Abstract
Purpose
This study aims to clarify the effect of intra-articular platelet-rich plasma (PRP) in total knee arthroplasty (TKA) in preventing postoperative bleeding.
Methods
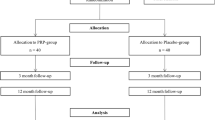
There were 315 knees that underwent TKA and were included in this study. The subjects were randomized by paramedical staffs. These were divided into the PRP group who received intra-articular PRP intraoperatively (n = 109) and the control group who did not (n = 206). We measured postoperative blood loss (drain bag volume), estimated blood loss, and change in hemoglobin (Hb) value at postoperative day 1, 2, 4, and 7. The clinical data were compared between the PRP group and the control group.
Results
The mean postoperative blood loss of 446.9 ± 149.7 mL in the PRP group was significantly less than that in the control group (550.7 ± 178.1 mL, p < 0.001). The mean postoperative estimated blood loss of 437.5 ± 221.3 mL in the PRP group was significantly less than that in the control group (552.2 ± 336.3 mL, p < 0.01). The mean change in Hb value (mg/dL) from baseline was −1.45 in the PRP group and −1.85 in the control group at postoperative 1 day (p < 0.05), −1.74 in the PRP group and −2.11 in the control group at postoperative day 2 (p < 0.05), −2.30 in the PRP group and −2.47 in the control group at postoperative day 4 (p < 0.05), and −1.98 in the PRP group and −2.46 in the control group at postoperative day 7 (p < 0.01).
Conclusion
In this prospective randomized study, those that received PRP after TKA had significantly less postoperative blood loss and change in Hb level. PRP appears to be effective in reducing postoperative bleeding in TKA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) is a commonly performed orthopedic procedure for osteoarthritis, rheumatoid arthritis, and osteonecrosis. TKA is an established procedure that can effectively reduce pain and improve function and quality of life. However, some of the complications accompanying TKA are infection, deep vein thrombosis, and bleeding. A perioperative blood loss of 800–1500 mL is associated with a unilateral TKA [1, 2]. Allogeneic red blood cell transfusion is often needed in routine TKA [2–4]; the transfusion rate in TKA was 11 % in a prospective observational study [1]. Allogeneic blood transfusion may lead to increased postoperative bacterial infection rates [5]. Many methods have been reported to reduce bleeding, including autologous re-transfusion drainage, and administration of intra-articular and intravenous tranexamic acid [2, 6].
Platelet-rich plasma (PRP) is plasma with a high concentration of platelets; PRP is extracted by centrifuging whole blood. PRP contains platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β), which have been associated with the beneficial wound healing cascade [7, 8]. There are many potential clinical applications for PRP; it is used for wound healing in the field of plastic surgery, implant treatment in the field of dentistry, and treatment of lateral elbow tendinosis in orthopedics [9, 10]. Additionally, PRP contains fibrinogen that relates to the coagulation of blood, blood coagulation factor V, adenosine diphosphate (ADP), and thromboxane (TX) A2; it is suggested that these components could potentially reduce bleeding. Some previous studies have reported that the administration of PRP resulted in decreased blood loss, less postoperative pain, and a shorter hospital stay [11–13]. However, other studies support that PRP has not been shown to be effective in TKA [14–16].
The efficacy of PRP for reducing blood loss in TKA is controversial. The purpose of this study is to clarify the effect of PRP in reducing postoperative bleeding after TKA.
Materials and methods
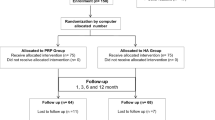
We investigated the clinical course and background variables of patients with osteoarthritis and rheumatoid arthritis who underwent TKA with or without PRP. This was a randomized, single-blinded, prospective clinical study. The subjects were randomized by paramedical staff, and the surgeon was not blinded to the patients’ groups during the TKA procedures. We prospectively enrolled 315 knees that underwent TKA by the same surgeon in our hospital. The exclusion criteria were ankylosed knee and intraoperative lateral patellar release. The patients were divided into the PRP group (n = 109) and the control group (n = 206). The PRP group and the control group had no significant differences in baseline demographics and characteristics (age, sex, disease, body weight, body mass index, preoperative Hb, preoperative extension angle, preoperative flexion angle, preoperative range of motion, and operation time) (Table 1).
The surgery was done via the medial parapatellar approach using the gap-balancing technique and an intraoperative tourniquet. We opened the tourniquet and used cauterization for a short period intraoperatively. All TKA were done using cemented posterior stabilized implants (Optetrak®; Exactech Inc., Florida, USA). PRP was prepared by adding 5 mL of sodium citrate to 55 mL of blood to make 60 mL in total. This solution was centrifuged at 3500 rpm for 15 min. The PRP that sank to the bottom was then collected. In the PRP group, we closely sutured the joint capsule and then injected PRP into the capsule. Subsequently, 0.5 mL thrombin and 0.5 mL calcium chloride were injected into the capsule (Fig. 1). After the operation, drain clamping was done for 1 h in the PRP group and the control group.
Postoperative blood loss (drain bag volume), estimated blood loss, and change in hemoglobin (Hb) value were measured at postoperative day 1, 2, 4, and 7; range of motion was recorded at postoperative day 7 and 14. The clinical data were compared between the PRP group and the control group. Estimated blood loss was calculated as follows: patient’s blood volume = k1 × height (m)3 + k2 × weight (kg) + k3; k1 = 0.3669, k2 = 0.03219, and k3 = 0.6041 for male patients; k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833 for female patients. Estimated blood loss = patient’s blood volume × (preoperative Hct − postoperative Hct) [17]. The postoperative blood loss and estimated blood loss were calculated by paramedical staff.
Univariate analysis was used to compare the PRP group and the control group. The Mann–Whitney U test was used to assess the age, body weight, body mass index, preoperative Hb, preoperative extension angle, preoperative flexion angle, preoperative range of motion, operation time, blood loss, estimated blood loss, and change in Hb. The Fisher exact probability test was used to assess sex and disease. Significance was established at a p value of <0.05.
This study was conducted in compliance with the ethical principles stated in the Declaration of Helsinki and was approved by the ethics committee of Kamagaya General Hospital. All patients provided informed consent.
Results
The mean postoperative blood loss was 446.9 ± 149.7 mL in the PRP group and 550.7 ± 178.1 mL in the control group. The mean postoperative blood loss of the PRP group was significantly less than that of the control group (p < 0.001, Fig. 2).
The mean postoperative estimated blood loss was 437.5 ± 221.3 mL in the PRP group and 552.2 ± 336.3 mL in the control group. The mean postoperative blood loss of the PRP group was significantly less than that of the control group (p < 0.01, Fig. 3).
The mean change in Hb value from baseline was −1.45 mg/dL in the PRP group and −1.85 mg/dL in the control group at postoperative day 1 (p < 0.05), −1.74 mg/dL in the PRP group and −2.11 mg/dL in the control group at postoperative day 2 (p < 0.05), −2.30 mg/dL in the PRP group and −2.47 mg/dL in the control group at postoperative day 4 (p < 0.05), and −1.98 mg/dL in the PRP group and −2.46 mg/dL in the control group at postoperative day 7 (p < 0.01, Fig. 4).
The mean range of motion was 106.1° ± 13.3° in the PRP group and 108.2° ± 15.7° in the control group at postoperative day 7 (p > 0.05), and 118.5° ± 10.8° in the PRP group and 120.1° ± 10.7° in the control group at postoperative day 14 (p > 0.05).
Discussion
Platelets play an important role in wound healing and hemostasis. Treatment with PRP delivers high concentrations of platelets and growth factors (which play a critical role in wound healing) directly to the wound site, which initiates and enhances wound healing [18, 19]. Further, when there is tissue damage with bleeding, von Willebrand factor (vWF) combines with the collagen in the tissue under vascular endothelial cells and megakaryocytes [20]. Glycoprotein (GP)Ia/IX compound in platelet membrane glycoprotein combines with and attaches to vWF and is then activated by the presence of Ca ions, ADP, and TXA2; ADP and TXA2 are contained in the granules that are released by activated platelets. ADP further activates platelets, while TXA2 promotes hemostasis by causing platelet aggregation and vasoconstriction [21, 22]. Further activated platelets produce GPIIb/IIIa complex and combine with fibrinogen; platelets then condensate with one another to form a blood clot and cause primary hemostasis. Secondary hemostasis occurs when fibrin covers the primary blood clot. This secondary hemostasis, or coagulating system, plays a critical role in efficient blood clotting by phospholipid in platelets. PRP is created by centrifuging autologous blood and contains factor V, ADP and TXA2. Clinically, PRP is 4–7 times more effective than standard platelets [23] and is considered to be effective in reducing postoperative bleeding.
PRP usage in TKA has been previously reported. Everts et al. [11] reported that perioperative application of PRP and fibrin sealant may reduce the incidence of allogeneic blood transfusions. They reported that the PRP and fibrin sealant group (n = 85) maintained significantly higher Hb levels than the control group (n = 80) (11.3 vs. 8.9 g/dL; p < 0.001) at postoperative day 1 and had a significantly lower incidence of allogeneic transfusion (0.17 vs. 0.52 units; p < 0.001) [11]. Gardner et al. [12] also reported that the group using PRP (n = 61) had a significantly smaller change in Hb from baseline (−2.68 vs. −3.16 g/dL; p < 0.05) at postoperative day 3, and decreased pain and reduced hospitalization period compared with the control group (n = 37). Meanwhile, Peerbooms et al. [14] reported that 3 months after surgery, the Hb in the PRP group (n = 52) was not different to baseline (−1.58 vs. −1.75 mmol/L; p > 0.05). Horstmann et al. [15] reported that there was no difference in the mean Hb and flexion angle of the PRP group (n = 20) at postoperative day 1 compared with the control group (n = 20).
In our study, the use of PRP significantly reduced postoperative bleeding and significantly suppressed the decrease in Hb at postoperative day 1, 2, 4, and 7. PRP was therefore effective in reducing postoperative bleeding after TKA. This result may have been due to the 1-h drain clamping time after the operation and the larger sample size used in our study. We consider that in the previous studies on PRP in TKA, the PRP may have overflown out from the joint, and therefore the effect of PRP was not manifested; hence, in our study we clamped the drain for 1 h to ensure that the PRP remained in the intra-articular compartment of the knee. The PRP is active by adding thrombin and calcium. The potential beneficial efficacy including reduction of blood loss associated with PRP use may have been due to the growth factors, cytokines, and fibrin products present in the PRP. Moreover, in addition to the growth factors in PRP, TXA2, thrombin and ADP release from platelets. PRP provide a concentrated and directed supply of the growth factor that stimulates the maturation of mesenchymal cells [24]. Collagen aggregation involved in platelet aggregation induced by ADP [25]. These agents lead to earlier hemostasis by enhancing the activity of platelets and forming a platelet plug.
The effect of PRP is a function of many variables, including the platelet concentration and the procedures implemented after injection of PRP. The concentration rate of PRP is increased 3.6 times by single-spinning [26], and 7.9 times by double-spinning [27]. Single centrifugation is simple, but the concentration of the resultant PRP may be low. This study used PRP prepared by single-spinning, so the results using PRP prepared by double-spinning may have been different. However, the role of PRP may not be concentration-dependent, as a previous study reported that the effect of PRP in vitro for neurotrophic function and migration of Schwann cells was not concentration-dependent [28].
PRP is considered to be effective in reducing postoperative bleeding. In the future, it is necessary to determine more effective methods of PRP usage for reducing postoperative blood loss by investigating optimal spin time, concentration rate, volume, and drain clamping time.
Conclusion
The results of this prospective study suggest that PRP was effective in reducing postoperative bleeding after TKA. Future studies are needed to compare the effectiveness of PRP, tranexamic acid, and autologous transfusion in reducing postoperative bleeding. Moreover, we need to investigate more effective methods of PRP preparation and usage.
References
Carling MS, Jeppsson A, Eriksson BI, Brisby H (2015) Transfusions and blood loss in total hip and knee arthroplasty: a prospective observational study. J Orthop Surg Res 10:48. doi:10.1186/s13018-015-0188-6
Horstmann W, Kuipers B, Ohanis D, Slappendel R, Kollen B, Verheyen C (2014) Autologous re-transfusion drain compared with no drain in total knee arthroplasty: a randomised controlled trial. Blood Transfus 12:176–181
Mylod AG Jr, France MP, Muser DE, Parsons JR (1990) Perioperative blood loss associated with total knee arthroplasty. A comparison of procedures performed with and without cementing. J Bone Joint Surg Am 72:1010–1012
Levy O, Martinowitz U, Oran A, Tauber C, Horoszowski H (1999) The use of fibrin tissue adhesive to reduce blood loss and the need for blood transfusion after total knee arthroplasty. A prospective, randomized, multicenter study. J Bone Joint Surg Am 81:1580–1588
Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP (2003) Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma 54:908–914
Whiting DR, Sierra RJ (2015) Efficacy of combined use of intraarticular and intravenous tranexamic acid in total knee arthroplasty. Ann Transl Med 3(Suppl 1):S39. doi:10.3978/j.issn.2305-5839.2015.03.37
Mustoe TA, Pierce GF, Thomason A, Gramates P, Sporn MB, Deuel TF (1987) Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science 237:1333–1336
Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A (1991) Role of platelet-derived growth factor in wound healing. J Cell Biochem 45:319–326
Georgakopoulos I, Tsantis S, Georgakopoulos P, Korfiatis P, Fanti E, Martelli M et al (2014) The impact of Platelet Rich Plasma (PRP) in osseointegration of oral implants in dental panoramic radiography: texture based evaluation. Clin Cases Miner Bone Metab 11:59–66
Ford RD, Schmitt WP, Lineberry K, Luce P (2015) A retrospective comparison of the management of recalcitrant lateral elbow tendinosis: platelet-rich plasma injections versus surgery. Hand 10:285–291
Everts PA, Devilee RJ, Brown Mahoney C, Eeftinck-Schattenkerk M, Box HA, Knape JT et al (2006) Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand 50:593–599
Gardner MJ, Demetrakopoulos D, Klepchick PR, Mooar PA (2006) The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty. An analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop 31:309–313
Berghoff WJ, Pietrzak WS, Rhodes RD (2006) Platelet-rich plasma application during closure following total knee arthroplasty. Orthopedics 29:590–598
Peerbooms JC, de Wolf GS, Colaris JW, Brujin DJ, Verhaar JA (2009) No positive effect of autologous platelet gel after total knee arthroplasty. Acta Orthop 80:557–562
Horstmann WG, Slappendel R, van Hellemondt GG, Wymenga AW, Jack N, Everts PA (2011) Autologous platelet gel in total knee arthroplasty: a prospective randomized study. Knee Surg Sports Traumatol Arthrosc 19:115–121
Morishita M, Ishida K, Matsumoto T, Kuroda R, Kurosaka M, Tsumura N (2014) Intraoperative platelet-rich plasma does not improve outcomes of total knee arthroplasty. J Arthroplasty 29:2337–2341
Sehat KR, Evans RL, Newman JH (2004) Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 86:561–565
Adler SC, Kent KJ (2000) Enhancing wound healing with growth factors. Facial Plast Surg Clin North Am 10:129–146
Anitua E, Andia I, Ardanza B (2004) Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 91:4–15
Ruggeri ZM (2007) The role of von Willebrand factor in thrombus formation. Thromb Res 120:5–9
Mills DC (1996) ADP receptors on platelets. Thromb Haemost 76:835–856
Hanasaki K, Arita H (1988) Characterization of thromboxane A2/prostaglandin H2 (TXA2/PGH2) receptorsof rat platelets and their interaction with TXA2/PGH2 receptor antagonists. Biochem Pharmacol 37:3923–3929
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62:489–496
Sánchez AR, Sheridan PJ, Kupp LI (2003) Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 18:93–103
Vargaftig BB, Chignard M, Benveniste J (1981) Present concepts on the mechanisms of platelet aggregation. Biochem Pharmacol 30:263–271
Eby BW (2002) Platelet-rich plasma: harvesting with a single-spin centrifuge. J Oral Implantol 28:297–301
Kakudo N, Kushida S, Kusumoto K (2009) Platelet-rich plasma: the importance of platelet separation and concentration. Plast Reconstr Surg 123:1135–1136
Zheng C, Zhu Q, Liu X, Huang X, He C, Jiang L et al (2013) Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J Tissue Eng Regen Med. doi:10.1002/term.1756
Authors’ contributions
T. Mochizuki, K. Ikari, T. Shirahata and S. Momohara designed the research. T. Mochizuki, Ryo Hiroshima, K. Kawakami, N. Koenuma and M. Ishibashi participated in the operations and data collection. K. Yano performed the statistical analysis. K. Ikari and S. Momohara participated in the editing and submission of the paper. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. The study sponsors were not involved in the following: study design; collection, analysis, and interpretation of data; writing of the paper; and/or the decision to submit the results for publication.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Mochizuki, T., Yano, K., Ikari, K. et al. Platelet-rich plasma for the reduction of blood loss after total knee arthroplasty: a clinical trial. Eur J Orthop Surg Traumatol 26, 901–905 (2016). https://doi.org/10.1007/s00590-016-1821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-016-1821-8