Abstract
Background
Tranexamic acid (TXA) is well established as a versatile intraarticular and intravenous (IV) antifibrinolytic agent that has been successfully used to control bleeding after total knee arthroplasty (TKA). The present meta-analysis aimed at assessing the effectiveness and safety of TXA in reducing blood loss and transfusion in TKA.
Methods
We searched the PubMed, Medline, Embase, Cochrane Central Register of Controlled Trials, and Google Scholar databases from 1966 to December 2013. Only randomized controlled trials (RCTs) were included in the present study. Two independent reviewers identified the eligible studies, assessed their methodological quality, and extracted data. The data were using fixed-effects or random-effects models with standard mean differences and risk ratios for continuous and dichotomous variables, respectively. Subgroup analysis was performed according to the IV or intraarticular administration of TXA.
Results
Thirty-four RCTs encompassing 2,594 patients met the inclusion criteria for our meta-analysis. Our meta-analysis indicated that when compared with the control group, the IV or intraarticular use of TXA significantly reduced total blood loss, postoperative blood loss, Hb loss, and transfusion rate as well as blood units transfused per patient after primary TKA, but did not reduce intraoperative blood loss. No significant difference in deep vein thrombosis (DVT), pulmonary embolism, or other adverse events among the study groups.
Conclusions
IV or intraarticular use of TXA for patients undergoing TKA is effective and safe for the reduction blood loss and blood transfusion requirements, yet does not increase the risk of postoperative DVT.
Level of evidence
Level II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) is widely acknowledged to be one of the effective treatments for severe osteoarthritis of the knee. However, this surgery is particularly prone to significant intraoperative and postoperative blood loss with long operation times and large wound surfaces. Estimated blood loss reported for TKA varies between 500 and 1,500 mL [1]. Despite the advances in techniques and perioperative management, however, TKA is associated with substantial bleeding [2]. Therefore, considerable blood loss after TKA is still problem, which can increase morbidity [3]. Postoperative blood transfusion may be a life-saving measure in those with hemorrhage, but it carries a substantial risk of transmitting infections (viral and bacterial), hemolytic transfusion reactions, transfusion-related diseases and increases hospital costs [4, 5]. Thus, it is very important to control perioperative bleeding for maintaining hemodynamic stability.
A variety of effective blood-conservation interventions have been used to reduce blood loss and the need for postoperative transfusion, including controlled hypotension, autologous blood transfusion, intraoperative blood salvage, navigation, minimally invasive surgery (MIS), use of tourniquet, and intravenous (IV)I or intraarticular administration of tranexamic acid (TXA) [6–8]. TXA is a synthetic antifibrinolytic drug that competitively blocks the lysine-binding sites of plasminogen, plasmin, and tissue plasminogen activator, thereby delaying fibrinolysis and blood clot degradation [9]. Currently, IV administration of TXA has been widely used in different settings and reduced the need for transfusion in cardiac, orthopedic, cranial and orthognathic, hepatic, and urological surgery [10, 11].
With respect to the IV administration of TXA in TKA patients, it has been reported to reduce blood loss and be safe [12]. However, TXA may be administered intravenously or topically in the surgical wound and different surgeons have their individual plans. Therefore, the optimal TXA treatment protocol in such conditions is still unknown. Besides, a number of clinical studies are conflicting on the effectiveness and safety of TXA [6, 13–21]. Because individual studies might have been underpowered to detect the overall effects and some studies are limited by their sample size and subsequently suffer from too low power to detect effects that may exist, and meta-analysis combining data from many randomized controlled trials (RCTs) is generally considered to provide the strongest evidence of clinical interventions, we deemed it important to perform a quantitative synthesis of the evidence. Therefore, we carried out this meta-analysis to evaluate the safety and efficacy of TXA in the reduction of blood loss in TKA.
Methods
Literature search
We identified RCTs from the previously published systematic review. We updated the list of studies by searching the PubMed, Medline, Embase, Cochrane Central Register of Controlled Trials, and Google Scholar databases. Two authors independently searched for relevant studies from 1966 to December 2013. The search strategy was created with the assistance of a librarian using a combination of terms including antifibrinolytics, tranexamic acid, cyklokapron, total knee arthroplasty, total knee replacement, TKA, TKR, RCT, prospective, meta, review, and random. We limited searches to randomized controlled trials, systematic reviews, and meta-analyses and imposed no language or other limitations. Reference lists of all the selected articles were hand-searched for any additional trials. Authors were contacted when possible to obtain missing information.
Inclusion and exclusion criteria
Studies were included if they met the following three inclusion criteria: (1) The patients underwent unilateral TKA; (2) the study involved the comparison of a TXA treatment group to a control group who received either a placebo or no treatment at all; and (3) the trial was RCT. Exclusion criteria included: (1) RCTs of low quality; (2) simultaneous bilateral primary TKA or revision TKA; (3) the procedure involved was described as minimally invasive or less invasive. Two authors independently assessed the articles for compliance with the inclusion criteria, and disagreement was followed by discussion until consensus was reached.
Selection of the literature
Two authors according to inclusion criteria independently reviewed the title and abstract and excluded the studies that did not meet the inclusion criteria obviously. A full text of any published article that potentially met the inclusion criteria was obtained to confirm. Any disagreements during the selection course were resolved by discussion with a third reviewer.
Methodological quality assessment
Two reviewers assessed the quality of the studies independently; revised Jadad Scale was used to perform the quality assessment. This scale includes the random sequence production (2 points), allocation concealment (2 points), appropriateness of blinding (2 points), and description of dropouts and withdrawals (1 point). The total score is 7 points, 0–3 points means poor quality and 4–7 points means high quality, and consolidated standards on reporting trials (CONSORT) checklist and scoring system were used to evaluate the quality of included trials: Scores of 18–22 are considered excellent study quality, 13–17 good, 8–12 fair, and <7 poor.
Data extraction
All data were extracted independently by two reviewers. The following data were extracted: postoperative venous thromboembolism (VTE), postoperative drainage loss, total blood loss, intraoperative blood loss, rate of patients who had allogeneic blood transfusion, units of transfusion, incidence of deep vein thrombosis (DVT) and/or pulmonary embolism (PE) complications and other adverse events. A consensus method was used to resolve disagreements, and a third reviewer was consulted if disagreements persisted. In order to understand the baseline of each included study, we extracted from trials included the following information: Number of patients enrolled, characteristics of participants, male/female ratio, dose of TXA, method of TXA administration, and transfusion criteria.
Data analysis
For each study, relative risks and 95 % confidence intervals were calculated for dichotomous outcomes, and standard mean differences (SMD) and 95 % confidence intervals were calculated for continuous outcomes. We assessed statistical heterogeneity for each study with the use of a standard Chi-square test with a significance set at a p value of 0.1, and the quantity of heterogeneity was measured by I 2 statistic. An I 2 statistic value of 50 % was considered to indicate substantial heterogeneity. We performed the meta-analysis using a fixed-effect model if no significant heterogeneity was present (I 2 < 50 %; p > 0.1). Otherwise, we adopted the random-effects model. The clinical heterogeneity prevented a direct quantitative meta-analysis; the data would be pooled by subgroup analysis according to the different (IV or topical) administration of tranexamic acid. To investigate whether publication bias might affect the validity of the estimates, funnel plot for transfusion rate (as this was the outcome that most studies included in meta-analysis) was generated to evaluate potential publication bias. Data analyses were performed using STATA version 11.0. A p value of <0.05 was considered statistically significant.
Results
Literature search
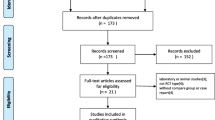
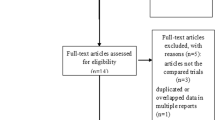
A search of the PubMed, Embase, Cochrane Central Register of Controlled Trials, and Google Scholar databases retrieved 658 articles. By screening the title, reading the abstract, 472 articles were excluded. Then, of the remaining 40 studies, two were excluded for bilateral TKA, two revision TKAs and one computer-assisted TKA. Therefore, a total of 34 studies were included by reading the whole paper.
Study characteristics
These 34 studies [4, 13–45] included a total population of 1,351 participants in the TXA group and 1,241 in control group. Thirty-three studies were published in English, and one study was Chinese. All included trials had compared the baseline preoperatively, and each had similar baseline. More characteristics of the 34 studies are described in Table 1 . All the studies were RCT. TXA was administered intravenously in 25 studies [13, 14, 16, 17, 19, 20, 23–32, 34, 35, 37, 38, 40–42, 44, 45], intraarticularly in seven studies [4, 15, 17, 22, 33, 39, 43]. Two studies [21, 36] reported the results on both IV and intraarticular use of TXA. To prevent DVT, seventeen trials used low molecular weight heparin [4, 13, 15, 16, 24, 27, 28, 30–34, 40, 42–45], three performed a mechanical ankle pump exercise regimen [13, 18, 26], two used aspirin [37, 38], two used compression stockinet and low molecular weight heparin [20, 36], one used bemiparin [23], one used fondaparinux [17] and eight did not mention any preventative measures [19, 21, 22, 25, 29, 35, 39, 41]. Postoperative drainage was quantified between 24 and 48 h, when drains were in most cases removed. The study by Onodera et al. [39], used TXA containing 50 mg of carbazochrome, was also included. The study by McConnell et al. [37], reported in 2012, included a group that received fibrin as well as TXA and control groups, was also included. The study [41] compared four IV bolus methods. Since there were five randomized controlled groups in this study, we regarded it as four independent studies in the following comparative research. Two studies [21, 39] compared efficacies of intraarticular and IV tranexamic acid, which had three independent randomized controlled groups, so we included them and regarded them as two independent studies in this review (Table 1).
Methodological quality
These 34 studies [4, 13–45] were relatively well designed, and the quality assessment score of most was high. For the revised Jadad Scale, no studies were 1–3 points with a poor quality; 34 studies were 4–7 points with a high quality. 35 RCTs were evaluated by the CONSORT checklist and scoring system; four studies were 8–12 scores; 19 studies were 13–17 scores; and 11 studies were 18–22 scores, all the RCTs had satisfied quality. The details are described in Table 2.
Publication bias
The funnel plot based on the studies with data on transfusion rate (as this was the outcome that most studies included in their meta-analysis) demonstrates only minimal asymmetry and a few outliers, indicating minimal publication bias.
Safety of TXA
Twenty-seven of the trials [4, 13, 15–17, 20, 21, 23, 25, 26, 28–32, 34–45] reported on the DVT. Among them, 61 patients in TXA group and 41 in control group developed DVT. The IV subgroup was proved to present higher DVT incidence (6.3 %) compared with the control group (3.8 %), although the difference did not reach levels of statistical significance, (RR = 0.92, p = 0.678, 95 CI 0.62, 1.38). The DVT incidence for the intraarticular subgroup was 3.8 % (12/325) and for the control was 3.6 % (12/329) (RR 1.02, 95 % CI 0.49, 2.15; p = 0.953) with no evidence of heterogeneity (I 2 = 4 %, p = 0.384) (Fig. 1a).
For PE, 15 trials [4, 15–17, 20, 21, 24, 26, 29, 31, 32, 38, 39, 41, 43] including 1,411 patients provided useful data on PE. The incidences of PE in the TXA and control groups were 2 of 705 and 7 of 706 patients, respectively. The rate of PE was not affected by the use of TXA when the TXA group was compared with the control group and evidence showed no heterogeneity (Fig. 1b).
Besides thromboembolic complications, other adverse events including hematoma, ecchymosis, infection and pneumonia et al were found in 16 studies [4, 14, 17, 21, 23–26, 29–32, 37, 38, 41, 42]. The IV subgroup was proved to present lower adverse events incidence (4.1%) compared with the control group (4.3 %), although the difference did not reach levels of statistical significance (RR = 0.96, p = 0.849, 95 CI 0.60, 1.53). Evidence showed no heterogeneity (I 2 = 0 %, p = 0.445). The adverse events incidence for the intraarticular subgroup was 0.78 % (1/129) and for the control was 3.9 % (5/128) (RR 0.2, 95 % CI 0.02, 1.65; p = 0.134) (Fig. 1c).
Transfusion rate
The 27 eligible studies [4, 13–17, 20, 21, 23–32, 34, 36, 38–45] that reported a total of 2,235 patients provided information on transfusion requirements. The transfusion rates for the intraarticular was 7.4 % (24/325) and for the control was 26.4 % (87/329) (RR = 0.35, 95 % CI 0.18, 0.67; p = 0.002) with evidence of heterogeneity (I 2 = 42.3 %, p = 0.109), showing that there was significant difference between the two groups. In the IV subgroup, the pooled analysis also found significant difference in transfusion rates between two groups (RR = 0.40, 95 % CI 0.31, 0.52; p = 0.00). Evidence showed substantial heterogeneity (I 2 = 1.5 %, p = 0.000) (Fig. 2).
Blood units transfused per patient
The units of blood transfused to patients were recorded in 14 trials [14, 15, 23, 25–28, 30–32, 34, 38, 41, 42], including 503 patients in TXA group and 479 patients in control group. In the intraarticular group, there was only one studies reported the data of blood units transfused per patient [15]. This study documented the mean number of transfused units was lower in patients receiving TXA (0 U) than in the control group (0.14 U). In the IV subgroup, the combined SMD for patients undergoing IV administration was found to be −1.14 (p = 0.000; 95 % CI −1.53, −0.76). The pooled analysis indicates that blood units transfused per patient was less in the IV TXA groups in comparison with the control group at a statistically significant level. There was significant heterogeneity between studies (p = 0.000, I 2 = 83.8 %) (Fig. 3).
Total blood loss
Eighteen trials [4, 13, 15, 16, 22–26, 29, 31, 32, 34–36, 38, 39, 44] described total blood loss, including 756 patients in TXA group and 780 patients in control group. In TXA group, five studies [4, 15, 22, 36, 39] provided data on transfusion rate in intraarticular application of TXA and 14 studies [13, 16, 23–26, 29, 31, 32, 34, 35, 38, 39, 44] were IV administration. The combined SMD for patients undergoing intraarticular application was found to be −0.86 (p = 0.000; 95 % CI −1.14, −0.59). This indicates that total blood loss was less in the intraarticular TXA groups in comparison with the control group at a statistically significant level. In the IV subgroup, the use of TXA significantly reduced total blood loss (SMD = −1.01, 95 % CI −1.43, −0.60; p = 0.00). There was a high level of statistical heterogeneity between studies (p = 0.000, I 2 = 92.7 %) (Fig. 4).
Postoperative blood loss
Sixteen trials [4, 13, 14, 17, 20, 21, 23–25, 29, 31–33, 36, 44, 45] involving 1,420 patients described postoperative blood loss. Four studies [4, 20, 21, 36] provided data on postoperative blood loss in intraarticular application of TXA and 14 [13, 14, 17, 20, 21, 23–25, 29, 31–33, 44, 45] were in IV administration. The combined SMD for patients undergoing intraarticular application was found to be −1.32 (p = 0.001; 95 % CI −2.08, −0.55). This indicates that postoperative blood loss was less in the intraarticular TXA groups in comparison with the control group at a statistically significant level. There was heterogeneity between studies (p = 0.000, I 2 = 90.4 %). In the IV subgroup, the use of TXA significantly reduced postoperative blood loss (SMD = −1.11, 95 % CI −1.61, −0.61; p = 0.000). There was a high level of statistical heterogeneity between studies (p = 0.000, I 2 = 92.6 %) (Fig. 5).
Intraoperative blood loss
Intraoperative blood loss was recorded in six trials [15, 20, 24, 31, 32, 44] which included 223 patients in TXA group and 221 patients in control group. In intraarticular subgroup, the combined SMD for patients undergoing intraarticular application was found to be −0.67 (p = 0.098; 95 % CI −1.47, 0.12). This indicates that intraarticular TXA did not significantly reduce intraoperative blood loss. In the IV subgroup, there was no decrease intraoperative blood loss associated with the use of TXA compared with the control group (SMD = −0.14, p = 0.244, 95 CI −0.37, 0.09) (Fig. 6).
Hb loss
Eleven trials [14–17, 20, 21, 25, 26, 29, 38, 39] reported postoperative reduction of Hb level. In intraarticular subgroup, the combined SMD for patients undergoing intraarticular application was found to be −0.65 (p = 0.000; 95 % CI −0.96, −0.35). This indicates that postoperative reduction of Hb level was lower in intraarticular group in comparison with the control group at a statistically significant level. In the IV subgroup, postoperative reduction of Hb level was lower compared with the control group (SMD = −0.85, p = 0.000, 95 CI −1.26, −0.44) (Fig. 7).
Discussion
In this meta-analysis, we assessed the evidence from RCTs that compared outcomes with TXA or not in TKA. The most important findings of our meta-analysis demonstrate a statistically significant benefit for TXA in reducing total blood loss, postoperative blood loss, Hb loss, blood units transfused per patient, and the number of patients receiving allogeneic transfusions in TKA. Only for the intraoperative blood loss, TXA has a trend of reduced intraoperative blood loss, but not statistically significant between TXA and control groups. At the same time, TXA did not appear to increase the risk of thromboembolic complications and other adverse events. We found the effect was even greater or patients in our review.
Our findings were basically consistent with the recent meta-analysis by Tan et al. [46] which included 19 trials, and another systematic review by Zhang et al. [12], which included 15 trials; however, more studies with higher amount cases were included in this analysis. This study was a complete meta-analysis about clinical results in the topical or IV use of TXA for prevention of bleeding associated with TKA procedures. More comprehensive evaluating indicators were discussed in this study which included total blood loss, postoperative blood loss, Hb loss, intraoperative blood loss, and blood units transfused per patient, the number of patients receiving allogeneic transfusions, DVT, PE, and other adverse events. What is more, previous meta-analysis only included 19 RCTs, which intervention was the administration of IV TXA, while our review included 34 RCTs. There had been a growing interest in the intraarticular use of TXA for prevention of bleeding in TKA. So, this study provided a more credible and stable evidence in comparing the effectiveness and safety of TXA in reducing blood loss and transfusion in TKA.
The methodological quality assessment identified some limitations of the current evidence bases. The quality assessment score for most of the studies included was high, which contributes to the strength of point estimates. The majority of included trials were of good methodological quality, they were relatively well designed, and the quality assessment score was high in most of them. There are several issues related to quality control in conducting a meta-analysis, in particular study selection and the homogeneity of the studies. Hence, our study focused on the use of TXA and the data would be pooled by subgroup analysis according to the IV or intraarticular use of TXA. Accordingly, this review of meta-analysis should be considered as appropriate.
Heterogeneity is a potential problem when interpreting the results of the present meta-analysis. In this meta-analysis, notable heterogeneity was observed in some comparisons. Although the overall methodological quality of the included studies was high relatively, some degree of clinical heterogeneity was induced by the following factors: first, clinical heterogeneity may be caused by blood transfusion protocol, surgical technologies of different centers, and the type of surgical hemostasis (i.e., gauzes packing, electrical cauterization). Second, the total blood loss and the amount of transfused blood per person may be affected by the operation time, type of surgery procedure, dose regimen of TXA, different follow-ups etc. Finally, the baseline of patients of individual studies, such as age, preexisting comorbidities, and economic condition, may also be confounding factors, which may exert influence on the stability of the pooled results.
There were several strengths of our review and meta-analysis. First, we performed exhaustive searches of the English and non-English languages literature to limit publication bias and pooled data from 34 manuscripts, including only RCTs. Second, all included studies were assessed rigorously by revised Jadad Scale and CONSORT checklist and scoring system, which indicated that most articles were of high quality. When came to heterogeneity, a random-effects model and sensitivity analysis were performed to control the veracity and stability of pooled results.
Although this study was believed to be the most comprehensive meta-analysis of RCT-based evidence for the safety and efficacy of TXA in the reduction of blood loss in TKA, we acknowledged that this study has some limitations. First, the general lack of random sequence production and allocation concealment methods in the included RCTs made it difficult to assess their methodological quality; thereby, the risk of bias and potential to overestimate the effect may be existent. Second, we did not limit the language in the process of the literature retrieval, but only Chinese- and English-language trials were included in the meta-analysis, publication and potential English language biases may have occurred. Third, the results of the other outcome measures (functional outcome scores, quality of life, length of stay, and cost effective) could not be pooled, either because too few data were available or because the methods varied too much; therefore, no definite conclusions can be drawn from these data.
Conclusion
This meta-analysis demonstrated that intraarticular or intraoperative application of TXA significantly reduced total blood loss, postoperative blood loss, Hb loss and transfusion rate as well as blood units transfused per patient after primary TKA. However, there were no statistically significant differences in reducing intraoperative blood loss. Patients receiving TXA had no significant increase in the risk of thromboembolic complications or other adverse events. For exploring the cost effective and optimal dose, well-designed RCTs are still needed to be run.
References
Park JH, Rasouli MR, Mortazavi SM, Tokarski AT, Maltenfort MG, Parvizi J (2013) Predictors of perioperative blood loss in total joint arthroplasty. J Bone Joint Surg Am 95(19):1777–1783
Banerjee S, Kapadia BH, Issa K, McElroy MJ, Khanuja HS, Harwin SF, Mont MA (2013) Postoperative blood loss prevention in total knee arthroplasty. J Knee Surg 26(6):395–400
Vielpeau C (2012) Perioperative bleeding and early mortality in hip and knee surgery. Orthop Traumatol Surg Res 98(5):475–476
Alshryda S, Mason J, Vaghela M, Sarda P, Nargol A, Maheswaran S, Tulloch C, Anand S, Logishetty R, Stothart B, Hungin AP (2013) Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am 95(21):1961–1968
Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ (1996) The risk of transfusion transmitted viral infections: the retrovirus epidemiology donor study. N Engl J Med 334(26):1685–1690
Muñoz M, Cobos A, Campos A (2014) Low vacuum re-infusion drains after total knee arthroplasty: is there a real benefit? Blood Transfus 12(Suppl 1):s173–s175
Horstmann W, Kuipers B, Ohanis D, Slappendel R, Kollen B, Verheyen C (2014) Autologous re-transfusion drain compared with no drain in total knee arthroplasty: a randomised controlled trial. Blood Transfus 12(Suppl 1):s176–s181
Perazzo P, Viganò M, De Girolamo L, Verde F, Vinci A, Banfi G, Romagnoli S (2013) Blood management and transfusion strategies in 600 patients undergoing total joint arthroplasty: an analysis of pre-operative autologous blood donation. Blood Transfus 11(3):370–376
Hardy JF, Desroches J (1992) Natural and synthetic antifibrino-lytics in cardiac surgery. Can J Anaesth 39(4):353–365
Abrishami A, Chung F, Wong J (2009) Topical application of antifibrinolytic drugs for on-pump cardiac surgery: a systematic review and meta-analysis. Can J Anaesth 56(3):202–212
Ker K, Edwards P, Perel P, Shakur H, Roberts I (2012) Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 344:e3054
Zhang H, Chen J, Chen F, Que W (2012) The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 20(9):1742–1752
Aguilera X, Martinez-Zapata MJ, Bosch A, Urrútia G, González JC, Jordan M, Gich I, Maymó RM, Martínez N, Monllau JC, Celaya F, Fernández JA (2013) Efficacy and safety of fibrin glue and tranexamic Acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am 95(22):2001–2007
Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C (2012) Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord 13:124
Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW (2013) A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty 28(8 Suppl):78–82
Kim TK, Chang CB, Kang YG, Seo ES, Lee JH, Yun JH, Lee SH (2014) Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 22(8):1870–1878
Lee SH, Cho KY, Khurana S, Kim KI (2013) Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 21(11):2611–2617
Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH (2014) Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty 29(5):889–894
Pachauri A, Acharya KK, Tiwari AK (2013) The effect of tranexamic acid on hemoglobin levels during total knee arthroplasty. Am J Ther 21(5):366–370
Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON (2012) Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 20(12):2494–2501
Seo JG, Moon YW, Park SH, Kim SM, Ko KR (2013) The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21(8):1869–1874
Abrishami A (2010) Timing and volume of topical tranexamic acid administration for postoperative blood loss in total knee arthroplasty. Can J Anesth 51:S142–S143
Alvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F (2008) Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion 48(3):519–525
Benoni G, Fredin H (1996) Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br 78(3):434–440
Camarasa MA, Ollé G, Serra-Prat M, Martín A, Sánchez M, Ricós P, Pérez A, Opisso L (2006) Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth 96(5):576–582
Charoencholvanich K, Siriwattanasakul P (2011) Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res 469(10):2874–2880
Ellis MH, Fredman B, Zohar E, Ifrach N, Jedeikin R (2001) The effect of tourniquet application, tranexamic acid, and desmopressin on the procoagulant and fibrinolytic systems during total knee replacement. J Clin Anesth 13(7):509–513
Engel JM, Hohaus T, Ruwoldt R, Menges T, Jürgensen I, Hempelmann G (2001) Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg 92(3):775–780
Gautam PL, Katyal S, Yamin M, Singh A (2011) Effect of tranexamic acid on blood loss and transfusion requirement in total knee replacement in the Indian population: a case series. Indian J Anaesth 55(6):590–593
Good L, Peterson E, Lisander B (2003) Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth 90(5):596–599
Hiippala ST, Strid LJ, Wennerstrand MI, Arvela JV, Niemelä HM, Mäntylä SK, Kuisma RP, Ylinen JE (1997) Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 84(4):839–844
Hiippala S, Strid L, Wennerstrand M, Arvela V, Mäntylä S, Ylinen J, Niemelä H (1995) Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth 74(5):534–537
Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, Kubo S, Matsumoto T, Matsushita T, Chin T, Iguchi T, Kurosaka M, Kuroda R (2011) Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop 35(11):1639–1645
Jansen AJ, Andreica S, Claeys M, D’Haese J, Camu F, Jochmans K (1999) Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth 83(4):596–601
Kakar PN, Gupta N, Govil P, Shah V (2009) Efficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trial. Indian J Anaesth 53(6):667–671
Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR (2012) Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res 470(9):2605–2612
McConnell JS, Shewale S, Munro NA, Shah K, Deakin AH, Kinninmonth AW (2012) Reducing blood loss in primary knee arthroplasty: a prospective randomised controlled trial of tranexamic acid and fibrin spray. Knee 19(4):295–298
Molloy DO, Archbold HA, Ogonda L, McConway J, Wilson RK, Beverland DE (2007) Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br 89(3):306–309
Onodera T, Majima T, Sawaguchi N, Kasahara Y, Ishigaki T, Minami A (2012) Risk of deep venous thrombosis in drain clamping with tranexamic acid and carbazochrome sodium sulfonate hydrate in total knee arthroplasty. J Arthroplasty 27(1):105–108
Orpen NM, Little C, Walker G, Crawfurd EJ (2006) Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee 13(2):106–110
Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S (2001) Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br 83(5):702–705
Veien M, Sørensen JV, Madsen F, Juelsgaard P (2002) Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand 46(10):1206–1211
Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, Syed KA, Muhammad Ovais Hasan S, De Silva Y, Chung F (2010) Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am 92(15):2503–2513
Zhang F, Gao Z, Yu J (2007) Clinical comparative studies on effect of tranexamic acid on blood loss associated with total knee arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 21(12):1302–1304
Zohar E, Ellis M, Ifrach N, Stern A, Sapir O, Fredman B (2004) The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg 99(6):1679–1683
Tan J, Chen H, Liu Q, Chen C, Huang W (2013) A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res 184(2):880–887
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Q., Zhang, HA., Liu, SL. et al. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol 25, 525–541 (2015). https://doi.org/10.1007/s00590-014-1568-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-014-1568-z