Abstract
Purpose
The three most commonly used autografts for anterior cruciate ligament reconstruction (ACL) are: bone–patellar tendon–bone (BTB), hamstring tendons (HT), and quadriceps tendon (QT). A cadaveric study was performed to determine if there were any differences in mechanical and structural properties under biomechanical testing.
Methods
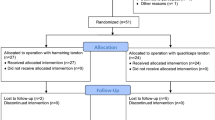
Twenty-seven graft specimens were harvested from 9 human cadaveric legs. Mean donor age was 75.2 years (range 53–85 years). Twenty-two specimens (8 HT, 7 QT, and 7 BTB) completed cyclic preconditioning from 50 to 800 N for 200 cycles and a load to failure test at an extension rate of 1 mm/s. Structural and mechanical properties of BTB, HT, and QT grafts were compared using a one-way ANOVA and Tukey’s honest significant difference.
Results
There was no difference in the ultimate load to failure (N) across all 3 graft types (p = 0.951). Quadriceps tendon demonstrated greater cross-sectional area (mm2) when compared to both HT and BTB (p = 0.001) and was significantly stiffer (N/mm) than HT but not BTB (p = 0.004). Stress (N/mm2) of the HT at ultimate load was greater than QT but not BTB (p = 0.036). Elastic modulus (MPa) of HT was greater than both QT and BTB (p = 0.016).
Conclusion
There was no difference in the ultimate load to failure of BTB, HT, and QT grafts harvested from the same specimens. All 3 grafts had similar loads to failure with a significant increase in stiffness when compared to the native ACL. Furthermore, QT demonstrated more favourable structural properties compared to HT and BTB with greater cross-sectional area to both HT and BTB and greater stiffness compared to HT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) reconstruction is one of the most commonly performed orthopaedic procedures [1, 2]. The choice of autograft for ACL reconstruction is often influenced by surgeon preference and patient characteristics but continues to be extensively studied and subject to debate.
Historically, bone–patellar tendon–bone (BTB) had been considered the gold standard autograft for ACL reconstruction. While its popularity has decreased more recently, it continues to remain a popular graft choice, especially within the USA [3, 4]. Concerns regarding persistent anterior knee pain, patellar fracture, and patellar tendon rupture have led some to prefer other graft options [5,6,7,8].
Hamstring tendon (HT) autograft is a commonly used graft choice worldwide with proponents pointing to less donor site morbidity and avoidance of extensor mechanism disruption with similar outcomes and re-rupture rates when compared to BTB [9,10,11]. However, variability in graft size, graft truncation during harvest, and increased re-ruptures rates in younger highly active individuals remain problematic [12,13,14].
More recently, quadriceps tendon (QT) has re-emerged as a viable alternative graft option [15]. Initially introduced by Marshall et al. [16] in 1979, it fell out of favour after Noyes et al. [17] noted graft relative weakness when compared to BTB in a biomechanical study. However, further biomechanical studies a decade later demonstrated improved biomechanical properties of QT when compared to BTB [18, 19]. The advantages of a more dependable graft size with less donor site morbidity to BTB and comparable outcomes have led to a growing enthusiasm for use of QT for primary ACL reconstruction [5, 15, 20].
There have been multiple biomechanical studies examining the various properties of BTB, HT, and QT as graft options, but to our knowledge, there have been no studies with direct mechanical and biomechanical comparisons between BTB, HT, and QT harvested from the same cadaveric knee. The use of grafts harvested from the same knee adds better controls of donor demographics (age, sex, side-to-side differences, etc.) across all study groups.
The purpose of this study was to compare the biomechanical properties and ultimate load to failure of 3 commonly used graft options, BTB, HT, and QT, for which all were prepared from the same cadaveric specimens. Our hypothesis is there will be no difference in ultimate load between graft types.
Methods
Nine fresh-frozen (− 20 °C) human cadaveric knee specimens (mid-femur to mid-tibia) were utilized from a single source, Science Care Inc. (Phoenix, AZ). Specimen information included age, height, weight, sex, race, and cause of death. Each specimen was inspected for any signs of bone or soft tissue disorder that would exclude the graft from analysis.
Graft preparation
Hamstring (HT) graft: An open-ended tendon stripper was utilized followed by release of the tendons from their tibial attachment. The remaining muscle was removed and both ends of the tendons were whipped stitched to each other with a No. 2 FiberWire (Arthrex, Naples, FL) and looped to create a 4-strand HT graft.
Quadriceps (QT) graft: A harvest knife was used (10 mm width and 7 mm depth) to incise the central portion of the tendon starting distally at the level of superior pole of patella. A grasping suture was placed in the free end of the QT. Metzenbaum scissors were used to dissect proximally along the tendon trying to ensure a partial thickness graft was taken. A No. 2 FiberWire (Arthrex, Naples, FL) was utilized to whip-stitch each end of the graft.
Bone–patellar tendon–bone (BTB) graft: The central third of the tendon (10 mm) was marked and cut in-line with its fibres. A bone block of 10 × 25 mm was obtained from the tibia and a 10 × 20 mm bone block from the patella was obtained with combination of an oscillating saw and osteotome (Fig. 1).
Mechanical loading protocol
A custom soft tissue cryo-clamp was used to secure the grafts to the materials testing machine (ElectroPuls E10000, Instron, Norwood, MA) at both the distal and proximal ends for the QT and BTB grafts. HT grafts were secured to the testing machine using a similar method described previously [21] in which the HT were folded over a pin with the free ends pre-tensioned with a 453.6 g mass on each end. The masses were removed after the graft was secured to the clamp (Fig. 2).
The clamps were tightened with a torque wrench and the grafts were allowed to freeze for 3 min before the testing procedure began [22, 23]. The testing procedure began with a preconditioning step of cyclic loading from 50 to 800 N at 0.5 Hz for 200 cycles. The grafts were then loaded to failure at a rate of 1 mm/s. This loading protocol has been previously described by Staubli et al.[19].
To simulate the intra-articular length of the ACL, an initial clamp-to-clamp distance of approximately 30 mm was used for testing [21]. In the case of the HT grafts, the clamp-to-pin distance was approximately 60 mm. Variables analysed included; cyclic elongation (mm), linear stiffness (N/mm), ultimate load (N), ultimate stress (N/mm2), ultimate strain (%), elastic modulus (MPa) and cross-sectional area (mm2). Cyclic elongation was defined as the change in crosshead position from the bottom of the last cycle relative to the first cycle. Linear stiffness (N/mm) was calculated as the slope of the linear portion of load–displacement plots. Similarly, elastic modulus (MPa) was calculated as the slope of the linear portion of stress–strain plots. Ultimate load (N) was the peak load recorded during the load to fail portion of the loading procedure. Ultimate stress (N/mm2) was the ultimate load divided by cross-sectional area of the graft. Strain was calculated as grip-to-grip strain using the extension of the crosshead of the testing machine divided by initial graft clamp-to-clamp and clamp-to-pin lengths. Cross-sectional area was defined as the product of the thickness and width of the graft measured with a digital caliper while the graft was under 50 N tension (the low end of cyclic loading).
Statistical analysis
Mechanical and structural properties for each graft type were compared with one-way ANOVA and Tukey’s honest significant difference using SPSS statistical software version 24 (SPSS Inc., Chicago, IL). Statistical significance was set at p < 0.05. An a priori calculation determined a sample size of 9 specimens in each group was adequate to detect a difference of 500 N while achieving a 1 − β of 0.83 assuming a standard deviation of 400 N in the primary outcome measure of ultimate load.
Results
Of the 27 grafts that were harvested and prepared, 22 were used for final analysis. Five grafts (HT; 1, QT; 2, BTB; 2) had to be discarded due to an error with the load cell, fixation mechanism during testing, or failure during cyclic loading. Failures during cyclic loading were influenced by small tendon thickness (< 5 mm). The mean age of the donors was 75.2 years (range 53–84).
The ultimate load (N) to failure did not demonstrate any significant differences between all 3 grafts. Stiffness (N/mm) of QT (672 ± 210) was significantly greater than HT (397 ± 91) but not BTB (543 ± 73) (p = 0.004). Hamstring tendon (557 ± 305) was noted to have a significantly greater elastic modulus (MPa) than both QT (269 ± 72) and BTB (297 ± 65) (p = 0.016). Stress (N/mm2) at ultimate load was greater in HT (44.3 ± 16.8) than QT (26.5 ± 8.6) but not BTB (34.6 ± 8.2) (p = 0.036). Quadriceps tendon (81.4 ± 19.2) demonstrated a significantly larger cross-sectional area (mm2) than both HT (49.1 ± 12.2) and BTB (61.8 ± 8.3) (p = 0.001). Complete breakdown of structural and mechanical properties can be found in Table 1. Averaged load–displacement and stress–strain behaviour are illustrated in Figs. 3 and 4, respectively.
The modes of graft failure observed were tearing initiated in the tendon mid-substance, tearing initiated at the insertion into the clamp, and fracture of the bone end in BTB grafts (Fig. 5). The majority of graft failures within all 3 grafts were noted to be insertion tears. Mid-substance tears occurred in only 2 HT, 1 QT, and no BTB grafts. Fracture of the bone end was noted to occur in 3 of the BTB grafts. No grafts were noted to have slipped out of the clamp during testing. Breakdown of graft failure can be seen in Table 2.
Discussion
QT grafts demonstrated favourable structural properties to HT and BTB grafts in terms of a significantly greater cross-sectional area when compared to both HT and BTB and a significantly higher stiffness than HT but not BTB. No difference in ultimate load to failure was noted between grafts and all demonstrated similar load to failure as that of the native ACL (2,160 ± 157 N) [24]. Stress was significantly greater in HT than QT in the current study. Clinically, ultimate load to failure is often considered the most important biomechanical property as it represents the ability of a graft to withstand the anticipated load that initially caused injury [17, 25,26,27].
There has been wide variability in the reported ultimate load to failure of HT that can likely be attributed to variable biomechanical testing protocols as well preparations of HT graft from single to doubled to quadrupled to most recently 6-stranded graft preparations [21, 27,28,29]. The ultimate load to failure within the current study was 2046 ± 455 N which is similar to that reported by Wilson et al. [30] using a similar 4-stranded graft. The failure load of a 4-stand graft has also been reported as high as 4590 ± 674 N [21]. What is becoming evident is increasing graft size leads to increased biomechanical properties within HT. Significant differences in ultimate load with increasing graft diameter have been reported [29]. A graft diameter larger than 9 mm demonstrated a load to failure of 4360 ± 606 N [29]. More recent biomechanical studies compared the properties of a 6-stranded HT graft to QT and reported an ultimate load to failure of 2641 ± 662 N and was also found to be significantly stiffer than a soft tissue QT (1,148 ± 339 vs. 809 ± 173 N/mm) [27].
The ultimate load to failure of a 10 mm wide BTB was 2129 ± 521 N in the current study. Similar-sized grafts have been reported to have an ultimate load of 2977 N [31]. The ultimate load of 13 mm wide grafts was found to be 3424 N [32] and 2900 N for 13.8 mm wide grafts [17]. In all 3 previously mentioned studies, the method of fixation was different from the current study as the bone blocks were anchored to the tensile testing machine by encasing them within materials that cured around them. This method of fixation exposes the graft to potentially fail by bony avulsion but does seem to provide strong fixation to the machine as evidenced by the reported ultimate loads. This method also reduces the potential of applying too much pressure to the tissue at the fixation point as is the risk with using clamps. Studies that have used clamps as a means of fixing BTB bone ends to the testing machine have reported ultimate loads of 1,580 ± 479 N with a cryo effect [33] and 413.3 ± 120.4 N without a cryo effect [34].
Within this study, the ultimate load to failure of an all soft tissue QT was 2097 ± 567 N. This is consistent with other recently reported values [22, 25, 27]. Proponents of QT graft for ACL reconstruction argue QT provides a thicker graft with more favourable tensile properties when compared to HT and BTB [18, 19, 25]. Our study reiterates some of these findings as QT was found to be have a significantly larger cross-sectional area than both HT and BTB (81.4 vs. 49.1 vs. 61.8 mm2; p = 0.001). Some argue the larger cross-sectional area increases the collagen content and may help mitigate the windshield wiper and bungee effect as well as tunnel-graft mismatch that may be seen with BTB due to its thin and flat morphologic shape that may result in inflow of synovial fluid and cytokines leading to bone resorption [5, 35, 36]. The ability to predict a graft size is crucial in planning ACL reconstruction. Historically, BTB was considered most predictable when considering graft volume but recently QT has also been shown to demonstrate similar predictability [37].
Stiffness represents the resistance of a structure to deformation [38]. All grafts within this study were considerably stiffer than the reported stiffness of the native ACL [24]. QT demonstrated a significantly increased linear stiffness when compared to HT (672 ± 210 vs. 397 ± 91 N/mm; p = 0.004) in the current study. Conversely, Urchek et al. [27] when testing of a thicker 6-stranded HT graft noted a statistically increased stiffness of HT opposed to QT (1,148 ± 339 vs. 809 ± 173 N/mm). Shani et al. [25] noted a significant increase in stiffness of QT when compared to BTB (466 ± 133 vs. 278 ± 75 N/mm). While obtaining a stiffer and stronger graft is felt to be advantageous for ACL reconstruction, the clinical implications of a stiffer graft are still not well elucidated.
The mean age (75.2; range 53–84) of the specimens in this study were considerably older than many of the previously mentioned biomechanical studies. The effect of age and orientation of the femur-ACL-tibia complex has been examined and reported significant decreases in load, stiffness, and energy absorbed with increasing age [24]. The effect of age has also been studied on patellar tendons [32, 39]. Higher ultimate tensile stress in younger specimens (29–50 years) has been seen in one study [39] while no difference in failure load, stress, modulus, or elongation for specimens aged 18–55 has been seen in another [32]. Despite the older age of specimens in this study, the ultimate failure loads were similar to that of native ACLs in patients aged 22 to 35 [24]. Moreover, the ultimate failure loads within this study are similar to other biomechanical studies with younger cadaveric specimens [22, 25, 27, 30].
There were several limitations within this study. Sample size within each group is relatively small which may have led some comparisons in this study to be underpowered. In order to try and minimize variability due to fixation method and allow for comparisons across groups, a single fixation device was used for 3 different graft types. Cryogenic fixation for soft tissue grafts is well described but is not as commonly used for grafts with bone blocks which are typically fixated with bone cement. It may be optimal in future studies to use a unique fixation method for different graft types to achieve the greatest potential peak tensile load. Strains were calculated as the grip-to-grip elongation and may not be indicative of local strains in the region of rupture of the graft. While age has been discussed previously, the age of our specimens should still be considered with interpretation of our results. Lastly, testing of isolated grafts is more representative of the direct postoperative phase prior to any tissue remodelling that may occur which could affect the mechanical properties.
Conclusion
The ultimate load to failure of BTB, HT, and QT grafts within this study was not significantly different between each other. All three grafts had similar loads to failure with a significant increase in stiffness when compared to the native ACL. Furthermore, QT demonstrated favourable structural properties to HT and BTB with an increased cross-sectional area to both HT and BTB along with increased stiffness compared to HT. All 3 grafts would be viable graft options for ACL reconstruction.
Data availability
Data and materials are available upon request.
References
Shelton WR, Fagan BC (2011) Autografts commonly used in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg 19:259–264
Junkin D, Johnson D, Fu F, et al (2009) Knee ligament injuries. In: orthopaedic knowledge update : sports medicine 4. American Academy of Orthopaedic Surgeons, pp 135–154
Marx RG, Jones EC, Angel M et al (2003) Beliefs and attitudes of members of the American academy of orthopaedic surgeons regarding the treatment of anterior cruciate ligament injury. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 19:762–770
Budny J, Fox J, Rauh M, Fineberg M (2017) Emerging trends in anterior cruciate ligament reconstruction. J Knee Surg 30:63–69. https://doi.org/10.1055/s-0036-1579788
Mouarbes D, Menetrey J, Marot V et al (2019) Anterior cruciate ligament reconstruction: a systematic review and meta-analysis of outcomes for quadriceps tendon autograft versus bone-patellar tendon-bone and hamstring-tendon autografts. Am J Sports Med 47:3531–3540. https://doi.org/10.1177/0363546518825340
Bonamo JJ, Krinick RM, Sporn AA (1984) Rupture of the patellar ligament after use of its central third for anterior cruciate reconstruction. A report of two cases. J Bone Joint Surg Am 66:1294–1297
Christen B, Jakob RP (1992) Fractures associated with patellar ligament grafts in cruciate ligament surgery. J Bone Joint Surg Br 74:617–619
Sachs RA, Daniel DM, Stone ML, Garfein RF (1989) Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med 17:760–765
Beasley LS, Weiland DE, Vidal AF et al (2005) Anterior cruciate ligament reconstruction: a literature review of the anatomy biomechanics surgical considerations and clinical outcomes. Anat ACL Reconstr Part 1 Related Basic Clin Sci 15:5–19. https://doi.org/10.1053/j.oto.2004.11.003
Samuelsson K, Andersson D, Karlsson J (2009) Treatment of anterior cruciate ligament injuries with special reference to graft type and surgical technique: an assessment of randomized controlled trials. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 25:1139–1174. https://doi.org/10.1016/j.arthro.2009.07.021
Mohtadi NG, Chan DS, Dainty KN, Whelan DB (2011) Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005960.pub2
Conte EJ, Hyatt AE, Gatt CJ, Dhawan A (2014) Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 30:882–890. https://doi.org/10.1016/j.arthro.2014.03.028
Magnussen RA, Lawrence JTR, West RL et al (2012) Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 28:526–531. https://doi.org/10.1016/j.arthro.2011.11.024
Kvist J, Kartus J, Karlsson J, Forssblad M (2014) Results from the Swedish national anterior cruciate ligament register. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 30:803–810. https://doi.org/10.1016/j.arthro.2014.02.036
Slone HS, Romine SE, Premkumar A, Xerogeanes JW (2015) Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 31:541–554. https://doi.org/10.1016/j.arthro.2014.11.010
Marshall JL, Warren RF, Wickiewicz TL, Reider B (1979) The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop. https://doi.org/10.1097/00003086-197909000-00014
Noyes FR, Butler DL, Grood ES et al (1984) Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am 66:344–352
Harris NL, Smith DA, Lamoreaux L, Purnell M (1997) Central quadriceps tendon for anterior cruciate ligament reconstruction. Part I: morphometric and biomechanical evaluation. Am J Sports Med 25:23–28. https://doi.org/10.1177/036354659702500105
Stäubli HU, Schatzmann L, Brunner P et al (1999) Mechanical tensile properties of the quadriceps tendon and patellar ligament in young adults. Am J Sports Med 27:27–34. https://doi.org/10.1177/03635465990270011301
Ajrawat P, Dwyer T, Whelan D et al (2019) A comparison of quadriceps tendon autograft with bone-patellar tendon-bone autograft and hamstring tendon autograft for primary anterior cruciate ligament reconstruction: a systematic review and quantitative synthesis. Clin J Sport Med Off J Can Acad Sport Med. https://doi.org/10.1097/JSM.0000000000000765
Hamner DL, Brown CH, Steiner ME et al (1999) Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am 81:549–557. https://doi.org/10.2106/00004623-199904000-00013
Hangody G, Szebényi G, Abonyi B et al (2017) Does a different dose of gamma irradiation have the same effect on five different types of tendon allografts? - a biomechanical study. Int Orthop 41:357–365. https://doi.org/10.1007/s00264-016-3336-7
Pearsall AW, Hollis JM, Russell GV, Scheer Z (2003) A biomechanical comparison of three lower extremity tendons for ligamentous reconstruction about the knee. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 19:1091–1096. https://doi.org/10.1016/j.arthro.2003.10.015
Woo SL, Hollis JM, Adams DJ et al (1991) Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med 19:217–225
Shani RH, Umpierez E, Nasert M et al (2016) Biomechanical comparison of quadriceps and patellar tendon grafts in anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 32:71–75. https://doi.org/10.1016/j.arthro.2015.06.051
Butler DL, Noyes FR, Grood ES (1980) Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am 62:259–270
Urchek R, Karas S (2019) Biomechanical comparison of quadriceps and 6-strand hamstring tendon grafts in anterior cruciate ligament reconstruction. Orthop J Sports Med 7:2325967119879113. https://doi.org/10.1177/2325967119879113
Wilson SF, Marks R, Collins N et al (2004) Benefits of multidisciplinary case conferencing using audiovisual compared with telephone communication: a randomized controlled trial. J Telemed Telecare 10:351–354. https://doi.org/10.1258/1357633042602026
Boniello MR, Schwingler PM, Bonner JM et al (2015) Impact of hamstring graft diameter on tendon strength: a biomechanical study. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 31:1084–1090. https://doi.org/10.1016/j.arthro.2014.12.023
Wilson TW, Zafuta MP, Zobitz M (1999) A biomechanical analysis of matched bone-patellar tendon-bone and double-looped semitendinosus and gracilis tendon grafts. Am J Sports Med 27:202–207. https://doi.org/10.1177/03635465990270021501
Cooper DE, Deng XH, Burstein AL, Warren RF (1993) The strength of the central third patellar tendon graft. A biomechanical study. Am J Sports Med 21:818–823. https://doi.org/10.1177/036354659302100610
Flahiff CM, Brooks AT, Hollis JM et al (1995) Biomechanical analysis of patellar tendon allografts as a function of donor age. Am J Sports Med 23:354–358. https://doi.org/10.1177/036354659502300319
Schimoler PJ, Braun DT, Miller MC, Akhavan S (2015) Quadrupled hamstring graft strength as a function of clinical sizing. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc 31:1091–1096. https://doi.org/10.1016/j.arthro.2015.01.013
Cavaignac E, Pailhé R, Reina N et al (2016) Can the gracilis replace the anterior cruciate ligament in the knee? A biomechanical study. Int Orthop 40:1647–1653. https://doi.org/10.1007/s00264-015-3027-9
Han HS, Seong SC, Lee S, Lee MC (2008) Anterior cruciate ligament reconstruction : quadriceps versus patellar autograft. Clin Orthop 466:198–204. https://doi.org/10.1007/s11999-007-0015-4
Hersekli MA, Akpinar S, Ozalay M et al (2004) Tunnel enlargement after arthroscopic anterior cruciate ligament reconstruction: comparison of bone-patellar tendon-bone and hamstring autografts. Adv Ther 21:123–131. https://doi.org/10.1007/bf02850339
Xerogeanes JW, Mitchell PM, Karasev PA et al (2013) Anatomic and morphological evaluation of the quadriceps tendon using 3-dimensional magnetic resonance imaging reconstruction: applications for anterior cruciate ligament autograft choice and procurement. Am J Sports Med 41:2392–2399. https://doi.org/10.1177/0363546513496626
Woo SL, Debski RE, Withrow JD, Janaushek MA (1999) Biomechanics of knee ligaments. Am J Sports Med 27:533–543. https://doi.org/10.1177/03635465990270042301
Johnson GA, Tramaglini DM, Levine RE et al (1994) Tensile and viscoelastic properties of human patellar tendon. J Orthop Res Off Publ Orthop Res Soc 12:796–803. https://doi.org/10.1002/jor.1100120607
Acknowledgements
The authors would like to acknowledge the Orthopaedic Innovation Centre in Winnipeg, Manitoba, Canada, for their assistance with mechanical testing and clamp design and fabrication in this study.
Funding
This study was funded by the University of Manitoba Alexander Gibson Fund and the Pan Am Clinic Foundation. The institution of authors DH, SM, and PM has received general funds for research and education from Arthrex, CONMED Linvatec, and Zimmer Biomet.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the project. DH, TG-D, JL, and PM designed the study. DH conducted the data acquisition. DH and SM participated in the analysis of the data. DH, TG-D, JL, RL, ASE, SM, and PM conducted the data interpretation. DH, TG-D, JL, RL, ASE, SM, and PM drafted and reviewed the manuscript. All of the authors approved the content of the manuscript before the submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
Ethical approval for this study was obtained from the University of Manitoba Research Ethics and Compliance Health Research Ethics Board. Ethics# HS24041(H2020:301).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hart, D., Gurney-Dunlop, T., Leiter, J. et al. Biomechanics of hamstring tendon, quadriceps tendon, and bone–patellar tendon–bone grafts for anterior cruciate ligament reconstruction: a cadaveric study. Eur J Orthop Surg Traumatol 33, 1067–1074 (2023). https://doi.org/10.1007/s00590-022-03247-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-022-03247-6