Abstract
Introduction
Heterotopic ossification (HO) is a known complication after total hip arthroplasty (THA). Radiotherapy is an effective prophylactic treatment for high-risk patients. However, there is no treatment for patients who did not receive prophylactic treatment and subsequently develop HO postoperatively. This study was to determine whether late radiotherapy treatment can prevent the progression of HO following THA.
Methods
A chart review was performed to identify patients who developed HO following THA and were treated with late radiotherapy. All these patients received radiotherapy after their 6- or 12-week postoperative follow-up. Patients were evaluated radiographically pre- and 2 years post-radiotherapy using ImageJ software to measure the difference in the area of HO that formed.
Results
Nine patients with a mean age of 64.5 years were identified. All patients developed HO within 6- or 12-week postsurgery and received later radiotherapy. Eight of the nine hips (89%) treated with late radiotherapy demonstrated no further progression in the amount of bone formed. Overall, there was an increase in the mean total area of HO by 19 mm2 (2%), (p = 0.12).
Conclusion
Late, low-dose radiotherapy is effective in preventing the progression of HO in patients who unexpectedly develop significant HO following THA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterotopic ossification (HO) is characterized by the formation of mature lamellar bone in non-osseous tissues [1]. It is a well-known complication following total hip arthroplasty (THA) with an incidence of 1–2%, in patients with no known risk factors [2,3,4,5]. The HO usually develops between the peri-acetabular region of the pelvis and the proximal femur [6, 7]. It can vary from small bone islands that are clinically asymptomatic to bridging ossifications in the soft tissue that can lead to severe pain and/or stiffness, resulting in functional impairment [8, 9]. Thirty percent of the patients that develop HO following THA have functional impairment, some of which require secondary surgery for its treatment [7, 10, 11].
Several treatment modalities have been developed in order to prevent the formation of HO following THA, including nonsteroidal anti-inflammatory drugs (NSAIDs) and perioperative radiotherapy [6, 12, 13]. Radiotherapy has been shown to be an effective prophylactic treatment to prevent the formation of HO, and in cases where it does form, it is usually minimal and does not lead to any significant clinical impairment [6, 9, 14, 15]. Several studies have shown that radiotherapy treatment is most effective when given within 24 h postoperatively, but can be effective up to 72 h postoperatively [6, 15,16,17]. Treatment after this time frame has been shown to be ineffective [18, 19]. In order to avoid the risk of radiation exposure, radiotherapy treatment is only used in patients that have an extremely high likelihood of developing HO, based on well-established risk factors [13, 15]. However, some patients without any known risk factors will go on to develop HO following their THA. In 2001, the senior author reported a pilot study demonstrating that late radiotherapy was effective in preventing the progression of heterotopic bone formation that occurred 6 weeks following THA [20]. These results have encouraged the continued use of this treatment in cases of substantial HO formation after THA in patients with no known risk factors. The purpose of this study was to evaluate the effectiveness of late radiotherapy in preventing the progression of HO following THA, since the pilot study in 2001. In these patients, we quantified the amount of heterotopic bone formed before and after receiving late radiotherapy and assessed their clinical outcomes.
Materials and methods
We used prospectively collected data from our computerized database to identify the study cohort. Institutional Review Board approval was obtained before the onset of the study. We identified all patients at our institution that underwent a primary or revision THA by a single surgeon (MT), between January 2001 and August 2016. The inclusion criteria were all cases in which the patients developed Brooker II or higher HO early in the postoperative period (by 6–12 weeks), despite not having any risk factors preoperatively such as previous HO, contralateral hip HO, hyperostotic disease, or ankylosing spondylitis, and underwent late radiotherapy treatment [4]. None of these patients underwent any prophylactic treatment for HO preoperatively or immediately postoperatively, and all patients had at least a 2-year follow-up.
Nine hips in nine patients were found to meet the inclusion criteria. All the patients underwent a cementless primary or revision THA through minimally invasive posterior approach. None of the patients were prescribed nonsteroidal anti-inflammatory drugs postoperatively, and pain was managed with opioids and acetaminophen as needed. On their routine 6- or 12-week postoperative follow-up, they were noted to have at least Brooker II HO on their radiographs. These nine hips underwent a standard 7 Gy radiation treatment within 1–2 weeks of their postoperative follow-up visit in which the HO was identified and noted to be at least Brooker II [21]. The dose of 7 Gy was given to the central axis at mid-plane depth, in one treatment. The target volume was the entire joint capsule, including all surrounding periarticular soft tissue with appropriate shielding of the porous-coated acetabular component, the immediately adjacent bone, and the entire pelvic retroacetabular bone stock. On the femoral side, the proximal portion of the porous-coated implant was shielded along with the entire proximal femur, including the lesser and greater trochanters.
Radiographic analysis was done on the digital anteroposterior (AP) hip X-rays, using ImageJ software to determine the difference in the area of HO formed before the radiotherapy treatment and at 2-year follow-up. All AP radiographs were done using a standardized protocol—the central ray was perpendicular to the hip joint and 1–2 inches distal to a point midline between anterior superior iliac spine and symphysis pubis, the leg was internally rotated 15–20, and at least 1 inch of femur below, the femoral stem was included. After calibrating each digital radiograph by using the known size of the femoral head, the imageJ software calculated the area of the newly formed bone in millimeter squared (mm2). The newly formed HO was measured from the acetabulum to the intertrochanteric region. The region(s) of HO on the digital radiograph was drawn manually for the software to calculate the area. In order to increase the accuracy of our measurements, each digital radiograph was measured three times and the average was used as the final area of HO. The measurement error was found to be 5%.
Clinical outcomes including range of motion (ROM) measured using goniometer, Harris Hip Score (HHS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and 36-Item Short Forum survey (SF-36) were compared preoperatively and 2 years following surgery [22]. All statistical analyses were performed using SPSS version 2.0. Statistical analyses were performed by paired t test with p < 0.05 considered statistically significant.
Results
Of the nine patients that were identified to match the inclusion criteria, seven developed HO after their primary cementless THA and two developed HO after their cementless revision THA (Table 1). The hips that underwent a revision THA did not develop HO after their primary procedure. There were two women and seven men with an average age of 65 years (Table 1).
Eight hips developed significant HO by their 6-week postoperative follow-up visit, while one hip demonstrated HO 12 weeks after a primary THA. At 6 weeks postoperatively, five of the eight hips (62%) demonstrated Brooker II HO, while the remaining three hips (38%) had Brooker III HO (Table 2). The one hip at 12 weeks had Brooker III HO. None of the nine hips developed Brooker IV HO post-radiation, and at 2-year follow-up, none of the hips progressed to a higher Brooker grade.
The mean of HO area pre-radiation was 873 mm2, while the mean post-radiation was 892 mm2, showing an increase in the mean total area by 19 mm2 (2%), (p = 0.12). (Table 3). Eight of the nine hips (88%) treated with late radiotherapy demonstrated no further progression in the amount of bone formed (Fig. 1). One patient (patient 6, Table 1) showed an increase in bone formation at 2 years; however, this increase did not change the final Brooker classification and did not result in any loss of motion. Review of this one patient’s radiotherapy planning demonstrated that the region directly above the greater trochanter was inadvertently shielded, allowing HO to progressively form in this region (Fig. 2).
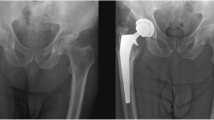
a An anteroposterior (AP) radiograph of the hip of a 48-year-old male 6 weeks following his revision THA demonstrating Brooker II HO formation (patient 8, Table 1). b The AP radiograph of the hip taken 2 years postoperatively demonstrating maturation of the previously identified HO, however, no further progression of the formed HO
a An anteroposterior (AP) radiograph of the hip of a 63-year-old male 6 weeks following his primary THA demonstrating Brooker II HO formation (patient 6, Table 1). b The AP radiograph of the right hip used by the radiotherapist to plan for the radiotherapy treatment. The non-shielded zone that is to be radiated is delineated by hash mark. Note that the region above the tip of the greater trochanter is inadvertently shielded from the radiotherapy. c An AP radiograph of the hip taken 2 years postoperatively demonstrating maturation of the previously identified HO, as well as new bone formation at the greater trochanteric area that was not shielded
Overall, the Harris Hip Score improved from a mean of 60 preoperatively to 96 at 2-year follow-up. The one patient (patient 2, Table 4) with a poor HHS (74) at final follow-up had Brooker III HO, which did not change post-radiotherapy, but did not decrease his hip range of motion (Table 2). Overall, the nine patients (100%) either maintained or improved their ROM (Table 2). The mean WOMAC and S-36 score improved from 44.9 and 46.6 to 2.6 and 52.9 at 2-year follow-up, respectively (Table 4). No patients developed Brooker IV HO, and no patients developed significant pain or limited hip motion from their HO. Moreover, none of the patients developed any apparent side effect following radiotherapy treatment including wound dehiscence, skin complications, implant loosening, or soft tissue sarcoma.
Discussion
This study contradicts the previously held belief that there is no role for radiotherapy in the management of heterotopic ossification more than 72 h after total hip arthroplasty. Although radiotherapy after 72 h has been shown to be ineffective for HO prophylaxis, this study highlights its usefulness in preventing HO progression [6, 13, 16, 19]. We demonstrated that patients who unexpectedly develop substantial HO following their hip arthroplasty can successfully be treated with radiotherapy to prevent significant progression of the already forming HO. In this study, the radiographic analysis at 2-year follow-up demonstrated that one radiation treatment with 7 Gy prevented the progression of heterotopic bone formation in 89% of the patients initially identified with at least Brooker II HO at 6- or 12-week post-THA. The only one hip that demonstrated progression of HO was inadvertently shielded from the radiation in the region where the ossification increased. Overall, the area of the HO that formed between 6 and 12 weeks postoperatively increased by only 2% at 2-year follow-up.
The results of this study are in agreement with the previous pilot study by Kantor et al. [20]. They evaluated eight patients who underwent cementless THA and were irradiated after their 6-week postoperative follow-up visit because they had formed significant HO. None of these patients showed any further progression of HO. These treated patients were then compared to a control group of nine patients who developed significant HO and were untreated. There was progression in the quantity of bone formed in 86% of the untreated patients, compared to only 32% of the treated group. They concluded that late radiotherapy was effective in preventing the progression of HO.
There are some limitations to this study. Firstly, this study includes only a small number of patients. However, the incidence of significant HO in patients without risk factors is low. All patients in the surgeon’s practice with known risk factors for HO are routinely treated with radiotherapy in the first 24 h following THA. As a result, this cohort only represents 0.006% of the patients operated on during this time period. Secondly, there was no control group to see the progression of the HO in this cohort. It is not clear how many of these patients would not have progressed without treatment. However, based on the previous pilot study where the treated group had only a 32% progression in the amount of bone formed compared with 86% in the control group, it was felt to be unethical to withhold radiation treatment [20]. Thirdly, the area was measured manually and not automated, which might affect the accuracy of the measurement. In order to minimize this potential error, we ensured that all the digital radiographs were performed in a standardized fashion and the images were calibrated based on the known femoral head size. In addition, each area was measured three times and averaged to give the final area. The measurement error was found to be 5%. All the measurements were performed by the same person in order to increase the precision and reproducibility of the results. Finally, the HO was only measured on the AP radiograph. Lateral radiographs were reviewed to ensure that no patient had developed Brooker IV HO, but were not analyzed for the area of HO. This is consistent with the Brooker classification which is only evaluated from the AP hip radiograph. However, it does not rule out that there was further progression on the lateral radiograph compared to the AP view.
These findings are contradictory to previous beliefs about the effectiveness of late radiotherapy for HO. However, these studies evaluated the effectiveness of radiotherapy in preventing the formation of HO, and not its role in preventing progression once HO has formed. Previously published studies have shown that early radiotherapy is effective in preventing the formation of HO if given within 72 h postoperatively, whereas radiation after 4 days is ineffective [6, 19]. In a multicenter study of 114 institutions, Seegenschmiedt et al. [18] found that the patients who were treated > 8 h before surgery or > 72 h after surgery experienced a higher radiologic failure rate. One can only speculate why late radiotherapy will not prevent the formation of HO, but once formed, it can prevent its progression. Although the exact mechanism is unknown, it is thought that HO results from the inappropriate differentiation of primitive mesenchymal cells into osteoblastic cells that produce osteoid and end up transforming into bone tissue [23, 24]. Immediately after THA, the trauma response to the surgery results in many actively dividing cells. These actively dividing cells are most sensitive to radiotherapy, but as time progresses, the rapidly dividing cells may become more numerous that the low-dose radiation can effectively deal with. However, by 6 and 12 weeks postoperatively, the trauma response to surgery is greatly diminished, leaving the rapidly dividing cells to create the HO as the most active cell population. This may help explain why radiotherapy treatment so long after the THA can almost eliminate the heterotopic bone growth.
This study confirms that late, low-dose radiotherapy is effective in preventing the progression of HO in patients who unexpectedly develop significant HO following THA. Further studies, perhaps a multicenter trial, are required to determine the time frame in which this late treatment is effective.
References
Kaplan FS, Glaser DL, Hebela N, Shore EM (2004) Heterotopic ossification. J Am Acad Orthop Surg 12(2):116–125
Neal B, Gray H, MacMahon S, Dunn L (2002) Incidence of heterotopic bone formation after major hip surgery. ANZ J Surg 72(11):808–821
Soballe K, Christensen F, Kristensen SS (1988) Ectopic bone formation after total hip arthroplasty. Clin Orthop Relat Res 228:57–62
Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr. (1973) Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am 55(8):1629–1632
Aljurayyan A, Tanzer D, Tanzer M (2016) Acute revision hip arthroplasty: a previously unrecognized risk factor for heterotopic ossification. Eur J Orthop Surg Traumatol 26(2):183–188. https://doi.org/10.1007/s00590-015-1733-z
Gregoritch SJ, Chadha M, Pelligrini VD, Rubin P, Kantorowitz DA (1994) Randomized trial comparing preoperative versus postoperative irradiation for prevention of heterotopic ossification following prosthetic total hip replacement: preliminary results. Int J Radiat Oncol Biol Phys 30(1):55–62
MacLennan I, Keys HM, Evarts CM, Rubin P (1984) Usefulness of postoperative hip irradiation in the prevention of heterotopic bone formation in a high risk group of patients. Int J Radiat Oncol Biol Phys 10(1):49–53
Fransen M, Neal B (2004) Non-steroidal anti-inflammatory drugs for preventing heterotopic bone formation after hip arthroplasty. Cochrane Database Syst Rev 3:Cd001160. https://doi.org/10.1002/14651858.cd001160.pub2
Noel G, Deutch E, Feuvret L, Mazeron JJ (1998) Prevention of heterotopic ossification about the hip: final results of two randomized trials in 410 patients using either preoperative or postoperative radiation therapy. Cancer Radiother 2(3):314–315
Thomas BJ (1992) Heterotopic bone formation after total hip arthroplasty. Orthop Clin North Am 23(2):347–358
Ritter MA, Vaughan RB (1977) Ectopic ossification after total hip arthroplasty. Predisposing factors, frequency, and effect on results. J Bone Joint Surg Am 59(3):345–351
Coventry MB, Scanlon PW (1981) The use of radiation to discourage ectopic bone. A nine-year study in surgery about the hip. J Bone Joint Surg Am 63(2):201–208
Board TN, Karva A, Board RE, Gambhir AK, Porter ML (2007) The prophylaxis and treatment of heterotopic ossification following lower limb arthroplasty. J Bone Joint Surg Br 89(4):434–440. https://doi.org/10.1302/0301-620x.89b4.18845
Vavken P, Castellani L, Sculco TP (2009) Prophylaxis of heterotopic ossification of the hip: systematic review and meta-analysis. Clin Orthop Relat Res 467(12):3283–3289. https://doi.org/10.1007/s11999-009-0924-5
Balboni TA, Gobezie R, Mamon HJ (2006) Heterotopic ossification: pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys 65(5):1289–1299. https://doi.org/10.1016/j.ijrobp.2006.03.053
Koelbl O, Seufert J, Pohl F, Tauscher A, Lehmann H, Springorum HW, Flentje M (2003) Preoperative irradiation for prevention of heterotopic ossification following prosthetic total hip replacement results of a prospective study in 462 hips. Strahlenther Onkol 179(11):767–773. https://doi.org/10.1007/s00066-003-1088-y
Kolbl O, Knelles D, Barthel T, Raunecker F, Flentje M, Eulert J (1998) Preoperative irradiation versus the use of nonsteroidal anti-inflammatory drugs for prevention of heterotopic ossification following total hip replacement: the results of a randomized trial. Int J Radiat Oncol Biol Phys 42(2):397–401
Seegenschmiedt MH, Makoski HB, Micke O (2001) Radiation prophylaxis for heterotopic ossification about the hip joint—a multicenter study. Int J Radiat Oncol Biol Phys 51(3):756–765
Pellegrini VD Jr, Konski AA, Gastel JA, Rubin P, Evarts CM (1992) Prevention of heterotopic ossification with irradiation after total hip arthroplasty. Radiation therapy with a single dose of eight hundred centigray administered to a limited field. J Bone Joint Surg Am 74(2):186–200
Kantor SR, Cummins J, Tanzer M (2005) Complications after total hip arthroplasty: heterotopic ossification. Semin Arthroplasty 16(2):105–113. https://doi.org/10.1053/j.sart.2005.06.001
Hashem R, Tanzer M, Rene N, Evans M, Souhami L (2011) Postoperative radiation therapy after hip replacement in high-risk patients for development of heterotopic bone formation. Cancer Radiother 15(4):261–264. https://doi.org/10.1016/j.canrad.2010.10.003
Harris WH (1969) Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 51(4):737–755
Urist MR, Nakagawa M, Nakata N, Nogami H (1978) Experimental myositis ossificans: cartilage and bone formation in muscle in response to a diffusible bone matrix-derived morphogen. Arch Pathol Lab Med 102(6):312–316
Naraghi FF, DeCoster TA, Moneim MS, Miller RA, Rivero D (1996) Heterotopic ossification. Orthopedics 19(2):145–151
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mina Morcos, Karen Smith, and Michael Tanzer declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Morcos, M., Smith, K. & Tanzer, M. The effect of late radiotherapy on the progression of heterotopic ossification following total hip arthroplasty. Eur J Orthop Surg Traumatol 28, 1125–1131 (2018). https://doi.org/10.1007/s00590-018-2185-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-018-2185-z