Abstract
Background
A scaphoid fracture is a common injury affecting the wrist joint. A fracture of the carpus scaphoid can heal uneventfully or be complicated by non-union. Scaphoid non-union can result in persistent wrist pain, and with functional difficulties affecting all activities of daily living of the patients, this disability is expected to be significant since most of these patients are young active adults.
Hypothesis
Extensive removal of the bone from the scaphoid, with the application of a large amount of cancellous bone graft and fixation with two to three wires, could lead to a high union rate and a good functional outcome.
Methods
Eighteen patients with scaphoid fracture non-union were recruited during their visit to the upper limb clinic at our institute. Demographic data were collected, and data regarding comorbidities, smoking, manual work, and others were recorded. Data regarding the interval between injury and surgery, time to radiographic union, and functional wrist scores were reported as well.
Results
A cohort of 18 patients was included. The mean age of patients was 30 years; most of our patients were healthy (83.3%), and more than two-thirds were smokers (72.2%). The mean follow-up time was 18 months (1.5 years), 15 patients (83.3%) achieved radiographic unions by 2–3 months, and the remaining 3 patients (16.7%) achieved radiographic unions by (4–5) months, i.e., all patients achieved successful radiographic unions by 5 months at maximum. The mean Mayo score for our series was 83.6 (± 12.4), with 5 patients (27%) achieved ≥ 95% which indicates a significantly high functioning wrist in our cohort.
Conclusion
Our modified technique with enhanced stability from using three k-wires can achieve full clinical and radiographic unions and result in enhanced recovery postoperatively with cast immobilization limited to 6 weeks total.
Level of evidence
IV Case series study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The scaphoid fracture is the most common fracture affecting the carpal bones, accounting for roughly 60% of total carpal bone fractures [1]. Scaphoid fractures in adults are predominately found in males, with a peak incidence in the age group from 20 to 29 years [2]. The scaphoid forms an important mechanical linkage between the proximal and distal carpal rows [3]. Approximately 80% of the surface of the scaphoid is covered with cartilage to articulate proximally with the distal radius, medially with the lunate and capitate, and distally with the trapezoid and trapezium bones [4, 5].
The dorsal carpal branch of the radial artery provides blood supply into the scaphoid through a capsular insertion on the dorsal ridge. They account for 70–80% of the scaphoid’s inter-osseous blood supply and represent the only vascular supply to the proximal pole [3]. On the palmar side, vessels from the superficial palmar branch of the radial account for 20–30% of the scaphoids inter-osseous blood supply, but solely in the distal pole area [6]. Given the inherent poor vascular supply of the scaphoid, it places it at a high risk for nonunion, delayed consolidation, or osteonecrosis [7].
Failure of a scaphoid fracture to heal may result in a predictable pattern of wrist arthritis, scaphoid nonunion and advanced collapse [8]. To minimize the risk of arthritis, the goal of treatment should be to consolidate the fracture with the scaphoid in anatomical alignment. Despite many reports, there is currently no consensus on the best form of management for scaphoid nonunion. Bone grafting has provided the mainstay of operative treatment for scaphoid nonunion, and the trend is to combine bone grafting with internal fixation [9]. Many proposed surgical techniques exist with a variable union rate.
The Matti-Russe technique has long been recognized as an effective method for managing scaphoid nonunions due to its simplicity, cost-effectiveness, and patient satisfaction rates [10]. Originally introduced by Hermann Matti in 1937, the technique involved the removal of necrotic bone, cartilage, and fibrous tissue at the fracture site through a dorsal incision, followed by the replacement with a plug of cancellous bone [11]. Otto Russe further refined the method in 1960 by adopting a palmar approach to minimize potential damage to the scaphoid's blood supply [12]. Building upon this established technique, our modification was a combination of extensive debridement, the use of three k-wires (if possible), and the introduction of an extremely large amount of cancellous bone graft at the resultant defect. It is a simple modification, yet a 100% union rate could be achieved at our institute with a short recovery period. The aim of our modification was to achieve union in chronic scaphoid non-union even in patients with the most unfavorable risk factors.
Patients and methods
All patients who presented to our department between 2012 and 2019 complaining of scaphoid non-union were enrolled in the current study. There were eighteen patients in total who were followed. The inclusion criteria were adult patients with a scaphoid non-union. We defined non-union as a failure to attain radiographic union for more than six months. Patients with advanced SNAC wrists were excluded (i.e., stages 3 and 4) [13]. Patients were not excluded for their previous treatments, number of previous surgical procedures, time between the fracture and presentation to our clinic, level of activity, smoking, steroid use, or pre-existing systemic diseases. We retrospectively reviewed all these patients radiologically and clinically. This study was done according to the guidelines of the institutional review board at our institute. The surgeries were done by a single upper-limb orthopedic surgeon. All patients were advised to quit smoking before the surgery; nonetheless, the surgery was performed regardless of their commitment.
In the assessment visit, Functional outcome was evaluated using the Mayo functional outcome score for each patient [14]. Preoperative radiographic evaluation included plain radiographs, computed tomography (CT) scan. Wrist radiographs and scaphoid series radiographs were ordered in the standard fashion (posteroanterior and lateral wrist views obtained in 90° shoulder abduction, 90° elbow flexion, neutral forearm rotation, and neutral wrist position). CT scans included axial, sagittal, and coronal reconstruction series. This preoperative radiological evaluation was aimed at determining the non-union site, the humpback deformity, the extent of bone resorption, the presence of arthritic changes, the presence of SNAC wrist, and the presence of cysts. We don’t do MRIs. The presence of avascular necrosis in the proximal pole doesn’t change our surgical protocol.
Bone union was assessed based on radiological union on AP and lateral wrist x-rays and clinically by the absence of tenderness at the anatomic snuffbox. Postoperative radiographs were used to assess the union. They were ordered at six weeks, three months, and six months postoperatively. The radiographs were assessed by the senior author, who is a consultant orthopaedic surgeon. The union was determined if no fracture line was visible on both views. When the union was questionable after four months of the procedure, a CT scan was ordered to confirm the union. The final clinical assessment was made in August 2021 for all patients, regardless of the time of the index procedure. The longest follow-up period was 63 months, and the shortest was 6 months, with an average follow-up of 18 months.
The surgical technique and our protocol
A standard volar Henry’s approach to the scaphoid was utilized in all patients, regardless of the site of the fracture or non-union in the scaphoid. The radiocarpal ligaments are tagged with PDS size 4/0 while incising them in the longitudinal axis of the scaphoid to facilitate their closure at the conclusion of the surgery. Confirming the fracture site is done through intraoperative fluoroscopy. Extensive curettage of the bone in the proximal part of the scaphoid is performed until we completely evacuate the proximal piece of the scaphoid from all bone, leaving an eggshell of the subchondral bone. Distally, curettage is performed until we get a clear margin of healthy bone. The bony margins of the cortical bony shell are trimmed gently proximally and distally, removing less than 1 mm via mini-bone nibblers. Usually, we end with a large defect at the conclusion of the refreshment. In revision cases, the defect can reach half the size of the scaphoid. If needed, we do radial styloidectomy.
Then, reduction is performed. In reduction, we don’t aim for bony apposition. Our target is to regain perfect alignment and length. This is accomplished by means of wrist extension, thumb traction, and mini-bone levers. Internal fixation of the scaphoid is accomplished via three or two Kirshner’s wires (K-wires) with a size of (1.6 mm–1.8 mm) depending on the available space at the fracture site. Our target is to use three K-wires if possible. We check the position of the K-wires via antero-posterior and lateral intraoperative imaging. The scaphoid should move as a single piece at the conclusion of fixation. After that, we harvest a bone graft from the ipsilateral iliac crest. We do a cortical bone window in the superior aspect of the iliac crest by reflecting a 4 cm-by-2 cm cortical piece, which is returned to its place after harvesting the graft. A purely cancellous bone graft is harvested. We aim to harvest a volume two-to-three times the size of the scaphoid. This cancellous bone graft is impacted gradually in the resulting bone defect at the scaphoid fracture site. We use mini-bone impactors to introduce this large amount of bone graft, starting from the proximal pole and followed by the distal piece defect. After confirming the scaphoid bony pieces are filled with a fully impacted bone graft, we fill the bone defect at the fracture site between the proximal and distal scaphoid pieces. Again, this is accomplished gradually with the sequential introduction and impaction of the cancellous bone graft. The defect should be filled with a well-impacted bone graft. K-wires are cut and buried inside the skin. Any extra bone graft is removed. Closure of the radio-carpal ligaments by PDS 4/0. Then, skin closure is done in a routine way. The two parts of the scaphoid, in addition to the bone graft filling the defect, should move as one piece at the conclusion of the procedure. We apply an above-elbow scaphoid cast, which is kept for 6 weeks. The resultant defect in this technique is extremely large, to the degree that we used an above elbow cast to offer extra stability. Although the existing literature doesn’t show a benefit for an above-elbow cast in scaphoid fractures [1, 3], we are unaware of any literature discussing defects as big as those resulting from this technique. Radiographs are taken postoperatively and repeated at six weeks, three months, and six months. Occupational therapy was commenced at six weeks postoperatively and continued according to the range of motion and strength of the wrist and hand. Wires are taken out after six months in all patients to ensure solid union. The wires were buried inside the skin and didn’t limit patients’ ability to mobilize their wrists or to participate in occupational therapy. Figures 1, 2, 3, 4, 5 demonstrate the results for a patient with scaphoid waist non-union. Figure 1 is a preoperative radiographs, Fig. 2 is the postoperative radiograph, Fig. 3 at 6 weeks follow-up visit, Fig. 4 at 5 months follow-up, and Fig. 5 at 6 months follow-up after removal of k-wires. Figures 6, 7, 8 are for another patient with distal pole scaphoid non-union. Figure 6 is the preoperative radiographs and CT scan, Fig. 7 is the postoperative radiographs, and Fig. 8 is the final radiograph after removal of k-wires.
A AP view of the right wrist after undergoing open curettage, bone grafting and retrograde fixation with three K-wires penetrating the distal pole and into the proximal pole of the bone. The fracture site is well aligned with adequate bone graft filling. Thumb spica cast is shown as well. B Lateral view is shows that surgery was done through a palmar (volar) approach. The K-wires appear buried under the skin on both views
Results
Descriptive statistical analysis was done using the Statistical Package for Social Sciences (SPSS) version 22. The current study includes a total of 18 patients. The mean age of patients was 30 years. Most of the patients were male (88.9%) and free of chronic medical illnesses (83.3%). However, thirteen patients (72.2%) were smokers. It is worth noting that smokers were counseled regarding smoking cessation before undergoing surgery, but none had quit smoking. Two patients (11.1%) of our sample were on chronic steroids (one primary and one revision case). Regarding mechanism of injury, fifteen patients (83.3%) sustained a fall on an outstretched hand; the remaining three patients (16.7%) sustained direct wrist trauma. (Table 1).
Regarding prior treatment, fifteen patients had a thumb spica cast acutely after the fracture. Three patients presented with a failed open reduction and internal fixation (ORIF) with a bone graft. The mean time from injury to surgery was 37 months (around 3 years). For the three revision cases, the time gap was 12, 10, and 8 years. One of them had two failed surgeries. (Table 1).
All patients had the same procedure as mentioned above. All patients had an above-elbow scaphoid cast for a total of six weeks. Thirteen patients had three k-wires, and five patients had two k-wires. The mean follow-up time for our patients was 18 months (1.5 years). In terms of radiographic union, fifteen patients (83.3%) achieved radiographic unions by two to three months, and the remaining three patients (16.7%) achieved radiographic unions by four to five months, i.e., all patients achieved successful radiographic unions by five months at maximum (Table 2). The radiographic patterns of the scaphoid fracture were as follows: Eight patients (44.4%) had a scaphoid waist fracture, and ten patients (55.6%) had a proximal pole fracture. Six patients (33.3%) were manual workers; the remaining were office-based employees. All patients had the same procedure as mentioned above. All patients had an above-elbow scaphoid cast for a total of six weeks.
The patients in this cohort had surgery between 2012 and 2021. All were clinically evaluated in August 2021 for the outcome measures. All patients reported high satisfaction. Assessment of the wrist function was done using the Mayo functional wrist score [15]. Scores range from 0 to 100, with a score of 0 indicating the worst possible wrist condition and a score of 100 indicating the best possible wrist condition. The mean Mayo score for our cohort was 83.6 (± 12.4), with 5 patients (27%) achieved ≥ 95% which indicates a significantly high functioning wrist in our cohort. Also, an assessment of the range of motion of the operated wrist was done. The mean flexion range of motion was 60 degrees, and the mean extension range was 56 degrees (Table 2). Correlation between Mayo score with age, previous surgeries, radiographic union, and postoperative range of motion is provided in Table 3. All our cohort at the time of visit and examination achieved full radiographic union, excellent clinical function of the wrist, no tenderness at the anatomic snuffbox, and full functional range of motion of the affected wrist.
Gender, steroid treatment, and the site of the fracture didn’t show significant differences in the outcome measures: radiographic union, Mayo functional outcome score, flexion arc, and extension arc (Table 4).
Smoking has negatively affected the Mayo functional outcome score (74 in smokers vs. 87 in non-smokers, p value 0.03). However, there was no difference in the union and the flexion and extension arcs. Also, smoking didn’t lower the reported patient satisfaction after the procedure (Table 4).
Two patients had infections at the site of the K-wires. Both healed uneventfully after removal. When the infection was noticed, only the infected K-wire was removed while keeping the others in place for the full treatment protocol.
Discussion
Scaphoid non-union remains a difficult situation to deal with. Various operations were described to deal with this condition, with a reported success rate of 60–90% [16]. Bone graft alone, bone graft with K-wire fixation, and bone graft with headless screws were described in the literature [8]. Even the use of mini plates and external fixators were also described previously [17, 18]. The bone grafts varied according to donor areas (iliac crest, distal radius, or olecranon), the nature of the graft harvested (cancellous or cortico-cancellous), and whether they were vascularized or not.
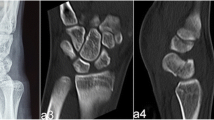
A AP view of the left wrist of a 15-year-old male patient showing distal scaphoid pole fracture non-union with obvious displacement of the fractured pieces. B Lateral X-ray shows a displaced fracture with flexed distal piece. C Coronal CT-Scan of the same patient showing the fracture non-union at the distal pole of the scaphoid with cystic changes and sclerosis mostly appreciated on the distal piece. Open growth plates of the distal radius and ulna can be visualized on all of these images
Three well-documented historical procedures were recorded to manage scaphoid non-union, with the first one described in literature by Adams and Leonard [19] as the use of cortical autograft at the non-union site. Eight years later (1936), Matti described another technique through a dorsal approach and the creation of a cavity within the non-united fragments, which was filled with cancellous bone [20]. Russe in 1960 mentioned the creation of a cavity; however, it utilized a volar approach, and the defect void was filled by both cortical and cancellous bone [12]. Authors refer to both techniques as Matti-Russe technique [21, 22]. In the original procedure, no osteosynthesis was used [23]. However, it required a prolonged immobilization period of the wrist, which could result in stiffness, leading to patient non-compliance and a poor outcome [22]. With the implementation of osteosynthesis, there is no need for such a long period of immobilization, and patients can start range of motion early postoperatively [24].
A long time interval between the time of injury and surgery was claimed to be a negative prognostic factor for union [25]. However, this current study showed no such effect (the mean time for the whole cohort was around three years, and for the revision cases, it was ten years). Similar to our results, Zoubos et al. and Meisel et al. reported no effect of the time interval on healing [26, 27].
Previous literature suggested the use of either screws or k-wires for fixation of the graft at the non-union site. Pinder et al. [28] in a review of the method used for fixation, reported that there is a general trend toward using screw fixation over k-wires in recent years; however, the series utilized k-wires had a higher estimated incidence of union than screw groups (91% vs. 88%, respectively). In this current study using k-wires for fixation, we achieved a 100% union rate. A longer period of time between the injury and the surgical intervention could lead to more erosion and resorption at the fracture site [8]. Using screws with differential threads would cause compression at the fracture. This means a shortening of the scaphoid due to pre-existing bone resorption. To avoid this, the use of a cortical graft at the fracture site is warranted when a compression screw is used. This shifted us away from the use of compressive screws towards the use of K-wires.
AP view of the left wrist status post open curettage, bone grafting and retrograde fixation with three K-wires penetrating the distal pole and into the proximal pole of the scaphoid. The fractured pieces are well reduced with adequate filling of the gap by bone graft. A cast is illustrated on X-ray as well
The number of K-wires used in fixation in the literature is variable. Raju et al. utilized the Matti-Russe inlay bone grafting technique with 2 K-wires in a cohort, and they utilized the Kohlman modification of vascularized muscle pedicle grafts using one K-wire in another group [29]. Meisel et al. [27] also used two K-wires to hold the graft. Ole et al. [30] in a series of 85 wrists with non-union scaphoids, utilized two K-wires and three K-wires for some patients. Stuart et al. [31] in a series of twenty-seven patients, used two 1.6-mm K-wires. In the current study, the target was the use of three K-wires. If this was difficult due to the dimensions of the scaphoid or the non-union site, two K-wires would be used. The aim is to have a stable construct to decrease the post-operative time spent in a cast and to allow early range of motion.
A AP view of the left wrist after removing the K-wires (6 months from the original surgery). Disappearance of the fracture site indicates a state of union in the scaphoid. Significant filling of the fracture site can be easily appreciated on this view. B The lateral view shows that the distal piece is no longer flexed and union of the fracture site with excellent alignment is evident
Zoubos et al. [26] kept the cast for 3 months duration after surgery. Ole et al. [30] reported using a thumb spica cast for 12 weeks. Sherief et al. [32] in a series of 50 patients, reported post-operative casting for 6 weeks, then another 4 weeks with a wrist splint. However, in our series, cast immobilization was kept for only 6 weeks, and then the patients started occupational therapy and the active use of the operated hand and wrist. This significantly enhanced functional recovery.
Smoking was shown to decrease the union rate after scaphoid non-union surgery [33]. This detrimental effect on union was documented regardless of the type of bone graft used (vascularized or non-vascularized) [7]. Even cessation of smoking prior to scaphoid non-union surgery didn’t improve the outcome [34]. Many authors have advised using a vascularized bone graft if the patient is a smoker [35]. In some series, the success rate in smoker patients was 27% [36] which improved to 65% if a vascularized bone graft was used [37]. Nonetheless, this success rate is still humble in comparison to our results. In this current cohort, thirteen patients were smokers, and all achieved a successful union and a good functional outcome. In this cohort, we had a 100% union rate regardless of smoking, chronic steroid use time interval, the site of the non-union, or being a revision procedure. This could be multifactorial. A large amount of cancellous bone graft was used and gradually impacted (two to three times the size of the scaphoid). This could introduce myriad viable osteoblasts. Also, performing an extensive and complete evacuation of the proximal pole coupled with an extensive evacuation of the distal pole could be a factor. This evacuation would make a sizable room to cope with the massive bone graft. On the other hand, this evacuation would enable us to get rid of all non-viable and sclerotic bones, which could hinder bone healing. Lastly, using two to three K-wires (1.6–1.8 mm) could make the final construct stiff enough to promote bone healing. The number and size of the K-wires depend on the dimensions at the non-union site. Possibly, the cross-sectional area of the metal crossing the non-union site is more important than the type of osteosynthesis used (K-wires vs. screws, or steel vs. titanium). We cannot attribute the success in the current cohort to either factor. Nonetheless, we think the use of these factors collectively resulted in this good outcome.
The main weakness of the study is the number of patients. Nonetheless, it could pave the way for larger studies. Due to the favorable results of this technique, more patients are getting this surgery in our center. After completing data collection, more patients received this procedure with the same favorable results. We aim to publish larger-scale studies on this procedure in the near future.
Conclusion
Healing of the scaphoid non-union was possible utilizing the current technique regardless of smoking, duration, site, revision or other risk factors. No major complications were encountered. We believe this could add to our knowledge and understanding of scaphoid non-union treatment. Larger-scale studies are warranted.
Change history
21 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00590-023-03732-6
References
Alshryda S, Shah A, Odak S, Al-Shryda J, Ilango B, Murali SR (2012) Acute fractures of the scaphoid bone: systematic review and meta-analysis. Surgeon 10(4):218–229. https://doi.org/10.1016/J.SURGE.2012.03.004
Jorgsholm P, Ossowski D, Thomsen N, Björkman A (2020) Epidemiology of scaphoid fractures and non-unions: a systematic review. Handchir Mikrochir Plast Chir 52(5):374–381. https://doi.org/10.1055/A-1250-8190
Clementson M, Björkman A, Thomsen NOB (2020) Acute scaphoid fractures: guidelines for diagnosis and treatment. EFORT Open Rev 5(2):96. https://doi.org/10.1302/2058-5241.5.190025
The anatomy of the scaphoid-PubMed. https://pubmed.ncbi.nlm.nih.gov/11775465/. Accessed 14 Nov 2022
Langer MF, Unglaub F, Breiter S, Ueberberg J, Wieskötter B, Oeckenpöhler S (2019) Anatomy and pathobiomechanics of the scaphoid. Unfallchirurg 122(3):170–181. https://doi.org/10.1007/s00113-018-0597-1
Gelberman RH, Menon J (1980) The vascularity of the scaphoid bone. J Hand Surg Am 5(5):508–513. https://doi.org/10.1016/S0363-5023(80)80087-6
Steinmann SP, Adams JE (2006) Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci 11(4):424. https://doi.org/10.1007/S00776-006-1025-X
Kawamura K, Chung KC (2008) Treatment of scaphoid fractures and nonunions. J Hand Surg Am 33(6):988–997. https://doi.org/10.1016/J.JHSA.2008.04.026
Kazuteru D, Tatsunori O, Tan SH, Nanda V (2000) Free vascularized bone graft for nonunion of the scaphoid. J Hand Surg Am 25(3):507–519. https://doi.org/10.1053/JHSU.2000.5993
Jones DB, Bürger H, Bishop AT, Shin AY (2009) Treatment of scaphoid waist nonunions with an avascular proximal pole and carpal collapse. Surgical technique. J Bone Joint Surg Am 91(Suppl 2):169–183. https://doi.org/10.2106/JBJS.I.00444
Shakir I, Okoroafor UC, Panattoni J (2018) Clinical and radiologic outcomes of the matti-russe technique for scaphoid nonunions in pediatric patients. Hand (N. Y) 14(1):73–79. https://doi.org/10.1177/1558944718797340
Russe O (1960) Fracture of the carpal navicular. Diagnosis, non-operative treatment, and operative treatment. J Bone Joint Surg 42-A:759–768. https://doi.org/10.2106/00004623-196042050-00002
Penteado FT, dos Santos JBG, Caporrino FA, de Moraes VY, Belloti JC, Faloppa F (2012) Scaphoid nonunion advanced collapse classifications: a reliability study. J Hand Microsurg 4(1):12. https://doi.org/10.1007/S12593-012-0062-2
Slutsky DJ (2013) Outcomes assessment in wrist surgery. J Wrist Surg 2(1):1. https://doi.org/10.1055/S-0033-1333892
Free online Mayo Wrist Score calculator-OrthoToolKit. https://orthotoolkit.com/mayo-wrist/. Accessed 17 Dec 2022
Bervian MR, Ribak S, Livani B (2015) Scaphoid fracture nonunion: correlation of radiographic imaging, proximal fragment histologic viability evaluation, and estimation of viability at surgery: Diagnosis of scaphoid pseudarthrosis. Int Orthop 39(1):67–72. https://doi.org/10.1007/S00264-014-2579-4/TABLES/3
Korompilias AV, Lykissas MG, Kostas-Agnantis IP, Gkiatas I, Beris AE (2014) An alternative graft fixation technique for scaphoid nonunions treated with vascular bone grafting. J Hand Surg Am 39(7):1308–1312. https://doi.org/10.1016/J.JHSA.2014.04.021
Schormans PMJ, Brink PRG, Poeze M, Hannemann PFW (2018) Angular stable miniplate fixation of chronic unstable scaphoid nonunion. J Wrist Surg 7(1):24. https://doi.org/10.1055/S-0037-1603202
Adams JD, Leonard RD (1928) Fracture of the carpal scaphoid. N Engl J Med 198(8):401–404. https://doi.org/10.1056/NEJM192804121980803
M H (1936) Uber die behandlung der navicularfracture und der refractura patellae durch Plombierung mit spongiosa. Zentralbl Chir, 1936, [Online]. https://scholar.google.com/scholar_lookup?journal=Zentralbl+Chir&title=Uber+die+behandlung+der+navicularfracture+und+der+refractura+patellae+durch+Plombierung+mit+spongiosa&author=H+Matti&volume=64&publication_year=1937&pages=2353-59&
Hooning JF, van Duyvenbode LC, Keijser EJH, Obermann WR, Rozing PM (1991) Pseudarthrosis of the scaphoid treated by the Matti-Russe operation. A long-term review of 77 cases. J Bone Joint Surg Br Vol 73-B(4):603–606. https://doi.org/10.1302/0301-620X.73B4.2071643
Stark A, Broström LÅ, Svartengren G (1989) Surgical treatment of scaphoid nonunion-review of the literature and recommendations for treatment. Arch Orthop Trauma Surg 108(4):203–209. https://doi.org/10.1007/BF00936202
Long-term results after Russe bone-grafting: the effect of m... : JBJS. https://journals.lww.com/jbjsjournal/Abstract/1992/74080/Long_term_results_after_Russe_bone_grafting__the.12.aspx. Accessed 17 Nov 2022
Parkinson RW, Hodgkinson JP, Hargadon EJ (1989) Symptomatic non-union of the carpal scaphoid: Matti-Russe bone grafting versus Herbert screw fixation. Injury 20(3):164–166. https://doi.org/10.1016/0020-1383(89)90090-9
[Treatment results in fractures and pseudarthrosis of the scaphoid bone] - PubMed. https://pubmed.ncbi.nlm.nih.gov/689506/. Accessed 17 Nov 2022
Zoubos AB, Triantafyllopoulos IK, Babis GC, Soucacos PN (2011) A modified matti-russe technique for the treatment of scaphoid waist non-union and pseudarthrosis. Med Sci Monit 17(2):MT7–MT12. https://doi.org/10.12659/MSM.881376
Meisel E, Seal A, Yao CA, Ghiassi A, Stevanovic M (2017) Management of scaphoid nonunion with iliac crest bone graft and K-wire fixation. Eur J Orthop Surg Traumatol 27(1):23–31. https://doi.org/10.1007/S00590-016-1876-6
Pinder RM, Brkljac M, Rix L, Muir L, Brewster M (2015) Treatment of scaphoid nonunion: a systematic review of the existing evidence. J Hand Surg Am 40(9):1797-1805.e3. https://doi.org/10.1016/J.JHSA.2015.05.003
Raju PK, Kini SG (2011) Fixation techniques for non-union of the scaphoid. J Orthop Surg (Hong Kong) 19(1):80–84. https://doi.org/10.1177/230949901101900119
Reigstad O, Thorkildsen R, Grimsgaard C, Reigstad A, Røkkum M (2010) Healing of ununited scaphoid fractures by Kirschner wires and autologous structural bone grafts. J Plast Surg Hand Surg 44(2):106–111. https://doi.org/10.3109/02844310903528663
Kirkham SG, Millar MJ (2012) Cancellous bone graft and Kirschner wire fixation as a treatment for cavitary-type scaphoid nonunions exhibiting DISI. HAND (N. Y) 7(1):86. https://doi.org/10.1007/s11552-011-9375-z
Amin SN, Mansour AM, Abdelaal HM, Moustafa AS (2021) Operative treatment of scaphoid nonunion. Cairo Univ 89(5):2023–2033
Dinah AF, Vickers RH (2007) Smoking increases failure rate of operation for established non-union of the scaphoid bone. Int Orthop 31(4):503. https://doi.org/10.1007/S00264-006-0231-7
Porter SE, Hanley EN (2001) The musculoskeletal effects of smoking. J Am Acad Orthop Surg 9(1):9–17. https://doi.org/10.5435/00124635-200101000-00002
Konstantinidis I et al (2022) The influence of smoking on healing of scaphoid non-union after a vascularized pedicle bone flap operation: a review and meta-analysis. Orthop Rev (Pavia). https://doi.org/10.52965/001C.35446
Ramamurthy C, Cutler L, Nuttall D, Simison AJM, Trail IA, Stanley JK (2007) The factors affecting outcome after non-vascular bone grafting and internal fixation for nonunion of scaphoid. J Bone Jt Surg Ser B 89(5):627–632. https://doi.org/10.1302/0301-620X.89B5.18183/ASSET/IMAGES/LARGE/18183-3.JPEG
Barton NJ (1997) Experience with scaphoid grafting. J Hand Surg Br 22(2):153–160. https://doi.org/10.1016/S0266-7681(97)80051-4
Acknowledgements
The author would like to thank the nursing team at the orthopedic clinic who facilitate patients meeting for follow-up.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AK described the new modified approach, wrote the introduction and the surgical technique. MNA wrote the results, discussion, conclusion, and helped in the review process. KM, AA, MA, LH, HR perform the data collection and the patient follow-up. MSA perform the analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Appropriate Institutional Review Board (IRB) for this study was obtained by the Jordan University Hospital Medical Research Office, IRB number (Approval number 2018–2019/3).
Informed consents
Appropriate informed consents were obtained from all participants of the study. The Code of Ethics of the World Medical Association (Declaration of Helsinki) was followed while conducting the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khanfar, A., Alswerki, M.N., Mousa, K. et al. Scaphoid nonunion: a novel modification of Matti-Russe technique with enhanced recovery and full clinical and radiographic union. Eur J Orthop Surg Traumatol 34, 459–468 (2024). https://doi.org/10.1007/s00590-023-03676-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-023-03676-x