Abstract
Introduction
Reconstruction of the distal femur in children following resection of bone sarcoma is challenging. The main problem in children is the small size of bone and a possible limb-length discrepancy at the end of skeletal growth secondary to the loss of the physes. We reported the results of a new surgical technique for distal femur reconstruction after bone tumor resection in children.
Material and methods
We analyzed 5 patients with distal femoral sarcomas who underwent intra-articular resection and reconstruction with resurfaced allograft–prosthetic composite at a mean follow-up of 70 months. There were 2 males and 3 females, with a mean age of 10 years (range 8–12) at the time of the diagnosis. All patients were affected by high-grade osteosarcoma. The patients’ medical records were reviewed for clinical and functional outcomes as well as post-operative complications. The functional evaluation of the patients was done at the end of the follow-up using Musculoskeletal Tumor Society scoring system. The minimal follow-up was 24 months.
Results
At the last follow-up, 4 patients were continuously disease-free. We excluded one patient who died of disease secondary to lung metastases 16 months after the surgery. Complications occurred in 2 of 4 patients at 17 months and 24 months, respectively. One patient developed deep infection who required the removal of the original reconstruction and, once the infection was treated, the patient underwent reconstruction with an expandable prosthesis. An allograft fracture occurred in another of the 4 patients at 24 months after the first surgery, thus the original reconstruction was removed and the patient underwent reconstruction with modular prosthesis. In the two patients who retained the original reconstruction at the time of their latest follow-up, the mean implant survival time was 70 months. These patients had an excellent MSTS score (29.5 points) and walked without support or limitations with an active knee range of motion of > 90° and complete active extension of the knee. No degenerative changes of the articular surface of the proximal tibia and the patella were observed at the time of the last follow-up. Growth of the physis of the proximal tibia was observed in all the patients during follow-up and no angular deformity of the joint was observed. The limb discrepancy was 4 cm and 2 cm, respectively.
Conclusions
Resurfaced allograft–prosthetic composite may represent an alternative surgical technique for distal femur reconstruction in children with bone sarcomas. Although its success is limited by high risk of complications, resurfaced allograft–prosthetic composite seems to be a viable option to preserve the bone stock and the physis of the proximal tibia in selected young patients, minimizing a potential limb-length discrepancy at the end of the skeletal growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distal femur is the most common site for bone sarcomas in children [1,2,3,4,5,6]. Surgical reconstructions in children are often complicated by loss of a physis and clinically important leg-length discrepancy [7,8,9]. The physes of the knee usually contribute approximately 70% of the limb’s growth [10]. Reconstructive options following distal femur resection of bone sarcomas include rotationplasty, expandable or fixed-length megaprosthesis, massive bone allograft and allograft–prosthetic composite [1, 10, 11]. The evidence supporting the optimum treatment for limb salvage in children with bone tumor is limited [7]. Megaprosthesis is less technically demanding surgery and provide immediate fixation with possible rapid return to weight-bearing; however megaprosthesis may eliminate the otherwise unaffected physis and aseptic loosening is a major concern [1, 2, 10, 12]. Massive bone allograft is a complex biological reconstruction but offers restoration of bone stock and improved longevity of the reconstruction [3, 13]. The use of massive bone allograft allows the preservation of the adjacent physis, therefore preserving the potential remaining growth of the proximal tibia [3]. The preservation of the proximal tibial physis usually contributes approximately 30% of the limb’s growth [9, 10]. However, in children, the use of massive bone allograft is limited because the small joint size of patients does not allow for acceptable articular congruency with thereby increasing the risk for subchondral collapse or degenerative arthritis [1,2,3, 10]. In addition, massive bone allograft has a relatively high complication rate, such as fracture or nonunion [13]. Allograft–prosthetic composite shares the benefit of the prosthetic and biological reconstructions, restoring bone stock and providing a stable knee; however, it does not preserve the opposite physis, which is particularly important in skeletally immature patients [1, 14]. To obtain and maintain the potential advantage of allograft–prosthetic composite avoiding the problems related to the size of the massive bone allograft and preserving the opposite physis, we used an original surgical reconstruction technique consisting of an unconstrained femoral component cemented in an massive bone allograft, a resurfaced allograft–prosthetic composite (rAPC) [10, 15]. Following distal femur resection, the rAPC spares the proximal tibial physis and articular cartilage, maintains the bone stock of the femur and allows the graft to be adapted to the small knee dimension in children [15]. The purpose of this study was to report intermediate-term results of this original technique for reconstruction of distal femur after resection of bone sarcomas in children.
Materials and methods
We analyzed retrospectively the medical records of five children who underwent reconstruction with rAPC following surgical resection of distal femoral for bone sarcomas. Ethical approval for this study was obtained from the institutional review board of our Institute. There were 2 males and 3 females, with a mean age of 10 years (range 8–12) at the time of the diagnosis. All patients were affected by high-grade osteosarcoma and received neoadjuvant chemotherapy. The inclusion criteria for the indication to use the technical procedure of rAPC were: being a child or young teenager, having a meta-epiphyseal sarcoma of the distal femur, not having a tumor extension into the knee ligaments or capsule, and having a satisfactory clinical response to preoperative chemotherapy. Pre-operatively, all patients were evaluated with antero-posterior and lateral knee view radiographs and Magnetic Resonance imaging in order to assess the extension of the lesion and to exclude any joint contamination. The minimal follow-up was 24 months.
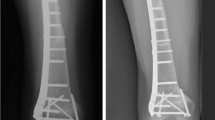
A lateral incision to the distal femur was performed in all patients. Level of osteotomy for bone resection was planned based on radiographs and Magnetic Resonance imaging. The extensor mechanism and menisci were spared, and surgical approach was enhanced by everting the patella. The soft tissue structures of the knee, including the anterior and posterior cruciate ligaments, medial and lateral collateral ligaments, and joint capsule, were dissected close to their femoral insertions. A fresh-frozen allograft was selected preoperatively on the basis of comparative patient and donor imaging and was matched as closely as possible to the host femur. The allograft was prepared for the femoral prosthesis according to the instructions provided by the implant manufacturer. The femoral component of an unconstrained cruciate retaining knee prosthesis was cemented in a massive bone allograft that was fixed to the host femur with a plate (Fig. 1). The first patient was treated in 2013 with a GENESIS II (Smith and Nephew). In the remaining four patients, treated between 2014 and 2018, a resurfacing platform Innex® Gender Solutions™ Primary Knee System (Zimmer) was used. The resurfacing femoral component allowed us to match the size of the articular part of the tibia and to remodel the allograft when the smallest graft was still oversized. A laterally based bridging plate (4.5 or 3.5 mm, depending on the size of the bone) was used to fix the rAPC to the host bone. The plate was long enough to cover the entire length of the allograft and accommodate three or four holes in the host femur. The ligaments and capsule on the allograft were sutured to their counterpart structures on the proximal tibia. The sutures of the most posterior part of the capsule were tied first, followed by those on the medial capsule. The posterior cruciate ligament was sutured to the posterior capsule of the allograft. Finally, sutures on the lateral part of the capsule were tied to close the joint. The sutures were tied with the knee in 45° of flexion. Varus–valgus stability, knee range of motion to confirm full extension, and patellar tracking were checked. The extremities were protected by long-leg casts or braces for 4–6 weeks. After this period, the cast was removed at intervals for the initiation of isometric quadriceps exercises and passive mobilization. At 8–10 weeks after the surgery, patients are kept partial weight-bearing until union is seen radiographically (bridging bone in at least three cortices seen on radiographs). Adjuvant chemotherapy was initiated 7–10 days postoperatively according to the specific protocol being followed. Patients were seen in the outpatient clinic at 4 weeks, 8 weeks and every 3 months until 2 years, every 4 months for the third year, every 6 months until 5 years and yearly thereafter to monitor healing of the graft–host junction and any progressive degenerative changes in tibial counterpart. At the last follow-up, radiographic and functional evaluation of the patients who retained their original rAPC reconstruction were obtained using the Musculoskeletal Tumor Society scoring system (MSTS) [16]. Our main objective of the study was to analyze the survival of reconstructions of children with distal femoral sarcoma treated with rAPC. A successful reconstruction was identified as the survival free from rAPC removal. Our secondary study goals were to analyze the MSTS score after this reconstruction. Therefore, we analyzed the patients’ medical records and radiographic images, searching for complications such as fracture or nonunion of the graft, implant breakage or infection. Radiologic union was assessed using antero-posterior and lateral radiographs of the surgically treated bone: we considered the allograft–host junction to be radiologically healed when the junction line was no longer visible or the junction was bridged with periosteal bone on at least three of the four cortices. Finally, we analyzed functional outcomes and possible deformities or knee instability, limb-length discrepancies and tibial osteoarthritis. The patients were functionally evaluated at the last follow-up using the MSTS score [11].
Results
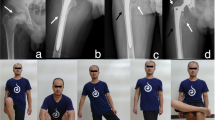
All resection margins were classified as wide. At the last follow-up, 4 patients were continuously disease-free. We excluded one patient who died of disease secondary to lung metastases 16 months after the surgery. In the remaining 4 patients no case of local recurrence or distant metastasis were observed. The overall survival of patients was 80%, with a mean follow-up of 60 months (range 16–99 months). At the time of diagnosis, the mean patient height was 140 cm (range 138–144 cm) and the mean patient weight was 35 kg (range 31–38 kg). Reoperation related to a complication of the rAPC occurred in 2 of 4 patients at 17 and 24 months, respectively. One patient had nonunion of the allograft–host junction and was treated with surgical revision with autogenous bone grafting 17 months after the surgery. Unfortunately, the patient developed deep infection who required two stage revision surgery: the rAPC was removed and replaced with a temporary cement spacer 54 months after the first surgery. Finally, once the infection was treated, the patient underwent reconstruction with an expandable prosthesis 59 months after the first surgery. Following to accidental fall, an allograft fracture occurred in another of the 4 patients at 27 months after the first surgery, thus the rAPC was removed and the patient underwent reconstruction with modular prosthesis. In the 2 remaining patients, complete radiographic healing of the osteotomy was assessed at 12 and 15 months after the first surgery, respectively. The overall implant survival rate was 50%. In the two patients who retained the original reconstruction at the time of their last follow-up, the mean implant survival time was 70 months (range 42–99 months). One of the two patients height and weight was 159 cm and 50 kg, while the other patient height and weight was 158 cm and 62 kg. These patients had an excellent MSTS score (29.5 points) and they walked without support or limitations with an active knee range of motion of > 90° and complete active extension of the knee. No degenerative changes of the articular surface of the proximal tibia and the patella were observed at the time of the last follow-up. Growth at the proximal tibia physis was observed in both of them during follow-up and no angular deformity of the joint was observed. The limb-discrepancy was 4 cm and 2 cm, respectively (Table 1).
Discussion
The options for the reconstruction of distal femur defects following the resection of bone sarcomas in children include biological or prosthetic implants [4, 8]. Various types of reconstructions have been reported, including megaprosthesis, massive bone allograft and allograft prosthesis composite [7]. The main problem in children is the small size of bone [4, 15]. In addition, the reconstruction of the distal femur in children is challenging because surgical resection of tumor in skeletally immature patients is complicated by the loss of a physis, with a resultant potential for clinically relevant limb-length discrepancy at the end of the growth [8, 10, 15]. The rationale of this reported surgical technique was to avoid loss of bone stock, to find an acceptable articular congruency, and to preserve the physis of the proximal tibia. The use of rAPC for reconstruction of the distal femur following resection of bone tumors in children could be an alternative surgical technique to a megaprosthesis or to massive bone allograft. Significant complications seem to be associated with rAPC: two of the four patients had a complication that required removal of the original reconstruction: an infection and a fracture of the graft, respectively. However, the other two patients retained their original reconstruction with good functional outcome and an acceptable limb-length discrepancy at the last follow.
Megaprostheses for reconstruction of the distal femur in children are one of the most used surgical techniques [12]. However, despite innovations in materials and designs, implant failure remains high: infection seems to be the most common cause of failure, followed by aseptic loosening, structural and soft-tissue failure [7, 12, 13]. The rate of revision of megaprostheses ranged from 15.4 to 55% [7]. The type of implant failure seems to be related with the anatomic location: aseptic loosening was more frequent in the distal femur reconstruction with an incidence of 6–13.2% [7, 12]. The rate of infection, structural failure and soft-tissue failure were 8.6%, 2.5% and 1.6%, respectively [7]. In order to address limb-length discrepancy following resection of a physis, expandable prostheses have been developed with an overall rate of limb-length discrepancy of 13.4% [7]. The average MSTS scores ranged from 76 to 82.5% across anatomical sites, with the distal femur reconstructions having 79.1% [7].
Allograft revisions following reconstruction of distal femur were reported from 38.7 to 45% [7, 13]. The most common mode of failure for allografts was structural failure, followed by infection [7]. The rate of infection, structural failure and soft-tissue failure were 12.9%, 22.6% and 3.2%, respectively [7]. Brigman et al. retrospectively reviewed 39 children who underwent reconstruction of distal femur with massive bone allograft following resection of bone sarcomas: allograft survival was 45% at 5 years and 37% at 10 years [13]. Toy et al. analyzed 26 patients who had osteoarticular allograft reconstruction of the distal femur after resection of bone tumors: the overall 5-year and 10-year allograft survival rates were 69% and 63%, respectively [1]. Progressive degenerative change was the most common complication requiring further surgery: 9 patients (35%) had been converted to allograft–prosthetic composites and 5 patients (19%) were converted to megaprostheses [1]. Joint deterioration is considered an inevitable late complication of osteoarticular allografts, but salvage is usually possible with a resurfacing procedure [17]. Attempts to reinforce the subchondral region with cement have been made without improving the results [3, 8]. The average MSTS scores ranged from 73 to 85% across anatomical sites, with the distal femur reconstructions having 75.9% [7, 13, 14].
Allograft–prosthetic composite represents an attempt to address the limitation of megaprosthesis and allograft [8]. The advantages of allograft–prosthetic composite are that the bone stock is replaced and the allograft does not need to be perfectly size matched to the host bone [2]. The rate of revision of allograft–prosthetic composite of the distal femur ranged from 40 to 44% [7, 13]. The most common modes of failure across all anatomic sites were aseptic loosening and infection [7]. Farfalli et al. retrospectively analyzed 45 patients with allograft–prosthetic composite reconstruction following resection of distal femur: the survival of the reconstruction was 73% at 5 years and 48% at 10 years [14]. The average MSTS scores ranged from 71 to 86.8% across anatomical sites, with the distal femur reconstructions having the lowest functional score [7]. Allograft–prosthetic composite is a useful reconstruction technique, but it necessitates the removal of the two physes of the joint, resulting in a possible limb-length discrepancy at the end of the growth.
The ideal treatment of distal femur following bone sarcoma resection in children remains a matter of debate. The literature is lacking in both quality and quantity [7]. The rate of failure of all three type of surgical reconstructions (megaprosthesis, allograft and allograft–prosthetic composite) was high, but data in functional outcome have shown satisfactory results [7].
Our study has several limitations. First, its retrospective nature is a major limitation. Second, it has been difficult to establish how the criteria were established for the indication of the use of rAPC. Third, we had a small series of patients because of the rarity of femur sarcomas treated with this new reconstruction; the outcome of the study could change with a larger patient population. Finally, although the mean follow-up duration was 70 months, with a longer follow-up duration, late complications may occur.
We found that reconstruction with rAPC after distal femur resection for bone tumors may be a reasonable reconstruction option for children with bone sarcomas. The appeal of this new surgical technique lies in the capacity to provide attachment sites for the capsule and tendons, to preserve bone stock, whereas the resurfacing prosthesis provides mechanical support against subchondral collapse and avoid the loss of the physis of the proximal tibia, minimizing a potential limb-length discrepancy at the end of the growth. The hemiarticular reconstruction did not affect the physis of the opposite side of the joint that grew normally during follow-up. Although its success is limited by complications, rAPC seems to be a viable option to preserve the bone stock and the physis of the proximal tibia in selected young patients with bone sarcomas of distal femur. The indication to perform rAPC reconstruction was related to the oncologic indication of an intra-articular resection of the distal femur that could allow the maintenance of capsule and ligaments. Our new technique avoids the mismatching of the distal femur reconstruction to the proximal part of the tibia because it is possible to undersize the allograft, obtaining a proper size for the resurfacing prosthesis. In addition, the rAPC spares the proximal tibial physis and articular cartilage. We consider the present technique an attempt to prolong the functional life of the knee and delay a total knee replacement. In fact, if revision is required after rAPC reconstruction, conversion to allograft–prosthetic reconstruction or megaprosthesis can be performed, which will still occur in non-growing patients. Multicenter studies with a larger number of patients and longer follow-up will be essential to confirm our preliminary results of this new surgical technique.
References
Toy PC, White JR, Scarborough MT et al (2010) Distal femoral osteoarticular allografts: long-term survival, but frequent complications. Clin Orthop 468:2914–2923. https://doi.org/10.1007/s11999-010-1470-x
Mo S, Ding Z-Q, Kang L-Q et al (2013) Modified technique using allograft-prosthetic composite in the distal femur after bone tumor resection. J Surg Res 182:68–74. https://doi.org/10.1016/j.jss.2012.08.012
Campanacci L, Manfrini M, Colangeli M et al (2010) Long-term results in children with massive bone osteoarticular allografts of the knee for high-grade osteosarcoma. J Pediatr Orthop 30:919–927. https://doi.org/10.1097/BPO.0b013e3181fa7981
Errani C, Ceruso M, Donati DM, Manfrini M (2019) Microsurgical reconstruction with vascularized fibula and massive bone allograft for bone tumors. Eur J Orthop Surg Traumatol Orthop Traumatol 29:307–311. https://doi.org/10.1007/s00590-018-2360-2
Tsukamoto S, Mavrogenis AF, Tanzi P et al (2020) Curettage as first surgery for bone giant cell tumor : adequate surgery is more important than oncology training or surgical management by high volume specialized teams. Eur J Orthop Surg Traumatol Orthop Traumatol 30:3–9. https://doi.org/10.1007/s00590-019-02535-y
Angelini A, Mavrogenis AF, Pagliarini E et al (2020) Rare aneurysmal bone cysts: multifocal, extraosseous, and surface variants. Eur J Orthop Surg Traumatol Orthop Traumatol 30:969–978. https://doi.org/10.1007/s00590-020-02640-3
Groundland JS, Ambler SB, Houskamp LDJ et al (2016) Surgical and functional outcomes after limb-preservation surgery for tumor in pediatric patients: a systematic review. JBJS Rev. https://doi.org/10.2106/JBJS.RVW.O.00013
Groundland JS, Binitie O (2016) Reconstruction after tumor resection in the growing child. Orthop Clin N Am 47:265–281. https://doi.org/10.1016/j.ocl.2015.08.027
Angelini A, Baracco R, Dolci A et al (2020) Limb lengthening for deformities in Ollier’s disease: a systematic review. Eur J Orthop Surg Traumatol Orthop Traumatol 30:1325–1332. https://doi.org/10.1007/s00590-020-02692-5
Campanacci L, Alì N, Casanova JMPS et al (2015) Resurfaced allograft-prosthetic composite for proximal tibial reconstruction in children: intermediate-term results of an original technique. J Bone Joint Surg Am 97:241–250. https://doi.org/10.2106/JBJS.N.00447
Errani C, Alfaro PA, Ponz V et al (2021) Does the addition of a vascularized fibula improve the results of a massive bone allograft alone for intercalary femur reconstruction of malignant bone tumors in children? Clin Orthop. https://doi.org/10.1097/CORR.0000000000001639
Henderson ER, Groundland JS, Pala E et al (2011) Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am 93:418–429. https://doi.org/10.2106/JBJS.J.00834
Brigman BE, Hornicek FJ, Gebhardt MC, Mankin HJ (2004) Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop. https://doi.org/10.1097/01.blo.0000127132.12576.05
Farfalli GL, Aponte-Tinao LA, Ayerza MA et al (2013) Comparison between constrained and semiconstrained knee allograft-prosthesis composite reconstructions. Sarcoma 2013:489652. https://doi.org/10.1155/2013/489652
Manfrini M, Donati D, Colangeli M, Campanacci L (2016) Resurfaced allograft-prosthetic composite for proximal tibial reconstruction in children. JBJS Essent Surg Tech. https://doi.org/10.2106/JBJS.ST.15.00010
Enneking WF, Dunham W, Gebhardt MC et al (1993) A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop 241–246
Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M (2006) Use of distal femoral osteoarticular allografts in limb salvage surgery: surgical technique. J Bone Joint Surg Am 88:305–321
Acknowledgements
We thank patients and their families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Errani, C., Tanzi, P., Ferra, L. et al. Resurfaced allograft–prosthetic composite for distal femur reconstruction in children with bone tumor. Eur J Orthop Surg Traumatol 31, 1577–1582 (2021). https://doi.org/10.1007/s00590-021-02995-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-021-02995-1