exercise groups reached statistical significance, there is no evidence that they are of clinical importance. There is also no evidence that resistance exercise differs from aerobic exercise in impact on cardiovascular risk markers or safety. Using one or the other type of exercise for type 2 diabetes may be less important than doing some form of physical activity. Future long-term studies focusing on patient-relevant outcomes are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Rationale
Exercise is considered a cornerstone of treatment for type 2 diabetes alongside diet and medication of proven efficacy [1, 2]. Although the effectiveness of exercise in improving glycaemic control, blood lipid profiles, and other outcomes in this group is well documented [3–5], there is less certainty about the relative effects of different types of exercise. Aerobic exercise is traditionally the most studied exercise [4], which recruits large groups of muscles and includes brisk walking, cycling, swimming, and jogging. However, 80 % of people with type 2 diabetes are overweight or obese [6], and many have mobility problems, peripheral neuropathy, visual impairment, or cardiovascular disease. For these patients, it may be infeasible to achieve the required volume and intensity of aerobic exercise [7], and resistance exercise may be more feasible. Resistance exercise uses muscular strength to move a weight or to work against a resistive load, causing isolated, brief activity of single muscle groups, and has received increasing attention in the last decade. There is a growing body of clinical evidence to support resistance exercise for those with type 2 diabetes [8, 9].
Indeed, most guidelines recommend both aerobic and resistance exercises for people with type 2 diabetes. However, lack of adherence to the recommendations is a concern, because even in research settings where people are instructed to follow just one type of exercise, the rate of regular exercise participation has been low and adherence to exercise protocols an ongoing problem [7, 10]. Possible reasons for this include personal preferences, physical limitations, and available facilities. Thus, for patients who are able to follow just one type of exercise, it would be important to know which type of exercise is more effective and/or safe. Previously, some systematic reviews have evaluated the efficacy of aerobic and resistance exercises, respectively, by pooling studies that compared either exercise with control [11]. Although it is possible to infer the relative efficacy of resistance versus aerobic exercise from these reviews by comparing their respective summary estimates, this is an indirect and less reliable approach compared with direct or head-to-head comparison of the two types of exercise.
1.2 Objectives
We set out to identify and review randomized controlled trials to date comparing the effects of resistance exercise with aerobic exercise on glycosylated hemoglobin (HbA1c) as well as other measures of cardiovascular risk and safety in patients with type 2 diabetes to better inform clinical decision-making and patient choice.
2 Methods
This review was carried out in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [12].
2.1 Data Sources and Searches
PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and SPORTdiscus were systematically searched from inception through March 2013. Both medical subject heading (MeSH) terms and text words related to diabetes, aerobic exercise, resistance exercise, and randomized controlled trials were used to identify potentially relevant studies, with no restrictions on the language of publication. The search strategy is reported in the Electronic Supplementary Material, Appendix 1. We also checked the reference lists of all identified trials and 36 relevant reviews or editorials, although no additional eligible trials were identified.
2.2 Study Selection
Initially, titles and abstracts were screened for relevance. The full-text of potentially eligible studies was obtained to review eligibility for inclusion. We used the following criteria to select studies for inclusion in the review: (i) the study was a randomized controlled trial; (ii) the participants were people with type 2 diabetes aged 18 years or more; (iii) the trial compared resistance exercise with aerobic exercise for a duration of at least 8 weeks, with pre-determined frequency, intensity, and duration; and (iv) the trial reported at least one of the following with available data: HbA1c, fasting blood glucose, insulin sensitivity measured by homeostasis model (HOMA-IR), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, systolic and diastolic blood pressures, weight, body mass index (BMI), waist circumference, waist-to-hip ratio, body fat percentage, peak oxygen consumption (VO2peak), maximum heart rate, health status, adverse events (all), and serious adverse events. The study selection was completed independently by three researchers (ZY, CM, CAS). The inclusion of each study was first based on their decisions and further confirmed by another reviewer (AJF).
2.3 Data Extraction and Quality Assessment
Data extracted from each eligible study included bibliographic information (author, publication year), baseline patient characteristics (mean age, sex, diabetes duration), details of interventions (frequency, duration, intensity, supervision), results of reported outcomes, and data to inform an assessment of risk of bias (generation of random sequence, allocation concealment, blinding of outcomes assessors, withdrawal, and use of intention-to-treat analysis). The assessment for risk of bias was based on the Cochrane Risk of Bias tool [13]. Data extraction and quality assessment were completed by three researchers (ZY, CAS, AJF).
2.4 Data Synthesis and Analysis
The primary outcome of interest was glycaemic control measured as HbA1c, with other measures of cardiovascular risk, health status, and adverse events as secondary outcomes. For continuous outcomes, including HbA1c, the difference in within-group pre-post change between resistance and aerobic groups, expressed as point estimate with 95 % confidence interval (CI), was extracted from reported data or calculated according to the method recommended by the Cochrane Handbook [14]. The differences from all relevant studies were then pooled to produce the weighted mean difference as a summary estimate. For each outcome, the within-group pre-post changes in resistance and aerobic groups, respectively, reported by different studies, were also pooled to estimate the effects of the two exercises. For dichotomous outcomes, including adverse events, relative risks (RRs) with 95 % CIs were pooled. As we anticipated heterogeneity between studies in patient characteristics (such as duration of diabetes and baseline HbA1c level), interventions (such as the frequency and duration of exercises), and clinical settings, all meta-analyses were conducted using a random-effects model [14].
The statistical heterogeneity across different studies in meta-analysis was assessed by the Cochran’s Q test and I 2 [15]. A p value of 0.10 or less for the Q test or an I 2 greater than 50 % was interpreted as indicating substantial heterogeneity [15]. Begg’s funnel plot and Egger’s test for asymmetry of the plot were used to detect small-study effects [14]. Exploratory subgroup analyses were carried out to examine whether the length of intervention and the use of co-intervention (e.g. dietary recommendation) would affect the estimates of effect size. Sensitivity analyses were carried out to test the robustness of the pooled results by removing trials with an assessed risk of bias. Funnel plot, subgroup, and sensitivity analyses were conducted only for the primary outcome. The quantitative syntheses of data were all performed with RevMan software, version 5.0 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012), or StataSE software, version 11.0 (StataCorp LP, College Station, TX, USA). For outcomes that could not be pooled, we provided a narrative summary of the findings.
3 Results
3.1 Literature Search and Trial Selection
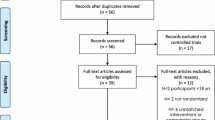
We identified a total of 2,988 references from all databases and initially excluded 1,094 duplicates. The flow diagram reporting trial selection is shown in Fig. 1. Briefly, by screening titles and abstracts, we excluded 1,850 references, leaving 44 for full-text review. Of these, 17 papers [16–32], representing 12 trials involving 626 patients with type 2 diabetes, were selected for inclusion. Four trials reported results in more than one paper [16, 20, 23, 25–30]. The researchers (ZY, CM, CAS) responsible for trial selection were initially uncertain about the eligibility of 11 papers and thus referred them to an additional reviewer (AJF), which led to three of them [18, 27, 32] being included and the remaining excluded.
3.2 Description of the Included Trials
3.2.1 Trial Settings and Participants
Included trial characteristics are summarized in Table 1. Each of the 12 trials was carried out in a different country, covering both developed and less developed regions. The exercise program settings included large public tertiary hospital, university health or research centre, commercial fitness centre, and community-based exercise facilities. The number of participants in each trial ranged from 20 to 145 [16, 19, 29]. One trial [24] included only male participants and another [22] only female. All trials were carried out in adults, and the mean age of exercise groups was between 48 and 59 years [25, 26, 31]. The mean duration of diabetes and baseline fasting blood glucose of patients reported in different trials varied markedly, respectively ranging from 2.6 to 12.0 years [25, 26, 31] and 6.3 to 11.6 mmol/L [21, 22]. Baseline HbA1c levels reported in different trials were all between 7 % (53.0 mmol/mol) and 9 % (74.9 mmol/mol), except Sukala et al. [31], in which the mean HbA1c of the resistance exercise group was 10.7 % (93.4 mmol/mol).
3.2.2 Interventions
A brief description of the exercise programs is given in Table 1, with a detailed account in the Electronic Supplementary Material, Appendix 2. The duration of interventions ranged from 8 weeks [16, 25, 26] to 12 months [32], with a median of 4 months. Three sessions of resistance exercise were taken per week in all but four trials [16, 21, 22, 25, 26, 29], each session lasting for 30–60 min. In all but one trial, the resistance exercises were conducted progressively [22]. The resistance exercises studied involved 5–10 muscle groups [22, 24], with an intensity varying from 2 to 6 sets (mostly 2–3 sets) of 6 to 20 repetitions (mostly 8–12 repetitions) of each exercise. In three trials, the resistance exercises had a low-intensity aerobic warm-up [16, 21, 24, 29].
The major forms of aerobic exercises studied were cycling, walking, and treadmill. Three sessions of aerobic exercises were taken per week in all but three trials [21, 22, 25, 26], each session lasting for 15–60 min (mostly 40–60 min). The intensity varied (Table 1): in six trials [17, 21, 23, 25, 26, 28, 30–32], at 60–85 % of heart rate reserve, with or without progressing; in one trial, at the heart rate corresponding to the lactate threshold [20, 27]; in three trials, at 50–85 % of VO2peak [18, 19, 24]; in another trial, at 3.6–5.2 metabolic equivalents [22].
The exercises were supervised in all except one trial where the aerobic exercise was not supervised [18] and another trial where it was unclear whether the aerobic exercise was supervised [16, 29]. The compliance with the exercise schemes was reported in seven trials, ranging from 67 to 99 % [20, 27, 31]. In addition to exercises, recommendations on dietary intake, daily activities, and/or medication taking were given to subjects in seven trials.
3.2.3 Methodological Quality Assessment
The methodological assessment of included trials is summarized in Table 1. Seven trials reported the randomization method by which the participants were allocated to different exercise groups [17, 19, 21, 23–26, 28, 30, 31]. Five of them clearly stated that the allocation process was concealed and the outcome assessors blinded [17, 19, 23–26, 28, 30], including the two trials that were focused on the health status of patients. One trial [18] reported that the participants were “consecutively divided” into different exercise groups. The remaining four trials did not report how the randomization was conducted [16, 20, 22, 27, 29, 32].
Three of the 12 trials had a run-in period before the commencement of exercise programs [19, 23, 28, 30, 32]. The withdrawal rates were below 20 % in ten trials (0–18.3 %, median 7.4 %) [16–30]. The remaining two had withdrawal rates of 20 and 31 %, respectively [31, 32]. Seven trials conducted intention-to-treat analyses [19, 20, 22–26, 30, 31], while the other five trials and the analyses on some outcomes in two of the seven trials were per-protocol [16–18, 21, 27–29, 32].
As a result of the identified potential for bias, seven trials [16, 18, 22, 27–29, 31, 32] were removed in sensitivity analyses.
3.3 Synthesis of the Results
The effects of resistance and aerobic exercises on the 18 selected outcomes (both effectiveness and safety), and the differences between the two regimens, are summarized in Table 2 and reported below, with the numerical values presented as ‘point estimate (95 % CI)’ or ‘mean ± standard deviation’.
3.3.1 Glycaemic Control
Twelve trials with a total of 595 participants provided data on HbA1c [17–22, 24, 25, 29–32]. The individual within-group pre-post changes in HbA1c in resistance and aerobic groups, respectively, ranged from −1.83 % (−20.00 mmol/mol) to −0.04 % (−0.44 mmol/mol) [19, 29] and from –1.33 % (−14.54 mmol/mol) to −0.1 % (−1.09 mmol/mol) [29, 31]. The pooled summary estimates of within-group pre-post changes in HbA1c in resistance and aerobic groups were −0.32 % (−0.45, −0.19) [−3.50 mmol/mol (−4.92, −2.08)] and −0.46 % (−0.64, −0.29) [−5.03 mmol/mol (−6.99, −3.17)], respectively. Differences in within-group change in HbA1c between resistance and aerobic groups in each of the trials ranged from −0.90 % (−9.84 mmol/mol) to 0.78 % (8.52 mmol/mol) [18, 32]. The pooled summary estimate of the difference in within-group change in HbA1c between resistance and aerobic groups was 0.18 % (0.01, 0.36) or 1.97 mmol/mol (0.11, 3.93) (Fig. 2); however, there was substantial statistical heterogeneity in this meta-analysis (I 2 = 51 %, p = 0.02).
Ten trials with a total of 326 participants provided data on fasting blood glucose [17, 18, 20–22, 24, 25, 29, 31, 32]. The within-group pre-post changes in fasting blood glucose in resistance and aerobic groups were −0.87 mmol/L (−1.46, −0.28) and −0.90 mmol/L (−1.69, −0.11), respectively. The difference in within-group change in fasting blood glucose between resistance and aerobic groups was −0.16 mmol/L (−0.75, 1.06), with substantial statistical heterogeneity (I 2 = 76 %, p < 0.001).
Four trials with a total of 125 participants provided data on HOMA-IR [18, 20, 21, 31]. The pooled within-group pre-post changes in HOMA-IR in resistance and aerobic groups were −0.73 (−1.72, 0.26) and −0.80 (−2.11, 0.50), respectively. The pooled difference in within-group change in HOMA-IR between resistance and aerobic groups was 0.56 (−0.30, 1.42), and the statistical heterogeneity was not substantial (I 2 = 45 %, p = 0.14).
3.3.2 Blood Lipids
Nine trials with a total of 400 participants [17, 18, 21, 24, 25, 27, 30–32] and ten trials [16–18, 20, 21, 24, 25, 30–32] with a total of 422 participants provided data on LDL and HDL cholesterol, respectively. The pooled within-group pre-post changes in LDL-cholesterol in resistance and aerobic groups were −0.05 mmol/L (−0.09, −0.01) and −0.08 mmol/L (−0.28, 0.12), respectively. The corresponding values for HDL cholesterol were 0.02 mmol/L (−0.03, 0.07) and 0.03 mmol/L (−0.02, 0.09), respectively. The difference in within-group change in LDL cholesterol and that of HDL cholesterol between resistance and aerobic groups were 0.02 mmol/L (−0.16, 0.19) and −0.03 mmol/L (−0.13, 0.08), respectively, with substantial statistical heterogeneity in both meta-analyses (I 2 = 66 %, p = 0.003 and I 2 = 93 %, p < 0.001, respectively).
Nine trials [16–18, 20, 21, 24, 25, 31, 32] with a total of 298 participants and ten trials [16–18, 20, 21, 24, 25, 30–32] with a total of 422 participants provided data on total cholesterol and serum triglycerides, respectively. The within-group pre-post changes in total cholesterol in resistance and aerobic groups were −0.22 mmol/L (−0.30, −0.14) and −0.11 mmol/L (−0.33, 0.12), respectively. The corresponding values for serum triglycerides were −0.33 mmol/L (−0.47, −0.18) and −0.14 mmol/L (−0.31, 0.03), respectively. The difference in within-group change in total cholesterol and that of serum triglycerides between resistance and aerobic groups were −0.10 mmol/L (−0.32, 0.11) and −0.19 mmol/L (−0.46, 0.09), respectively, with substantial statistical heterogeneity in both meta-analyses (I 2 = 62 %, p = 0.007 and I 2 = 86 %, p < 0.001, respectively).
3.3.3 Anthropometric Measures
Eleven trials with a total of 450 participants provided data on BMI [16–18, 20–22, 24, 25, 30–32]. The within-group pre-post changes in BMI in resistance and aerobic groups were −0.25 (−0.45, −0.0) and −0.41 (−0.58, −0.23), respectively. The difference in within-group change in BMI between resistance and aerobic groups was 0.22 (0.06, 0.39), with substantial statistical heterogeneity in this meta-analysis (I 2 = 43 %, p = 0.06).
Data on weight, waist circumference, and body fat percentage were provided by eight trials with a total of 465 participants [18, 19, 22, 25, 27, 30–32], eight trials with 460 participants [17, 19, 22, 24, 25, 27, 30, 31] and seven trials with 336 participants [18, 21, 25, 27, 30–32], respectively. The within-group pre-post changes in weight, waist circumference and body fat percentage in resistance versus aerobic groups were −0.65 kg (−1.21, −0.09) vs. −0.90 kg (−1.72, −0.08), −1.93 cm (−2.40, −1.47) vs. −1.82 cm (−2.85, −0.78) and −1.51 % (−2.49, −0.53) versus −1.21 % (−1.40, −1.02), respectively. The differences in within-group change in the three measures between resistance and aerobic groups was 0.32 kg (−0.19, 0.83), −0.41 cm (−1.18, 0.35) and −0.43 % (−1.42, 0.56), respectively; however, there was substantial statistical heterogeneity in the pooled results for body fat percentage (I 2 = 80 %, p < 0.001).
Four trials with a total of 154 participants provided data on waist-to-hip ratio [20, 21, 24, 25]. The within-group pre-post changes in waist-to-hip ratio in resistance and aerobic groups were −0.02 (−0.02, −0.02) and −0.01 (−0.03, 0.01), respectively. The difference in within-group change in waist-to-hip ratio between resistance and aerobic groups was −0.01 (−0.03, 0.01) (I 2 = 84 %, p < 0.001).
3.3.4 Blood Pressure
Ten trials with a total of 422 participants provided data on blood pressure [17, 18, 20, 21, 24, 25, 29–32]. The within-group pre-post changes in systolic blood pressure in resistance and aerobic groups were −7.02 mmHg (−11.03, −3.01) and −8.69 mmHg (−11.14, −6.23), respectively. The corresponding values for diastolic blood pressure were −4.40 mmHg (−6.53, −2.28) and −4.98 mmHg (−8.89, −1.08). The difference in within-group change for systolic and diastolic blood pressure between resistance and aerobic groups were 2.25 mmHg (−2.06, 6.56) and 0.28 mmHg (−2.92, 3.49), respectively, with substantial statistical heterogeneity in both meta-analyses (I 2 = 70 %, p < 0.001 and I 2 = 79 %, p < 0.001, respectively).
3.3.5 Fitness
Nine trials with a total of 530 participants provided data on VO2peak [17–21, 23–25, 32]. Within-group pre-post changes in VO2peak in resistance and aerobic groups were 0.57 mL/kg/min (0.21, 0.92) and 3.10 mL/kg/min (1.94, 4.26), respectively. The difference in within-group change in VO2peak between resistance and aerobic groups was −1.84 mL/kg/min (−3.07, −0.62), with substantial statistical heterogeneity in the meta-analysis (I 2 = 94 %, p < 0.001).
Four trials with 231 participants provided data on maximum heart rate [17, 18, 23, 32]. Within-group pre-post changes in maximum heart rate in resistance and aerobic groups were −0.45 beats per minute (−2.23, 1.33) and −3.82 beats per minute (−4.51, −3.12), respectively. The difference in within-group change in maximum heart rate between resistance and aerobic groups was 3.44 beats per minute (2.49, 4.39) (I 2 = 0 %, p = 0.90).
3.3.6 Health Status
Two trials provided data on health status which was measured by the Medical Outcomes Study Short Form-36 (SF-36) [26, 28]. In Ng et al. [26] (60 participants), the pre-post changes in SF-36 physical component summary score in resistance and aerobic groups were −0.3 ± 1.1 and −0.2 ± 1.0, respectively. The corresponding values for the mental component summary score were 1.9 ± 3.4 and 1.3 ± 2.7, respectively. The difference in within-group change in physical component summary score and that of mental component summary score between resistance and aerobic groups were −0.1 (−0.6 to 0.4) and 0.5 (−1.1 to 2.1), respectively.
In Reid et al. [28] (109 participants), the pre-post changes in SF-36 physical component summary score in resistance and aerobic groups were 2.9 ± 5.81 and 0.2 ± 5.70, respectively. The corresponding values for the mental component summary score were −0.8 ± 8.59 and 2.1 ± 8.46, respectively. The difference in within-group change in physical component summary score and that of mental component summary score between resistance and aerobic groups were 2.7 (0.1, 5.2) and −3.0 (−6.5, 0.5), respectively.
3.3.7 Adverse Events
Five trials reported the rates of adverse events [17, 20, 24, 30, 31]. In total, 45 events occurred in 121 participants of the resistance groups and 34 events in 117 participants of the aerobic groups. The pooled RR (resistance vs. aerobic groups) was 1.17 (0.77, 1.79). Three trials reported serious adverse events [19, 30, 31], with 9 events out of 150 patients in resistance groups and 10 out of 145 in aerobic groups. The pooled RR was 0.89 (0.18, 4.39). The statistical heterogeneity was low in both meta-analyses (I 2 = 14 %, p = 0.33 and I 2 = 39 %, p = 0.20, respectively).
The common types of adverse events reported did not differ much between the two exercise groups, and included hypoglycemia, back pain, shoulder pain, musculoskeletal injury, tendonitis, and other musculoskeletal discomforts. Two trials also reported other ‘medical’ adverse events, including diverticulitis, hysterectomy, lung cancer, cardiovascular disease events, blood clot, elective hernia repair, spinal stenosis, and worsening angina [19, 30]. One clearly stated that only one of these ‘medical’ events, which was non-cardiovascular, was associated with the exercise intervention [19], while the other trial reporting a case with worsening angina made no such statement [30].
3.4 Additional Analyses
As planned, funnel plot, subgroup, and sensitivity analyses were conducted for the meta-analysis on the primary outcome (i.e. HbA1c).
3.4.1 Funnel Plot
The funnel plot constructed based on the data presented in Fig. 2 was visually and statistically significantly asymmetrical (Egger’s test t = −2.81, p = 0.019), indicating the presence of small-study effects.
3.4.2 Subgroup Analyses
First, the 12 trials were grouped according to the length of intervention (≥4 months [six trials] [17–19, 21, 30, 32] vs. <4 months [six trials] [20, 22, 24, 25, 29, 31]). In the two subgroups, the difference in within-group pre-post change in HbA1c between resistance and aerobic exercises were 0.26 % (0.03, 0.48) [2.84 mmol/mol (0.33, 5.25)] (≥4 months) and 0.04 % (−0.20, 0.28) [0.44 mmol/mol (−2.19, 3.06)] (<4 months), respectively. The trials were then grouped according to the use of co-intervention in addition to exercise. In the subgroup with co-intervention (seven trials) and that without (five trials), the difference in within-group pre-post change in HbA1c between resistance and aerobic exercises were 0.30 % (0.11, 0.50) [3.28 mmol/mol (1.20, 5.46)] and 0.05 % (−0.14, 0.23) [0.55 mmol/mol (−1.53, 2.51)], respectively.
3.4.3 Sensitivity Analysis
Sensitivity analysis was conducted as described previously in the Methods section. The summary estimates for difference in change in HbA1c were unchanged, but became statistically non-significant; 0.18 % (0.01, 0.36) [1.97 mmol/mol (0.11, 3.93)] before and 0.15 % (−0.06, 0.37) [1.64 mmol/mol (−0.66, 4.04)] after removing the seven trials with important methodological limitations. The I 2 was 51 % before and 72 % after the sensitivity analysis.
4 Discussion
Previous research has shown that exercise improves glycaemic control in type 2 diabetes. A Cochrane review of 14 trials published in 2009 found a 0.6 % (95 % CI 0.3, 0.9) reduction in HbA1c when comparing any type of exercise (including aerobic, resistance, and both combined) with no exercise [5]. However, the comparative effectiveness of different types of exercise has not been adequately clarified. Neither existing systematic reviews nor clinical practice recommendations on the use of aerobic and resistance exercises address their relative benefits [4, 5, 10, 33]. This systematic review summarizes evidence directly comparing resistance with aerobic exercise in diabetes. In addition, it reports data on a range of cardiovascular risk factors and safety outcomes, thus providing a detailed comparison of the effects of the two exercises.
In line with other studies, our results show that both resistance and aerobic exercises are effective in diabetes control. We observed a fall of HbA1c of 0.32 % (3.50 mmol/mol) with resistance exercise and a fall of 0.46 % (5.03 mmol/mol) with aerobic exercise. There was a marginally greater reduction of HbA1c in the aerobic group than in the resistance group (0.18 % [95 % CI 0.01, 0.36]; 1.97 mmol/mol) which, compared with the overall effect size of exercise, is unlikely to be of clinical importance. There is no evidence that resistance exercise differs from aerobic exercise in impact on cardiovascular risk markers or safety.
In a previous systematic review by Umpierre et al. [11], aerobic and resistance exercises reduced HbA1c by 0.73 and 0.57 %, respectively. Indirect comparisons of these data, although limited in value because of potential for bias, shows that aerobic exercise is also slightly better than resistance exercise (the difference in absolute efficacy, 0.73−0.57 % = 0.16 %). This is consistent with and thus lends further support to the results of direct comparison in the present review (the difference in absolute efficacy, 0.18 %; Fig. 1).
Our finding, that either form of exercise appears to have comparable effects on glycaemic control, may be helpful to policy makers, clinicians, and individuals when advising and choosing appropriate lifestyle interventions. This is because many patients with type 2 diabetes are able to follow just one type of exercise, for example through physical limitations, personal preference, or availability of facilities, despite clinical guidelines advocating both aerobic and resistance exercises. For these patients, it would be important to know which type of exercise is better. Our work suggests that the two types of exercise are comparable and people may choose either one on the basis of feasibility and personal preference while achieving similar effect. It is possible that the easier the exercise is to maintain, the more likely it will be taken up. As resistance exercise could be feasible in a residential setting, it may be more appropriate for sedentary, elderly patients with type 2 diabetes, who tend to have worse muscle strength than those without diabetes [34].
However, there are three issues that need to be highlighted when extrapolating our findings to clinical practice. First, most were short-term trials (median: 4 months), and the only trial with a duration of 12 months had important methodological limitations, therefore it is difficult to generalize the findings to the impact of long-term exercise. Our subgroup analysis of short- (<4 months) or longer-term (≥4 months) trials indicated that the difference in effects between resistance and aerobic exercises might be more evident in a longer time period. As it is difficult to motivate people to incorporate exercise into their daily lives [5], it is likely that compliance with exercise regimens would reduce in the long term, thus reducing the effect on clinical outcomes. Conversely, it is plausible that clinical benefits may become more marked with increased duration of intervention. Furthermore, there may be more adverse events such as osteoarthritis or other long-term complications or mortality, which these short-term studies would not identify.
Second, nearly all of the exercise interventions in the included trials were supervised. However, in practice it is unlikely that most patients would have access to supervised exercise regimens of this intensity and there is evidence that supervised exercise is more effective than unsupervised [35, 36]. It is possible that either aerobic or resistance exercise may be easier to perform effectively without supervision, thus affecting the external validity of these results.
Finally, as with many studies in this field, we are relying on interpreting the results from surrogate markers, and are assuming that these changes translate to long-term benefit in clinical outcomes such as diabetic complications. To put the results in context, metformin lowers HbA1c levels by 1.12 % (12.24 mmol/mol) [37], and data from the United Kingdom Prospective Diabetes Study show that a 1 % rise in HbA1c represents a 21 % increase in risk for any diabetes-related endpoint [38].
Our data suggest that aerobic exercise may be associated with greater BMI reduction and better physical fitness (VO2peak, maximum heart rate) than resistance exercise. The biological mechanism underlying this difference is unclear. A possible explanation is that resistance exercise mainly increases the size and strength of muscles, while aerobic exercises forces the body to burn the stored fat for energy. Aerobic exercise may also enhance the strength of respiratory muscles and thus facilitate the body’s utilization of oxygen [39]. Additionally, the heart muscles may be strengthened and enlarged during aerobic exercise to improve the pumping efficiency and reduce the resting heart rate.
The present systematic review has some limitations. First, there are some methodological limitations of included trials that may have introduced bias (Table 1). Second, the meta-analyses for some of the secondary outcomes (Table 2) had a small number of participants or substantial statistical heterogeneity. The heterogeneity may indicate subgroups where aerobic exercise could be of clinical benefit in comparison with resistance exercise. Third, there were small-study effects in the main meta-analysis, which could be due to publication bias as well as other reasons, including poor methodological quality, true heterogeneity, and chance [14].
Further studies with longer exercise interventions and with post-intervention follow-up would provide information on whether either type of exercise is more likely to be maintained, and whether changes seen in these short-term studies are sustained, or even improved.
5 Conclusion
Although differences in some diabetic control and physical fitness measures between resistance exercise and aerobic exercise groups reached statistical significance, there is no evidence that they are of clinical importance. There is also no evidence that resistance exercise differs from aerobic exercise in impact on cardiovascular risk markers or safety. Using one or the other type of exercise for type 2 diabetes may be less important than doing some form of physical activity. Future long-term studies focusing on patient-relevant outcomes are warranted.
References
Sigal RJ, Armstrong MJ, Colby P, et al. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: physical activity and diabetes. Can J Diabetes. 2013;37(suppl 1):S40–4.
Myers J, Atwood JE, Froelicher V. Active lifestyle and diabetes. Circulation. 2003;107:2392–4.
Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:1228–37.
Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27.
Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968.
Bloomgarden ZT. American Diabetes Association Annual Meeting, 1999: diabetes and obesity. Diabetes Care. 2000;23:118–24.
Linke SE, Gallo LC, Norman GJ. Attrition and adherence rates of sustained vs. intermittent exercise interventions. Ann Behav Med. 2011;42:197–209.
Irvine C, Taylor NF. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: a systematic review. Aust J Physiother. 2009;55:237–46.
Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med. 2009;48:9–19.
Arikawa AY, O’Dougherty M, Schmitz KH. Adherence to a strength training intervention in adult women. J Phys Act Health. 2011;8:111–8.
Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–9.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. Chichester: Wiley; 2009.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Arora E, Shenoy S, Sandhu JS. Effects of resistance training on metabolic profile of adults with type 2 diabetes. Indian J Med Res. 2009;129:515–9.
Bacchi E, Negri C, Zanolin ME, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care. 2012;35:676–82.
Cauza E, Hanusch-Enserer U, Strasser B, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2005;86:1527–33.
Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253–62.
Jorge ML, de Oliveira VN, Resende NM, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60:1244–52.
Kadoglou NP, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with Type 2 diabetes. Diabet Med. 2013;30:e41–50.
Ku YH, Han KA, Ahn H, et al. Resistance exercise did not alter intramuscular adipose tissue but reduced retinol-binding protein-4 concentration in individuals with type 2 diabetes mellitus. J Int Med Res. 2010;38:782–91.
Larose J, Sigal RJ, Boulé NG, et al. Effect of exercise training on physical fitness in type II diabetes mellitus. Med Sci Sports Exerc. 2010;42:1439–47.
Moe B, Augestada LB, Asvoldb BO, Flanders WD. Effects of aerobic versus resistance training on glycaemic control in men with type 2 diabetes. Eur J Sport Sci. 2011;11:365–74.
Ng CL, Goh SY, Malhotra R, Østbye T, Tai ES. Minimal difference between aerobic and progressive resistance exercise on metabolic profile and fitness in older adults with diabetes mellitus: a randomised trial. J Physiother. 2010;56:163–70.
Ng CL, Tai ES, Goh SY, Wee HL. Health status of older adults with Type 2 diabetes mellitus after aerobic or resistance training: a randomised trial. Health Qual Life Outcomes. 2011;9:59.
de Oliveira VN, Bessa A, Jorge ML, et al. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl Physiol Nutr Metab. 2012;37:334–44.
Reid RD, Tulloch HE, Sigal RJ, et al. Effects of aerobic exercise, resistance exercise or both, on patient-reported health status and well-being in type 2 diabetes mellitus: a randomised trial. Diabetologia. 2010;53:632–40.
Shenoy S, Arora E, Jaspal S. Effects of progressive resistance training and aerobic exercise on type 2 diabetics in Indian population. Int J Diabetes Metab. 2009;17:27–30.
Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–69.
Sukala WR, Page R, Rowlands DS, et al. South Pacific Islanders resist type 2 diabetes: comparison of aerobic and resistance training. Eur J Appl Physiol. 2012;112:317–25.
Yavari A, Najafipoor F, Aliasgarzadeh A, Niafar M, Mobasseri M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes. Biol Sport. 2012;29:135–43.
Colberg SR, Sigal RJ, Fernhall B, American College of Sports Medicine; American Diabetes Association, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–67.
Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–8.
Nicolaï SP, Kruidenier LM, Leffers P, Hardeman R, Hidding A, Teijink JA. Supervised exercise versus non-supervised exercise for reducing weight in obese adults. J Sports Med Phys Fitness. 2009;49:85–90.
Olney SJ, Nymark J, Brouwer B, et al. A randomized controlled trial of supervised versus unsupervised exercise programs for ambulatory stroke survivors. Stroke. 2006;37:476–81.
Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35:446–54.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Plowman SA, Smith DL. Exercise physiology for health, fitness, and performance. 2nd ed. San Francisco: Benjamin Cummings; 2007.
Acknowledgments
The authors thank the Global Scholarship Programme for Research Excellence—CNOOC Grants for 2012–2013 offered by The Chinese University of Hong Kong.
AJF is an NIHR Senior Investigator and receives funding from NIHR Oxford Biomedical Research Centre. CAS is an NIHR funded GP Academic Clinical Fellow.
The authors declare that there are no conflicts of interest associated with this manuscript.
ZY, JT, and AJF contributed to the conception and design of the study. ZY, CAS, CM, and AJF contributed to the acquisition of data. ZY, CAS, and AJF contributed to the analysis and interpretation of data. All authors contributed to drafting the article or revising it critically. All authors approved the final version to be submitted.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Z., Scott, C.A., Mao, C. et al. Resistance Exercise Versus Aerobic Exercise for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sports Med 44, 487–499 (2014). https://doi.org/10.1007/s40279-013-0128-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-013-0128-8