Abstract
Aims
The role of metformin in the development of lactic acidosis (LA) in the setting of acute renal failure (ARF) is debated. Moreover, recent experiments suggested that metformin can also be nephrotoxic, but little clinical data exist about this topic. We sought to investigate these possible associations in a large cohort of patients with diabetes who developed ARF.
Methods
We analyzed data from patients with diabetes admitted to our emergency department between 2007 and 2011 with ARF and a previously normal renal function (n = 126). We compared acid–base balance and renal function of patients taking metformin (n = 74) with patients not taking it (n = 52).
Results
Patients taking metformin had decreased pH (7.31 ± 0.16 vs 7.39 ± 0.11, p = 0.008) and higher lactates (4.54 ± 4.30 vs 1.71 ± 1.14 mmol/L, p < 0.001). Both acidosis (pH < 7.35) and LA (lactates >5 mmol/L and pH < 7.35) were more frequently observed in this group (p = 0.0491 and p < 0.001, respectively). Multivariate analysis ruled out the role of some possible confounders, especially decreased renal function. The influence of metformin on pH and lactates grew significantly with higher doses of the drug (p = 0.259 and p = 0.092 for <1 g/day, p = 0.289 and p < 0.001 for 1–2 g/day, p = 0.009 and p < 0.001 for 2–3 g/day, for pH and lactates, respectively). Metformin influenced creatinine levels in a dose-related manner as well (p = 0.925 for <1 g/day, p = 0.033 for 1–2 g/day, p < 0.001 for 2–3 g/day).
Conclusions
In patients with diabetes who were admitted to our emergency department with ARF, the use of metformin was associated in a dose-related fashion with both LA and worse renal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is commonly believed that metformin can increase the risk of lactic acidosis (LA) in patients with renal failure and other conditions, such as hepatic insufficiency or hypoxia. This opinion arises from the fact that phenformin, another biguanide previously sold in the market, has been withdrawn for an increased incidence of fatal cases of LA in these settings. Moreover, a large number of case reports have described the occurrence of the so-called metformin-associated lactic acidosis (MALA) in patients with acute renal failure (ARF) [1–4].
Nevertheless, the link between metformin and LA has been questioned. The last Cochrane meta-analysis concluded that there is no evidence that therapeutic doses of metformin are associated with the development of LA, and that most of the reported cases have occurred in patients with other severe acute conditions, which could have been the real cause of LA. However, this meta-analysis failed to assess metformin safety in patients with renal failure, since this condition was listed as an exclusion criterion in most of the included studies [5]. Another limitation of the available studies lies in the fact that drug safety was assessed in a chronic setting: Data were obtained from outpatients who were on a steady state, and not from patients who developed acute renal function deterioration during the study period. Owing to this lack of data, the active role of metformin in inducing LA in the acute setting could have been missed, as suggested by MALA case reports. In order to test whether metformin can favor the development of LA in patients with ARF, we analyzed the acid–base balance of all the patients with diabetes who came to our emergency department with ARF in a 4-year period, comparing arterial blood gas (ABG) values between patients taking metformin and patients not taking it.
The mechanistic interpretation of MALA rests on the increased blood levels of the drug that occur in acute intoxications or when renal function rapidly deteriorates, since metformin is excreted unmetabolized by the kidneys [6]. These two events are somewhat different since metformin diffusion from blood to tissues is relatively slow: Patients suffering from inadvertent drug accumulation developing over days are expected to present with lower blood drug levels, but higher tissue drug levels and more severe LA than those with voluntary intoxication. However, patients with voluntary or accidental intoxications usually develop LA as well [7–10]. On a theoretical point of view, patients taking a higher dose of the drug should have increasingly higher blood and tissue levels of metformin when ARF develops.
Therefore, we investigated whether higher doses of metformin were related to an increased risk of developing LA in the setting of ARF. Curiously, intoxicated patients also develop ARF along with LA [11–13]: This might mean that metformin can have a sort of direct nephrotoxic effect. A recent experimental model showed indeed that metformin can impair renal mitochondrial function [14]. Thus, in order to understand the possible contribution of metformin to the development of ARF, we tested in our cohort the hypothesis that metformin can also be a nephrotoxic agent.
Materials and methods
The Humanitas Clinical and Research Center Ethics Committee approved the study and waived informed consent as data were collected retrospectively on an anonymized database.
Study population
Patients with diabetes mellitus type 2 arrived to our emergency department with ARF who had a previously normal renal function.
Inclusion and exclusion criteria
We collected data from all the patients with diabetes who were discharged from the medical wards of our hospital with the ICD-9 diagnosis code of “acute renal failure” from January 1, 2007, to December 31, 2011. Algorithm for patient selection is schematized in Fig. 1. Patients with chronic kidney disease (CKD) and type 1 diabetes mellitus were excluded.
Definitions
-
Diabetes was defined as the use of insulin and/or any oral antidiabetic drug (OAD) or a previous diagnosis of type two diabetes mellitus made by a physician.
-
CKD was defined as a previous serum creatinine greater than 1.5 mg/dL [5] or ultrasonographic signs of CKD (kidneys length <9 cm or a thin and hyperechoic cortex). For those patients in whom creatinine had not been tested previously, we ruled out CKD if patients were discharged with a serum creatinine <1.5 mg/dL, i.e., if they had a complete renal function recovery after the acute event.
-
Acidosis was defined as an arterial pH < 7.35.
-
LA was defined as an arterial pH < 7.35 along with lactates >5 mmol/L [15].
-
Comorbidities were approximated as the number of drugs taken daily.
Data collection
For each patient, demographic and clinical data were retrieved by accurate analysis of hospital archives. Clinical data collected included renal function before and after emergency department (ED) admission, use of metformin, the prescribed daily dose taken at home before ED admission, use of insulin or any other OAD, arterial blood gas analysis (ABG) values of the first sample taken after ED admission, glycaemia at ED admission, glycated hemoglobin, hemoglobin, total bilirubin, number of drugs taken daily, cause of ARF (expressed as pre-renal, intrinsic renal or post-renal on the basis of the work-up made after admittance), use of ACE-inhibitors (ACE-I) and/or angiotensin receptor blockers (ARBs), length of hospital stay, need for hemodialysis and hospital-survival.
Study aims
-
1.
To verify whether the occurrence of acidosis and LA was more frequent in patients taking metformin (Group A) than in patients taking any other OAD and/or insulin (Group B);
-
2.
To assess whether metformin influenced pH and lactate levels in an independent fashion from renal function, estimated as the creatinine value on ED admission, glycemia, number of drugs taken daily and causes of ARF through a multivariate analysis. To study whether different doses of the drug (<1, 1–2 and 2–3 g/day) had an influence on pH and lactate levels, considering as 0 g/day patients who were not taking it;
-
3.
To investigate whether metformin had an influence on renal function, estimated as the creatinine value on ED admission, in an independent fashion from causes of ARF, use of ACE-I and/or ARBs and the number of drugs taken daily through a multivariate analysis. To study whether different doses of the drug (<1, 1–2 and 2–3 g/day) had an influence on creatinine, considering as 0 g/day patients who were not taking it;
-
4.
To investigate whether the use of metformin had an influence on hospital-survival and need for hemodialysis.
Statistical analysis
Data were expressed as number and percentage, or mean and standard deviation, or median and range, where appropriate. Differences between patients taking or not metformin were explored with Fisher or Wilcoxon test, where appropriate. All possible association with pH and lactate levels was explored with a linear regression analysis. A p value <0.05 was considered as statistically significant. All analyses were performed with Stata11 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Results
From a cohort of 5841 patients, discharged between January 1, 2007, and December 31, 2011, we isolated 126 non-CKD ARF patients with diabetes (Fig. 1), 74 of which were taking metformin (group A), while the remaining 52 were taking insulin, any other OAD or both (group B). The median dose of metformin in group A was 1500 mg (400–3000 mg). Clinical data are listed in Table 1. Serum creatinine levels tended to be higher in patients taking metformin (361.7 ± 247.3 vs 264.9 ± 158.4 µmol/L or 4.11 ± 2.81 vs 3.01 ± 1.80 mg/dL, p = 0.071). Causes of ARF were similar between the two groups, with pre-renal ARF accounting for more than 80 % in both of them. ABG values showed a significant difference in pH and lactate levels among groups, with group A having lower pH (7.31 ± 0.16 vs 7.39 ± 0.11, p = 0.008) and higher lactates (4.54 ± 4.30 vs 1.71 ± 1.14 mmol/L, p < 0.001). Bicarbonate levels and base excess were decreased as well, with a trend toward a lower pCO2 (Table 1).
-
1.
Acidosis occurred in 57 cases, more frequently in group A (40 cases in group A, 17 cases in group B, odds ratio 2.11, p = 0.0491). LA, as defined earlier, occurred in 21 cases, all of which belonged to group A (p < 0.001).
-
2.
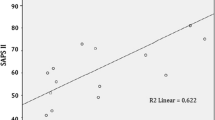
By multivariate analysis, metformin acted as an independent variable for pH (p = 0.042) and lactate levels (p < 0.001) when data were adjusted for creatinine on ED admission, number of drugs taken at home, glycaemia and causes of ARF (Table 2a). Creatinine had a strong influence on pH, as expected (p < 0.001), but a lesser effect on lactates (p = 0.059). We observed a good correlation between increasing doses of metformin and lactates, especially with doses greater than 1 g/day (p < 0.001). The effect on pH became significant only with doses above 2 g/day (p = 0.009) (Table 2b; Fig. 2).
Table 2 Multivariate analysis showing the influence of metformin, renal function (estimated as the creatinine value on ED admission), glycemia, cause of ARF and number of drugs taken at home, an estimate of comorbidities, on pH and lactate levels (panel a) -
3.
Renal function, estimated as creatinine value on ED admission, was worse among metformin users (Table 1). This has been confirmed by the multivariate analysis, when data were adjusted for causes of ARF, number of drugs taken at home and use of ACE-I and/or ARBs (Table 3a). Higher doses of metformin significantly influenced creatinine (p = 0.033 for 1–2 and p < 0.001 for 2–3 g/day) (Table 3b).
Table 3 Multivariate analysis showing the influence of metformin, cause of ARF, use of ACE-I and/or ARBs and number of drugs taken at home, an estimate of comorbidities, on renal function, estimated as the creatinine value on ED admission (panel a) -
4.
Thirteen patients deceased (10.3 %) and sixteen needed dialysis (12.7 %). There were no differences in hospital-survival and need for hemodialysis among groups A and B.
Discussion
These results demonstrated a strong association between the use of metformin and both lactate and pH levels in patients with diabetes admitted to our ED with ARF (Table 2a). This association was dose-related: The risk of lactic acidosis increased with escalating doses of the drug independently of renal function and the other studied confounders (Table 2b).
These data are in contrast with the common belief that LA in metformin users is due to the accompanying conditions [5, 16]. This idea was deducted from the fact that MALA has usually been observed in acutely ill patients and trials did not demonstrate any increased incidence of LA among metformin users. However, most of the trials had focused on stable outpatients and did not provide data about acid–base status during acute illnesses. Notably, 57 % of the studies considered by the Cochrane meta-analysis excluded patients with renal failure, 46 % excluded patients with cardiovascular, and 54 % excluded those with liver diseases [5]. In one of the biggest trials, the mean age was 53 and exclusion criteria included renal failure, myocardial infarction in the previous year, concurrent angina pectoris or heart failure and more than one major vascular event [17]: This setting is often much different from the clinical reality. By contrast, we chose to set ARF as the first inclusion criterion in our study in order to understand whether the data reported for the general population at the steady state are valid even in the acute setting. We chose to exclude patients with an already impaired kidney function for essentially two reasons: (1) These patients were unlikely to take metformin because in Italy, this drug was contraindicated with eGFR <60 mL/min/1.73 m2 in the study period, and (2) patients with CKD are likely to have a lower pH, i.e., a potential confounder, while we wanted to isolate a cohort of patients with a normal acid–base balance at the baseline.
Thereafter, we decided to test the hypothesis that metformin could exert a nephrotoxic effect. Metformin switches cell metabolism from an anabolic to a catabolic phenotype, reducing lipids, glucose and protein synthesis and increasing beta-oxidation. This is accomplished through the activation of AMP-activated kinase (AMPK), a crucial enzyme in cell metabolism that regulates the expression of many enzymes, especially those involved in gluconeogenesis. Metformin increases AMP/ATP ratio, thus activating AMPK, through a transient inhibition of mitochondrial respiration, being its molecular target the mitochondrial-respiratory chain complex one [18]. It has been recently demonstrated in an animal model that the mitochondrial dysfunction that follows this inhibition reduces oxygen consumption and determines an increase in lactic acid production. This is a widely spread process that is not limited to a single organ or apparatus, since metformin inhibits mitochondrial function in almost any cell that uptakes it, including renal cells [14]. Therefore, it is reasonable to think that this process is dose-dependent and has a therapeutic effect as long as the lactates produced are effectively cleared, preventing the occurrence of LA. Protti et al. [19] demonstrated that global oxygen extraction is reduced in metformin-intoxicated patients: This highlights that mitochondrial dysfunction is global and may resemble cyanide poisoning. This effect can theoretically be worse in those cells that have a high aerobic metabolism, such as renal tubular epithelial cells, and may explain why ARF is observed in voluntary or accidental intoxications [11–13].
Our results show indeed that metformin is associated with increased plasma creatinine values (Table 3a), and the strength of this association is higher when increasing doses of metformin are administered (Table 3b). This may underline an additive negative effect of the drug on renal function when ARF has already developed due to other causes. Most of our patients had a pre-renal failure (Table 1); thus, hypoperfusion is a likely trigger of ARF in our cohort. We can speculate that increased blood levels of metformin can worsen renal function when renal tubular epithelial cells are already suffering from hypoxia, as in most of our cases, through direct mitochondrial toxicity. Reasonably, the worse hypoperfusion and hypoxia are, the higher the toxicity will be. Our hypothesis is summarized in Fig. 3.
It is well known that metabolic acidosis is associated with a worse prognosis in terms of mortality, morbidity and need for hospitalization [20]. However, there was no significant difference in the overall survival and need for hemodialysis between patients who were given metformin and those who were not. This can be explained by the low rate of events in our cohort (mortality 10 % and need for hemodialysis 13 %).
Taken together, all these data support the hypothesis that in patients presenting with ARF and LA, metformin is not just an innocent bystander, but rather one of the major contributors to the acid–base disorder. The dose–effect relationship we have showed in our study could be explained by drug accumulation due to higher intake combined with a reduced excretion in the setting of renal failure. As showed previously, drug accumulation per se can lead to lactic acidosis [7–10], and the linear correlation we found between lactate production and the dose of the drug taken at home supports the theory that this could be the pathogenic mechanism underlying LA in these patients. The difference in results between our study and the ones reported in literature is probably due to the patients’ selection: We considered only patients in the setting of ARF, and not on a steady state. Notably, mean age of our patients (79 ± 10) was much higher than the ones reported before [5, 17]. This may account for the different results obtained, meaning that we explored a field that was previously neglected, i.e., the role of metformin in frail patients who develop an acute illness. According to our findings, a recently published population-based study from the Netherlands observed that patients taking high doses of metformin (>2 g/day) had greater risk to develop LA [21].
To our knowledge, the finding that metformin can exert a nephrotoxic effect has already been observed in animal models [14], but little clinical data exist about this topic. In a recently published MALA case series, a strong correlation between blood metformin and creatinine levels was noted [22]. However, this may only reflect the normal increase in blood metformin levels when renal function deteriorates. On the other hand, our data may suggest an active role of metformin in renal function worsening for two reasons: Firstly, we considered a control group who did not take metformin. Secondly, we observed a dose–effect relationship that may provide an additional meaning: The more metformin our patients took, the worse their renal function was on ED admission.
Of course, considering the retrospective nature of this study, there are some drawbacks that need to be addressed. First, blood metformin levels were not available: Further studies will be needed to better clarify the drug pharmacokinetic during ARF. Second, comorbidities were approximated as the number of drugs taken at home. Third, patients were selected through the ICD-9 code at discharge. An US study found a high specificity for ARF coding but a sensitivity as low as 35 % [23]. Thus, many ARF cases may have been missed and we cannot rule out selection bias, i.e., that metformin use could have influenced the ARF coding by the physician. Moreover, due to the size of the cohort we could analyze only few variables in the multivariate analysis. Therefore, our results should be interpreted with caution: The hypothesis generated with this retrospective study has to be further evaluated in both experimental and clinical models.
In conclusion, in a relatively large cohort of patients with diabetes who developed ARF, we observed a strong association between the dose of metformin and both pH and lactate levels. Physicians should be aware of its dangerous effects on acid–base balance when renal function acutely deteriorates and that the drug administration should be promptly stopped in the setting of ARF. To our knowledge, the possible nephrotoxic effect of metformin is currently being discovered and our findings reinforce this hypothesis.
References
Renda F, Mura P, Finco G, Ferrazin F, Pani L, Landoni G (2013) Metformin-associated lactic acidosis requiring hospitalization. A national 10 year survey and a systematic literature review. Eur Rev Med Pharmacol Sci 17(Suppl 1):45–49
Spiller HA, Quadrani DA (2004) Toxic effects from metformin exposure. Ann Pharmacother 38:776–780
Li Cavoli G, Tortorici C, Bono L, Giammarresi C, Ferrantelli A, Zagarrigo C et al (2011) Acute kidney injury associated with metformin. Am J Emerg Med 29:568–569
Runge S, Mayerle J, Warnke C, Robinson D, Roser M, Felix SB et al (2008) Metformin-associated lactic acidosis in patients with renal impairment solely due to drug accumulation? Diabetes Obes Metab 10:91–93
Salpeter SR, Greyber E, Pasternak GA, Salpeter EE (2010) Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev CD002967. doi:10.1002/14651858.CD002967.pub4
Frid A, Sterner GN, Löndahl M, Wiklander C, Cato A, Vinge E et al (2010) Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: clinical recommendations. Diabetes Care 33:1291–1293
Dell’Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW (2009) Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med 54:818–823
Wills BK, Bryant SM, Buckley P, Seo B (2010) Can acute overdose of metformin lead to lactic acidosis? Am J Emerg Med 28:857–861
Guo PYF, Storsley LJ, Finkle SN (2006) Severe lactic acidosis treated with prolonged hemodialysis: recovery after massive overdoses of metformin. Semin Dial 19:80–83
Bruijstens LA, van Luin M, Buscher-Jungerhans PMM, Bosch FH (2008) Reality of severe metformin-induced lactic acidosis in the absence of chronic renal impairment. Neth J Med 66:185–190
Rifkin SI, McFarren C, Juvvadi R, Weinstein SS (2011) Prolonged hemodialysis for severe metformin intoxication. Ren Fail 33:459–461
Lacher M, Hermanns-Clausen M, Haeffner K, Brandis M, Pohl M (2005) Severe metformin intoxication with lactic acidosis in an adolescent. Eur J Pediatr 164:362–365
Nguyen H-L, Concepcion L (2011) Metformin intoxication requiring dialysis. Hemodial Int 15(Suppl 1):S68–S71
Protti A, Fortunato F, Monti M, Vecchio S, Gatti S, Comi GP et al (2012) Metformin overdose, but not lactic acidosis per se, inhibits oxygen consumption in pigs. Crit Care 16:R75
Luft D, Deichsel G, Schmülling RM, Stein W, Eggstein M (1983) Definition of clinically relevant lactic acidosis in patients with internal diseases. Am J Clin Pathol 80:484–489
Nye HJ, Herrington WG (2011) Metformin: The safest hypoglycaemic agent in chronic kidney disease? Nephron Clin Pract 118:c380–c383
UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865
Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci 122:253–270
Protti A, Russo R, Tagliabue P, Vecchio S, Singer M, Rudiger A et al (2010) Oxygen consumption is depressed in patients with lactic acidosis due to biguanide intoxication. Crit Care 14:R22
Bakker J, Nijsten MW, Jansen TC (2013) Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care 3:12
Eppenga WL, Lalmohamed A, Geerts AF, Derijks HJ, Wensing M, Egberts A et al (2014) Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 37:2218–2224
Vecchio S, Giampreti A, Petrolini VM, Lonati D, Protti A, Papa P et al (2014) Metformin accumulation: lactic acidosis and high plasmatic metformin levels in a retrospective case series of 66 patients on chronic therapy. Clin Toxicol 52:129–135
Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O et al (2006) Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17:1688–1694
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Human and animal rights
The authors have followed the rules of good clinical and scientific practice.
Informed consent
The local ethics committee approved the study and waived informed consent as data were collected retrospectively on an anonymized database.
Additional information
Managed by Massimo Porta.
Rights and permissions
About this article
Cite this article
Cucchiari, D., Podestà, M.A., Merizzoli, E. et al. Dose-related effects of metformin on acid–base balance and renal function in patients with diabetes who develop acute renal failure: a cross-sectional study. Acta Diabetol 53, 551–558 (2016). https://doi.org/10.1007/s00592-016-0836-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0836-2