Abstract
Purpose
This study aimed to compare unilateral biportal endoscopic discectomy (UBED) with microdiscectomy (MD) for treating lumbar disk herniation (LDH).
Methods
A comprehensive literature search was conducted in the Embase, PubMed, Cochrane Library, CNKI, and Web of Science databases from database inception to April 2023 to identify studies comparing UBED and MD for treating LDH. This study evaluated the visual analog scale (VAS) score, Oswestry disability index (ODI), Macnab scores, operation time, estimated blood loss, hospital stay, and complications, estimated blood loss, visual analog scale (VAS) score, Oswestry disability index (ODI), and Macnab scores at various pre- and post-surgery stages. The meta-analysis was performed using RevMan 5.4 software.
Results
The meta-analysis included 9 distinct studies with a total of 1001 patients. The VAS scores for low back pain showed no significant differences between the groups at postoperative 1–3 months (P = 0.09) and final follow-up (P = 0.13); however, the UBED group had lower VAS scores at postoperative 1–3 days (P = 0.02). There were no significant differences in leg pain VAS scores at baseline (P = 0.05), postoperative 1–3 days (P = 0.24), postoperative 1–3 months (P = 0.78), or at the final follow-up (P = 0.43). ODI comparisons revealed no significant differences preoperatively (P = 0.83), at postoperative 1 week (P = 0.47), or postoperative 1–3 months (P = 0.13), and the UBED group demonstrated better ODI at the final follow-up (P = 0.03). The UBED group also exhibited a shorter mean operative time (P = 0.03), significantly shorter hospital stay (P < 0.00001), and less estimated blood loss (P = 0.0002). Complications and modified MacNab scores showed no significant differences between the groups (P = 0.56 and P = 0.05, respectively).
Conclusion
The evidence revealed no significant differences in efficacy between UBED and MD for LDH treatment. However, UBED may offer potential benefits such as shorter hospital stays, lower estimated blood loss, and comparable complication rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lumbar disc herniation (LDH) is a prevalent spinal column disorder caused by the rupture of the intervertebral disc's annulus fibrosus and disk degeneration, leading to nerve root compression [1, 2]. This condition results in symptoms such as lower back pain, lower extremity numbness, and reduced mobility, significantly affecting the patient's quality of life. Surgery is the primary intervention for individuals with ineffective conservative treatment, severe disease, or neurological damage [3]. The main goal of surgical treatment is to alleviate nerve compression caused by the herniated disks, thus reducing symptoms and improving function. However, traditional open surgery requires extensive paravertebral muscle dissection, which can cause significant trauma, spinal instability, and increased complication rates. Minimally invasive techniques have emerged as viable alternatives [4]. These methods use endoscopes or microscopes inserted through small incisions to provide surgeons with a superior visual field and reduce trauma and complications [5].
A surgical approach resembling the modern unilateral biportal endoscopic (UBE) technique was first documented by De Antoni in 1996 [6]. Despite the initial lack of attention, recent studies have demonstrated the potential of the UBE technique in achieving favorable outcomes for various lumbar spinal diseases, leading to renewed interest among spinal surgeons [7]. The UBE technique distinguishes itself from other minimally invasive procedures through its dual-channel design: one channel serves as the endoscopic portal; however, the other functions as the working portal [8]. This configuration allows the UBE technique to combine the advantages of minimally invasive surgery with a larger operative workspace while maintaining surgical techniques similar to traditional open surgery [9]. Because this endoscopic procedure can successfully decompressive central and foraminal stenosis, it is currently mainly used for the treatment of lumbar spinal stenosis. In the meantime, the procedure can easily remove a migrated disk fragment, therefore, some studies have also applied it to the treatment of lumbar disk herniation. As a new technique that can treat LDH, there are no relevant guidelines to guide clinical practice. Historically, the conventional surgical approach for treating LDH has been MD [10]. This systematic review and meta-analysis aimed to compare the effectiveness of UBED and MD in treating LDH based on the most recent evidence, with the aim of providing guidance for clinicians.
Methods
Search strategy
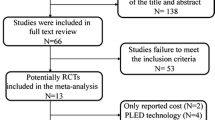
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 statement [11] and was registered in PROSPERO with the identifier CRD42022332774 before data extraction. Two authors independently searched CNKI, Embase, PubMed, Cochrane Library, and Web of Science databases using keywords such as "biportal,” "microdiscectomy," "lumbar disk herniation," and synonyms. The search, conducted until April 2023, did not impose any language restrictions. Furthermore, the literature was assessed based on pre-established inclusion and exclusion criteria, with disagreements resolved through discussion involving a third researcher. Figure 1 shows the PRISMA flow diagram.
Study selection
The inclusion criteria were as follows: (1) study population consisting of patients diagnosed with lumbar disk herniation; (2) interventions comparing UBED and MD, with the consistency of procedures ensured by referencing surgical procedure descriptions and images from the original literature; and (3) studies including at least two outcome indicators, such as operating time (OT), hospital stay, estimated blood loss, complications, visual analog score (VAS), Oswestry disability index (ODI), and MacNab score.
The exclusion criteria were as follows: (1) studies lacking relevant data or without accessible full text and (2) review articles, case reports, case series, or technical reports.
Quality assessment
The meta-analysis encompassed various study types, including randomized controlled trials (RCTs), case–control studies, and cohort studies, as detailed in the original literature. Quality assessment for case–control and cohort studies used the Newcastle–Ottawa Scale (NOS) [12], with higher scores indicating better quality. RCT quality was evaluated using the Cochrane Collaboration tool to assess the risk of bias [13].
Data extraction
Two investigators independently extracted data, including (1) basic characteristics: study ID (author + year), design, journal, country, surgical approach, sample size, age, follow-up time, and outcome indicators; and (2) clinical outcome indicators: operation time, hospital stay, estimated blood loss, complications, preoperative and postoperative VAS scores for back and leg pain, preoperative and postoperative ODI scores, and MacNab scores.
Statistical analysis
Statistical analyzes were performed using Review Manager software (version 5.4). Categorical variables were summarized using the Mantel–Haenszel odds ratio (OR) with 95% confidence intervals (CIs); nevertheless, continuous data were reported as mean differences (MDs) and 95% CI. The I2 test was employed to assess statistical heterogeneity, with I2 values ≥ 50% indicating significant heterogeneity and warranting a random-effects model for the meta-analysis. Otherwise, a fixed effects model was used.
Results
Search results and bias assessment
Figure 1 shows the PRISMA flow diagram outlining the study selection process. An initial search of multiple databases yielded 103 articles, and duplicates were excluded, leaving 34 articles for screening. These articles were assessed based on strict inclusion and exclusion criteria through title and abstract evaluation, which excluded non-relevant studies. A total of 16 articles underwent full-text evaluation, of which 7 were excluded because they did not meet the inclusion criteria. Finally, 9 studies were included in the meta-analysis [14,15,16,17,18,19,20,21,22]. The risk of bias was assessed using the NOS scale and the Cochrane Collaboration tool, as shown in Tables 1 and 2.
Characteristics of included studies
Table 3 shows the baseline characteristics of the included studies. A total of 1001 patients diagnosed with lumbar disk herniation were analyzed, with 477 patients undergoing UBED and 524 patients undergoing MD in 4 countries. Table 4 presents the meta-analysis results, and Table 5 provides a comprehensive list of the complications associated with UBED.
VAS of back pain (Preoperative/postoperative)
Six studies reported the preoperative VAS scores for back pain (262 patients in the UBED group and 319 in the MD group) (Fig. 2). A slightly lower preoperative VAS score for back pain was observed in the UBE group than in the MD (mean difference: − 0.29, 95% CI[ − 0.52, − 0.06], P = 0.01; heterogeneity: Tau2 = 0.01, Chi2 = 5.41, df = 5, P = 0.37, I2 = 8%).
The same 6 studies reported 1–3 days postoperative VAS scores for back pain. Meta-analysis revealed significantly lower scores in the UBED group than in the MD group. (mean difference: − 0.81,95% CI[ − 1.48, − 0.14], P = 0.02; heterogeneity: Tau2 = 0.63, Chi2 = 102.65, df = 5, P < 0.00001, I2 = 95%).
Five studies reported 1–3 months postoperative VAS scores for back pain (167 patients in the UBE group and 182 in the MD group), and four studies reported 1-year postoperative VAS scores for back pain (147 patients in the UBED group and 162 in the MD group). The meta-analysis did not reveal any statistically significant differences between the groups (1–3 months postoperative VAS of back pain: mean difference: − 0.39, 95% CI[ − 0.85, 0.07], P = 0.09; heterogeneity: Tau2 = 0.20, Chi2 = 21.17, df = 4, P = 0.0003, I2 = 81%; 1-year postoperative VAS of back pain: mean difference: − 0.32, 95% CI[ − 0.74, 0.10], P = 0.13; heterogeneity: Tau2 = 0.10, Chi2 = 8.50, df = 3, P = 0.04, I2 = 65%).
VAS of leg pain (Preoperative/postoperative)
Seven studies reported the preoperative VAS scores for leg pain (305 patients in the UBED group and 356 in the MD group) (Fig. 3). Furthermore, 6 studies reported 1–3 days postoperative VAS scores for leg pain (262 patients in the UBED group and 319 in the MD group). In addition, 5 studies reported 1–3 months postoperative VAS scores for leg pain (167 in the UBED group and 182 in the MD group). Moreover, 4 studies reported 1-year postoperative VAS scores for leg pain (147 in the UBED group and 162 in the MD group). There were no statistically significant differences between the two groups (1. preoperative VAS of leg pain: mean difference:-0.16, 95% CI[-0.33, 0.00], P = 0.05; heterogeneity: Tau2 = 0.00, Chi2 = 1.49, df = 6, P = 0.96, I2 = 0%; 2. 1–3 days postoperative VAS of leg pain: mean difference: − 0.19, 95% CI[ − 0.49, 0.12], P = 0.24; heterogeneity: Tau2 = 0.09, Chi2 = 15.45, df = 5, P = 0.009, I2 = 68%; 3. 1–3 months postoperative VAS of leg pain mean difference:-0.09, 95% CI[ − 0.70, 0.53], P = 0.78; heterogeneity: Tau2 = 0.37, Chi2 = 26.63, df = 4, P < 0.0001, I2 = 85%; 4. 1-year postoperative VAS of leg pain mean difference: − 0.23, 95% CI[ − 0.81, 0.34], P = 0.43; heterogeneity: Tau2 = 0.24, Chi2 = 16.45, df = 3, P = 0.0009, I2 = 82%).
ODI (Preoperative/postoperative)
Seven studies reported preoperative ODI (294 patients in the UBED group and 351 in the MD group) (Fig. 4). Furthermore, 3 studies reported the 1-week postoperative ODI (158 patients in the UBED group and 211 in the MD group). In addition, 4 studies reported 1–3 months postoperative ODI (125 patients in the UBED group and 139 in the MD group). The meta-analysis showed no statistically significant differences between the two groups in the above indicators. (1. preoperative ODI:mean difference: 0.21, 95% CI − 1.69, 2.11], P = 0.83; heterogeneity: Tau2 = 2.78, Chi2 = 12.34, df = 6, P = 0.05, I2 = 51%; 2. 1-week postoperative ODI: mean difference: − 1.09, 95% CI[ − 4.08, 1.90], P = 0.47; heterogeneity: Tau2 = 4.18, Chi2 = 5.01, df = 2, P = 0.08, I2 = 60%; 3. 1–3 months postoperative ODI: mean difference: − 2.15, 95% CI[ − 4.97, 0.67], P = 0.13; heterogeneity: Tau2 = 5.69, Chi2 = 16.55, df = 3, P = 0.0009, I2 = 82%). Additionally, 4 studies reported 1–year postoperative ODI (147 patients in the UBED group and 162 in the MD group), and the meta-analysis showed that the UBED group had better ODI than the MD (1-year postoperative ODI: mean difference: − 1.40, 95% CI[ − 2.68, − 0.11], P = 0.03; heterogeneity: Tau2 = 0.67, Chi2 = 5.10, df = 3, P = 0.16, I2 = 41%).
MacNab score
Five studies reported the MacNab score (254 patients in the UBED group and 294 in the MD group) (Fig. 5). The meta-analysis did not reveal any statistically significant differences between the groups. (odds ratio: 1.58, 95% CI [1.00, 2.50], P = 0.05; heterogeneity: Chi2 = 1.83, df = 4, P = 0.77, I2 = 0%).
Operation time
Nine studies reported the operating time in 1001 patients, with 477 in the UBED group and 524 in the MD group (Fig. 6). Specifically, the operating time was slightly longer in the MD group, with a statistically significant difference. (mean difference: 9.16 min, 95% CI[1.13, 17.20], P = 0.03; heterogeneity: Tau2 = 130.26, Chi2 = 112.00, df = 8, P < 0.00001, I2 = 93%).
Estimated blood loss
Four studies reported the estimated blood loss (200 and 256 patients in the UBED and MD groups, respectively) (Fig. 7). A lower estimated blood loss was observed in the UBED group than in the MD group, with a statistically significant difference. (mean difference: − 74.42, 95% CI[ − 114.11, − 34.73], P = 0.0002; heterogeneity: Tau2 = 1616.29, Chi2 = 408.49, df = 3, P < 0.00001, I2 = 99%).
Hospital stay
Among the 9 studies analyzed, 8 reported the hospital stays of 914 patients, with 435 in the UBED group and 479 in the MD group (Fig. 8). The results showed a statistically significant difference between the two groups, with a significantly shorter hospital stay observed in the UBED group compared to MD. (mean difference: − 2.32 days 95% CI[ − 3.30, − 1.35], P < 0.00001; heterogeneity: Tau2 = 1.69, Chi2 = 88.71, df = 7, P < 0.00001, I2 = 92%,).
Complications
Seven studies reported the incidence of complications (317 and 368 patients in the UBED and MD groups, respectively) (Fig. 9). The meta-analysis revealed no statistically significant difference in the incidence of complications between the two groups (odds ratio: 1.21, 95% CI[0.63, 2.33], P = 0.56; heterogeneity: Chi2 = 1.84, df = 6, P = 0.93, I2 = 0%).
Discussion
This study revealed that both UBED and MD demonstrated comparable therapeutic efficacy in treating LDH, as evidenced by analogous VAS, ODI, and MacNab scores and complication rates (minor discrepancies in the VAS score for back pain and ODI offer limited evidence for meaningful information). Additionally, the UBED group exhibited reduced hospital stays and estimated blood loss, albeit with marginally increased operative times, compared to the MD.
Efficacy of UBED
Lumbar disk herniation (LDH) is a common degenerative spinal disorder. Microdiscectomy (MD) has been the gold standard treatment for LDH for an extended period [23] and was introduced by Yasargil in 1967 using a microscope [24]. Nevertheless, the microscopic technique presents limitations, such as challenging instrument manipulation because of a single-port and the steep learning curve associated with tubular microsurgery [25]. Over the past decade, minimally invasive endoscopic spinal surgery has gained popularity for preserving normal anatomical structures, thus expediting postoperative recovery [26]. According to the North American Spine Society's (NASS) 2013 evidence-based guidelines, percutaneous endoscopic lumbar discectomy (PELD) is the primary recommended surgical approach for selected patients with LDH radiculopathy, which also indicates that the treatment concept of LDH is gradually changing to minimally invasive methods [3]. However, PELD involves a challenging learning curve [27, 28], and if not performed with the necessary skills and precision, complications such as dural and nerve root injuries can occur [29]. Due to the inevitable procedural defects of PELD, it is necessary to find another minimally invasive method to treat LDH. Recent studies have shown that the UBE technique [30], proved effective in LDH treatment [31, 32] and the findings of this study indicate that UBED exhibit therapeutic efficacy in the treatment of LDH by compared with MD, as demonstrated by similar VAS, ODI, and MacNab scores. Even more remarkable, this technique demonstrated versatility in addressing various spinal diseases, including high-grade migrated LDH, recurrent LDH, far-out syndrome, thoracic ossification of the ligamentum flavum, cervical radiculopathy, and lumbar interbody fusion [33,34,35,36,37,38,39].
Advantages and limitations of UBED
The recent orthopedic addition, the UBE technique [30], proved effective in LDH treatment [31, 32] and demonstrated versatility in addressing various spinal diseases, including high-grade migrated LDH, recurrent LDH, far-out syndrome, thoracic ossification of the ligamentum flavum, cervical radiculopathy, and lumbar interbody fusion [33,34,35,36,37,38,39]. Prior studies have documented numerous benefits of the UBE technique. Its utilization of instruments identical to those used in conventional spinal surgery presents a potential for cost reduction. Choi et al. substantiated that UBE is a more economically efficient alternative to MD, boasting a cost-effectiveness on par with other endoscopic lumbar discectomies Several benefits of the UBE technique have been previously documented. By employing instruments identical to those used in traditional spinal surgery, UBE has the potential to reduce overall costs. Choi et al. demonstrated that the UBE technique is a more cost-effective alternative to MD, with cost-effectiveness comparable to other endoscopic lumbar discectomies [16].
Many surgeons consider this technique as a superior minimally invasive approach for the following reasons. The biportal technique provides a broader visual access range than single-port endoscopes, akin to the amplified visual field achieved with a microscopeThe biportal technique offers a wider visual access range than single-port endoscopes, similar to the magnified visual field obtained using a microscope [40]. By separating the viewing and working portals, it allows unrestricted bimanual operation by surgeons, facilitating more straightforward instrument manipulation than uniportal or microscopic techniques. Additionally, the biportal technique enhances visualization of contralateral sublaminar and foraminal spaces, which can be further optimized using a 30˚ endoscopeSeparating the viewing and working portals allows unrestricted bimanual use by surgeons, enabling easier instrument manipulation than uniportal or microscopic techniques. The biportal technique also provides an enhanced visualization of the contralateral sublaminar and foraminal spaces, which can be further improved by using a 30˚ endoscope[41], fthereby promoting secure and efficient bilateral nerve decompressionacilitating secure and effective bilateral nerve decompression [42]. As a result, the biportal technique's provision of clear visual access and unrestricted instrument maneuverability is thought to lead to reduced radiation exposure.Consequently, clear visual access and unobstructed instrument maneuverability offered by the biportal technique is believed to contribute to shorter operative times and radiation exposure. This finding aligns with Merter et al.'s study, which ranked UBED above microendoscopic discectomy in terms of radiation exposure duration[43]. Subsequently, the UBE technique has gained popularity among spinal surgeons, showing faster recovery and shorter hospital stays in lumbar spinal stenosis treatment than in MD[44,45,46]. Our meta-analysis of UBED for LDH treatment supports this finding.
However, Jiang et al. identified several disadvantages of the UBE technique compared to PELD, including increased total, intraoperative, and hidden blood loss; longer operative time; and extended hospitalization periods [32]. Furthermore, meta-analyzes by Zhu et al. and Ma et al. observed no significant differences in the clinical effectiveness between UBED and PELD [47, 48]. However, PELD demonstrated superiority in operative duration, intraoperative hemorrhage, and immediate postoperative pain relief. This discrepancy may be because of the use of a double incision, the invasiveness of the procedure on the muscle tissue, and the steeper learning curve associated with this new surgical approach. Regarding potential paraspinal muscle injury, Choi et al.'s study used creatine phosphokinase (CPK) and C-reactive protein (CRP) as indicators to assess damage during surgery [14]. The PELD group showed significantly smaller cross-sectional areas of high-intensity lesions in the paraspinal muscles and lower CPK and CRP levels than the UBED group. Ahn et al. observed multifidus muscle status changes on MRI after UBE, which resolved spontaneously over time [49]. Wang et al. produced similar results, which suggested that radiological manifestations of paraspinal muscle invasiveness were comparable between UBE and percutaneous endoscopic techniques during final follow-ups of at least one year [50]. In addition, surgery duration and paraspinal muscle extent at specific levels may be considered risk factors for high blood loss in UBE surgery [51]. Consequently, reducing the surgery duration is crucial to address these issues, necessitating a focus on the learning process of the technique.
Learning curve of UBED
Choi et al. emphasized the importance of magnifying regional views through an endoscope and carefully controlling epidural bleeding to maintain a clear surgical field when learning the UBE technique [52]. Furthermore, Park et al. suggested that achieving proficiency in the UBE technique might require a significant learning duration, as demonstrated by a trainee with no prior UBE experience performing adequately in 58 cases [53]. Moreover, Xu et al.'s 2022 study identified three distinct learning curve phases for the UBE technique, requiring at least 54 cases to master the procedure, with the operation time and postoperative hospital stay decreasing as the learning curve progressed [54]. However, Chen et al. observed that mastery could be achieved with as few as 24 single-level procedures [55]. In general, there is a learning curve for this technique, so the summary of early evidence in our meta-analysis will be valuable for surgeons who are new or about to perform this technique. Notably, Sahin et al. proposed a cost-effective mobile training model to enhance biportal endoscopic skills for inexperienced practitioners [56]. This may be beneficial for shortening the learning process.
Complications and safety of UBED
The present study has identified a range of complications following UBED, such as dural tears, retinal hemorrhage, postoperative spinal epidural hematoma, recurrent intervertebral disk herniation, incomplete decompression, instability, ascites, burn injuries, infections, and neurological complicationsThe current study identified various complications after UBED, including dural tears, retinal hemorrhage, postoperative spinal epidural hematoma, recurrent intervertebral disk herniation, incomplete decompression, instability, ascites, burn injury, infection, and neurological complications [57]. Table 5 provides a summary of the complications discussed in all nine articles included in the study.
Dural tears
Table 5 summarizes the complications mentioned in all 9 included articles. Dural tears represent the most frequently occurring complication of the UBE technique, with multiple factors contributing to their incidence, including inappropriate instrument handling, inexperienced surgeons, limited visibility during the procedure, and the anatomy of the central dural fold Dural tears are the most common complication of the UBE technique, with several factors contributing to their occurrence, such as improper instrument handling, inexperienced surgeons, limited visibility during the procedure, and central dural fold anatomy [58,59,60,61]. Furthermore, Lee et al. observed that dural sac tears often occur during ligament flavum removal [58], which can be attributed to the meningovertebral ligament, a web-like anatomical interface joining the dorsal face of the dura with the lamina and ligament flavum [62]. Park et al. guided managing dural tears [59]; tears < 4 mm required no intervention; 4–12 mm tears should be treated with a fibrin sealant patch; tears > 12 mm with standard margins should undergo primary endoscopic closure using nonpenetrating titanium clips and a fibrin sealant patch; and irregular margin tears > 12 mm should be converted to direct microscopic repair. Kim et al. demonstrated that dural tears < 10 mm can be effectively treated using the fibrin seal patch technique [60]. Chun et al. reported a case of postoperative nerve root herniation caused by a dural tear in the UBE technique, suggesting an epidural blood patch as a treatment before open surgery [61].
Hematoma
Patient dissatisfaction is primarily associated with hematoma formation and incomplete decompression [63]. Kim et al. observed a higher prevalence of radiological hematoma identified through postoperative MRI than symptomatic postoperative hematoma. The risk factors for postoperative hematoma after UBED include female sex, age > 70 years, anticoagulation medication use, and intraoperative water infusion pump use [64]. Ahn et al. concluded that high e-SBP (≥ 170 mmHg) could influence postoperative spinal epidural hematoma development in UBED [65]. Furthermore, Kim et al. suggested that revision surgery may be necessary when canal encroachment by hematomas exceeds 50%, and the patient presents with related symptoms [66].
Incomplete decompression
Incomplete decompression represents a common concern across all minimally invasive procedures. This issue, particularly in UBED, often arises due to blurred vision induced by intraoperative bleedingIncomplete decompression because of intraoperative bleeding-induced blurred vision in UBED [63]. It can be effectively managed through continuous irrigation during the surgical procedure. This approach facilitates the removal of bone debris, minimizes bleeding, ensures a clear operating field, and helps in reducing intracranial pressure can be addressed through continuous irrigation during surgery to remove bone debris, minimize bleeding, maintain a clear operating space, and reduce intracranial pressure [67]. Notably, the irrigation pressure should be regulated (25–30 mmHg recommended) to avoid increased epidural hydrostatic pressure, which can delay postoperative recovery [68]. Limited depth perception during endoscopic surgery (because of 2D vision) may increase the risk of perioperative complications. However, Heo et al. introduced 3D endoscopy to improve the discrimination of anatomical structures with enhanced stereognosis and depth perception, potentially benefiting patient safety and well-being during endoscopic spinal surgery [69].
This systematic review and meta-analysis have certain limitations, such as the relatively limited number of studies comparing UBED with MD and variable findings among the reviewed studies. Furthermore, because certain studies employed distinct outcome units, they could not be incorporated into the meta-analysis. Notably, only one of the included studies was a randomized controlled trial; however, the other studies had observational designs. Therefore, to bolster the validity and reliability of this investigation, there is a pressing need for additional randomized controlled trials characterized by larger sample sizes.
Conclusion
Available evidence indicates no significant difference in efficacy between UBED and MD for lumbar disk herniation. Nonetheless, UBED has the potential to offer benefits, such as a shorter hospital stay, lower estimated blood loss, and lower complication rates. However, more robust evidence is required, and further multicenter randomized controlled trials (RCTs) are necessary to establish a definitive conclusion.
References
Benzakour T, Igoumenou V, Mavrogenis AF, Benzakour A (2019) Current concepts for lumbar disc herniation. Int Orthop 43:841–851. https://doi.org/10.1007/s00264-018-4247-6
Yu P, Mao F, Chen J, Ma X, Dai Y, Liu G, Dai F, Liu J (2022) Characteristics and mechanisms of resorption in lumbar disc herniation. Arthritis Res Ther 24:205. https://doi.org/10.1186/s13075-022-02894-8
Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, Cho CH, DePalma MJ, Dougherty P, Fernand R, Ghiselli G, Hanna AS, Lamer T, Lisi AJ, Mazanec DJ, Meagher RJ, Nucci RC, Patel RD, Sembrano JN, Sharma AK, Summers JT, Taleghani CK, Tontz WL, Toton JF (2014) An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J 14:180–191. https://doi.org/10.1016/j.spinee.2013.08.003
Alvi MA, Kerezoudis P, Wahood W, Goyal A, Bydon M (2018) Operative approaches for lumbar disc herniation: a systematic review and multiple treatment meta-analysis of conventional and minimally invasive surgeries. World Neurosurg. https://doi.org/10.1016/j.wneu.2018.02.156
Kim M, Lee S, Kim H-S, Park S, Shim S-Y, Lim D-J (2018) A comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for lumbar disc herniation in the Korean: a meta-analysis. Biomed Res Int 2018:9073460. https://doi.org/10.1155/2018/9073460
De Antoni DJ, Claro ML, Poehling GG, Hughes SS (1996) Translaminar lumbar epidural endoscopy: anatomy, technique, and indications. Arthroscopy 12:330–334
Chu P-L, Wang T, Zheng J-l, Xu C-Q, Yan Y-J, Ma Q-S, Meng-Chen Y, Da-Sheng T (2022) Global and current research trends of unilateral biportal endoscopy/biportal endoscopic spinal surgery in the treatment of lumbar degenerative diseases: a bibliometric and visualization study. Orthop Surg 14:635–643. https://doi.org/10.1111/os.13216
Hwa Eum J, Hwa Heo D, Son SK, Park CK (2016) Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine 24:602–607. https://doi.org/10.3171/2015.7.SPINE15304
Choi C-M (2020) Biportal endoscopic spine surgery (BESS): considering merits and pitfalls. J Spine Surg 6:457–465. https://doi.org/10.21037/jss.2019.09.29
Ruan W, Feng F, Liu Z, Xie J, Cai L, Ping A (2016) Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: a meta-analysis. Int J Surg 31:86–92. https://doi.org/10.1016/j.ijsu.2016.05.061
Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S (2013) Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 8:e83138. https://doi.org/10.1371/journal.pone.0083138
Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. https://doi.org/10.1007/s10654-010-9491-z
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Choi K-C, Shim H-K, Hwang J-S, Shin SH, Lee DC, Jung HH, Park HA, Park C-K (2018) Comparison of Surgical Invasiveness Between Microdiscectomy and 3 Different Endoscopic Discectomy Techniques for Lumbar Disc Herniation. World Neurosurg 116:e750–e758. https://doi.org/10.1016/j.wneu.2018.05.085
Kim S-K, Kang S-S, Hong Y-H, Park S-W, Lee S-C (2018) Clinical comparison of unilateral biportal endoscopic technique versus open microdiscectomy for single-level lumbar discectomy: a multicenter, retrospective analysis. J Orthop Surg Res 13:22. https://doi.org/10.1186/s13018-018-0725-1
Choi K-C, Shim H-K, Kim J-S, Cha KH, Lee DC, Kim ER, Kim MJ, Park C-K (2019) Cost-effectiveness of microdiscectomy versus endoscopic discectomy for lumbar disc herniation. Spine J 19:1162–1169. https://doi.org/10.1016/j.spinee.2019.02.003
Fishchenko I, Balan S, Blonskyi R, Borzykh N, Kravchuk L (2020) Our experience with the use of biportal endoscopic surgery in the treatment of herniated discs of the lumbar spine. Georgian Med News 301:21–27
Foocharoen T (2021) Early outcomes: a comparison between biportal endoscopic spine surgery and open lumbar discectomy for single-level lumbar disc herniation. J Med Assoc Thai 104:123–128. https://doi.org/10.35755/jmedassocthai.2021.01.11524
Weidong G, Xiaoping Z, Xiaoming B, Kang Y, Huanhuan Q, Haien Z, Xin D, Bo L (2022) Comparison of the efficacy of unilateral biportal endoscopic and microscopic discectomy in treatment of lumbar disc herniation. J Xi’an Jiaotong Univ (Med Sci) 43:430–435. https://doi.org/10.7652/jdyxb202203018
Dongsheng Y, Xiongjie S (2022) Effects of two kinds of surgical methods on lumbar disc herniation and lumbar spinal stenosis. Chinese J Med 57:1105–1108. https://doi.org/10.3969/j.issn.1008-1070.2022.10.017
Chang H, Xu J, Yang D, Sun J, Gao X, Ding W (2023) Comparison of full-endoscopic foraminoplasty and lumbar discectomy (FEFLD), unilateral biportal endoscopic (UBE) discectomy, and microdiscectomy (MD) for symptomatic lumbar disc herniation. Eur Spine J 32:542–554. https://doi.org/10.1007/s00586-022-07510-6
Park S-M, Lee H-J, Park H-J, Choi J-Y, Kwon O, Lee S, Kim H-J, Yeom JS (2023) Biportal endoscopic versus microscopic discectomy for lumbar herniated disc: a randomized controlled trial. Spine J 23:18–26. https://doi.org/10.1016/j.spinee.2022.09.003
Rickers KW, Pedersen PH, Tvedebrink T, Eiskjær SP (2021) Comparison of interventions for lumbar disc herniation: a systematic review with network meta-analysis. Spine J 21:1750–1762. https://doi.org/10.1016/j.spinee.2021.02.022
Yasargil MG, Vise WM, Bader DC (1977) Technical adjuncts in neurosurgery. Surg Neurol 8:331–336
Parikh K, Tomasino A, Knopman J, Boockvar J, Härtl R (2008) Operative results and learning curve: microscope-assisted tubular microsurgery for 1- and 2-level discectomies and laminectomies. Neurosurg Focus 25:E14. https://doi.org/10.3171/FOC/2008/25/8/E14
Ahn Y (2019) Endoscopic spine discectomy: indications and outcomes. Int Orthop 43:909–916. https://doi.org/10.1007/s00264-018-04283-w
Ahn Y, Lee S, Son S, Kim H, Kim JE (2020) Learning curve for transforaminal percutaneous endoscopic lumbar discectomy: a systematic review. World Neurosurg 143:471–479. https://doi.org/10.1016/j.wneu.2020.08.044
Tenenbaum S, Arzi H, Herman A, Friedlander A, Levinkopf M, Arnold PM, Caspi I (2011) Percutaneous posterolateral transforaminal endoscopic discectomy: clinical outcome, complications, and learning curve evaluation. Surg Technol Int 21:278–283
Pan M, Li Q, Li S, Mao H, Meng B, Zhou F, Yang H (2020) Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician 23:49–56
Kwon O, Yoo S-J, Park J-Y (2022) Comparison of unilateral biportal endoscopic discectomy with other surgical technics: a systemic review of indications and outcomes of unilateral biportal endoscopic discectomy from the current literature. World Neurosurg 168:349–358. https://doi.org/10.1016/j.wneu.2022.06.153
Cheng X, Bao B, Wu Y, Cheng Y, Xu C, Ye Y, Dou C, Chen B, Yan H, Tang J (2022) Clinical comparison of percutaneous transforaminal endoscopic discectomy and unilateral biportal endoscopic discectomy for single-level lumbar disc herniation. Front Surg 9:1107883. https://doi.org/10.3389/fsurg.2022.1107883
Jiang H-W, Chen C-D, Zhan B-S, Wang Y-L, Tang P, Jiang X-S (2022) Unilateral biportal endoscopic discectomy versus percutaneous endoscopic lumbar discectomy in the treatment of lumbar disc herniation: a retrospective study. J Orthop Surg Res 17:30. https://doi.org/10.1186/s13018-022-02929-5
Heo DH, Sharma S, Park CK (2019) Endoscopic treatment of extraforaminal entrapment of L5 nerve root (far out syndrome) by unilateral biportal endoscopic approach: technical report and preliminary clinical results. Neurospine 16:130–137. https://doi.org/10.14245/ns.1938026.013
Deng Y, Yang M, Xia C, Chen Y, Xie Z (2022) Unilateral biportal endoscopic decompression for symptomatic thoracic ossification of the ligamentum flavum: a case control study. Int Orthop 46:2071–2080. https://doi.org/10.1007/s00264-022-05484-0
Kang T, Park SY, Park GW, Lee SH, Park JH, Suh SW (2020) Biportal endoscopic discectomy for high-grade migrated lumbar disc herniation. J Neurosurg Spine. https://doi.org/10.3171/2020.2.SPINE191452
Kang M-S, Hwang J-H, Choi D-J, Chung H-J, Lee J-H, Kim H-N, Park H-J (2020) Clinical outcome of biportal endoscopic revisional lumbar discectomy for recurrent lumbar disc herniation. J Orthop Surg Res 15:557. https://doi.org/10.1186/s13018-020-02087-6
Song K-S, Lee C-W (2020) The biportal endoscopic posterior cervical inclinatory foraminotomy for cervical radiculopathy: technical report and preliminary results. Neurospine 17:S145–S153. https://doi.org/10.14245/ns.2040228.114
Liu G, Liu W, Jin D, Yan P, Yang Z, Liu R (2023) Clinical outcomes of unilateral biportal endoscopic lumbar interbody fusion (ULIF) compared with conventional posterior lumbar interbody fusion (PLIF). Spine J 23:271–280. https://doi.org/10.1016/j.spinee.2022.10.001
Choi D-J, Jung J-T, Lee S-J, Kim Y-S, Jang H-J, Yoo B (2016) Biportal endoscopic spinal surgery for recurrent lumbar disc herniations. Clin Orthop Surg 8:325–329. https://doi.org/10.4055/cios.2016.8.3.325
Park S-M, Park J, Jang HS, Heo YW, Han H, Kim H-J, Chang B-S, Lee C-K, Yeom JS (2020) Biportal endoscopic versus microscopic lumbar decompressive laminectomy in patients with spinal stenosis: a randomized controlled trial. Spine J 20:156–165. https://doi.org/10.1016/j.spinee.2019.09.015
Kim JY, Heo DH (2021) Contralateral sublaminar approach for decompression of the combined lateral recess, foraminal, and extraforaminal lesions using biportal endoscopy: a technical report. Acta Neurochir 163:2783–2787. https://doi.org/10.1007/s00701-021-04978-x
Lin G-X, Yao Z-K, Xin C, Kim J-S, Chen C-M, Hu B-S (2022) A meta-analysis of clinical effects of microscopic unilateral laminectomy bilateral decompression (ULBD) versus biportal endoscopic ULBD for lumbar canal stenosis. Front Surg 9:1002100. https://doi.org/10.3389/fsurg.2022.1002100
Merter A, Karaeminogullari O, Shibayama M (2020) Comparison of radiation exposure among 3 different endoscopic diskectomy techniques for lumbar disk herniation. World Neurosurg 139:e572–e579. https://doi.org/10.1016/j.wneu.2020.04.079
Liang J, Lian L, Liang S, Zhao H, Shu G, Chao J, Yuan C, Zhai M (2022) Efficacy and complications of unilateral biportal endoscopic spinal surgery for lumbar spinal stenosis: a meta-analysis and systematic review. World Neurosurg. https://doi.org/10.1016/j.wneu.2021.12.005
Pranata R, Lim MA, Vania R, July J (2020) Biportal endoscopic spinal surgery versus microscopic decompression for lumbar spinal stenosis: a systematic review and meta-analysis. World Neurosurg 138:e450–e458. https://doi.org/10.1016/j.wneu.2020.02.151
Theodore N, Arnold PM, Mehta AI (2018) Introduction: the rise of the robots in spinal surgery. Neurosurg Focus. https://doi.org/10.3171/2018.7.FocusVid.Intro
Ma X, Li W, Gao S, Cao C, Li C, He L, Li M (2022) Comparison of unilateral biportal endoscopic discectomy versus percutaneous endoscopic lumbar discectomy for the treatment of lumbar disc herniation: a systematic review and meta-analysis. Medicine 101:e30412. https://doi.org/10.1097/MD.0000000000030612
Zhu W, Yao Y, Hao J, Li W, Zhang F (2022) Short-term postoperative pain and function of unilateral biportal endoscopic discectomy versus percutaneous endoscopic lumbar discectomy for single-segment lumbar disc herniation: a systematic review and meta-analysis. Appl Bionics Biomech 2022:5360277. https://doi.org/10.1155/2022/5360277
Ahn J-S, Lee H-J, Park EJ, Kim SB, Choi D-J, Kwon Y-S, Chung H-J (2019) Multifidus muscle changes after biportal endoscopic spinal surgery: magnetic resonance imaging evaluation. World Neurosurg 130:e525–e534. https://doi.org/10.1016/j.wneu.2019.06.148
Wang L, Li C, Han K, Chen Y, Qi L, Liu X (2023) Comparison of clinical outcomes and muscle invasiveness between unilateral biportal endoscopic discectomy and percutaneous endoscopic interlaminar discectomy for lumbar disc herniation at L5/S1 level. Orthop Surg 15:695–703. https://doi.org/10.1111/os.13627
Guo S, Tan H, Meng H, Li X, Su N, Yu L, Lin J, An N, Yang Y, Fei Q (2022) Risk factors for hidden blood loss in unilateral biportal endoscopic lumbar spine surgery. Front Surg 9:966197. https://doi.org/10.3389/fsurg.2022.966197
Choi D-J, Choi C-M, Jung J-T, Lee S-J, Kim Y-S (2016) Learning curve associated with complications in biportal endoscopic spinal surgery: challenges and strategies. Asian Spine J 10:624–629. https://doi.org/10.4184/asj.2016.10.4.624
Park S-M, Kim H-J, Kim G-U, Choi M-H, Chang B-S, Lee C-K, Yeom JS (2019) Learning curve for lumbar decompressive laminectomy in biportal endoscopic spinal surgery using the cumulative summation test for learning curve. World Neurosurg 122:e1007–e1013. https://doi.org/10.1016/j.wneu.2018.10.197
Xu J, Wang D, Liu J, Zhu C, Bao J, Gao W, Zhang W, Pan H (2022) Learning curve and complications of unilateral biportal endoscopy: cumulative sum and risk-adjusted cumulative sum analysis. Neurospine 19:792–804. https://doi.org/10.14245/ns.2143116.558
Chen L, Zhu B, Zhong H-Z, Wang Y-G, Sun Y-S, Wang Q-F, Liu J-J, Tian D-S, Jing J-H (2022) The learning curve of unilateral biportal endoscopic (UBE) spinal surgery by CUSUM analysis. Front Surg 9:873691. https://doi.org/10.3389/fsurg.2022.873691
Sahin E, Erken HY (2023) A low-cost mobile training model for biportal endoscopic spinal surgery. Turk Neurosurg 33:53–57. https://doi.org/10.5137/1019-5149.JTN.36675-21.1
Fernandes-Breitenbach F, Peres-Ueno MJ, Santos LFG, Brito VGB, Castoldi RC, Louzada MJQ, Chaves-Neto AH, Oliveira SHP, Dornelles RCM (2022) Analysis of the femoral neck from rats in the periestropause treated with oxytocin and submitted to strength training. Bone 162:116452. https://doi.org/10.1016/j.bone.2022.116452
Lee HG, Kang MS, Kim SY, Cho KC, Na YC, Cho JM, Jin BH (2021) Dural injury in unilateral biportal endoscopic spinal surgery. Global Spine J 11:845–851. https://doi.org/10.1177/2192568220941446
Park H-J, Kim S-K, Lee S-C, Kim W, Han S, Kang S-S (2020) Dural tears in percutaneous biportal endoscopic spine surgery: anatomical location and management. World Neurosurg 136:e578–e585. https://doi.org/10.1016/j.wneu.2020.01.080
Kim J-E, Choi D-J, Park EJ (2020) Risk factors and options of management for an incidental dural tear in biportal endoscopic spine surgery. Asian Spine J 14:790–800. https://doi.org/10.31616/asj.2019.0297
Chun YM, Lee SH, Moon KS, Chang MC (2022) Treatment of dural tear with nerve root herniation after unilateral biportal endoscopic decompression using an epidural blood patch: a case report. J Int Med Res 50:3000605221144147. https://doi.org/10.1177/03000605221144147
Geers C, Lecouvet FE, Behets C, Malghem J, Cosnard G, Lengelé BG (2003) Polygonal deformation of the dural sac in lumbar epidural lipomatosis: anatomic explanation by the presence of meningovertebral ligaments. AJNR Am J Neuroradiol 24:1276–1282
Kim W, Kim S-K, Kang S-S, Park H-J, Han S, Lee S-C (2020) Pooled analysis of unsuccessful percutaneous biportal endoscopic surgery outcomes from a multi-institutional retrospective cohort of 797 cases. Acta Neurochir 162:279–287. https://doi.org/10.1007/s00701-019-04162-2
Kim J-E, Choi D-J, Kim M-C, Park EJ (2019) Risk factors of postoperative spinal epidural hematoma after biportal endoscopic spinal surgery. World Neurosurg 129:e324–e329. https://doi.org/10.1016/j.wneu.2019.05.141
Ahn DK, Kim YH, Ko YR, Jang SJ, Jung JS (2023) The influence of systolic blood pressure at the time of extubation on the development of postoperative spinal epidural hematoma. Clin Orthop Surg 15:265–271. https://doi.org/10.4055/cios22297
Kim J-E, Choi D-J, Park EJ (2019) Evaluation of postoperative spinal epidural hematoma after biportal endoscopic spine surgery for single-level lumbar spinal stenosis: clinical and magnetic resonance imaging study. World Neurosurg 126:e786–e792. https://doi.org/10.1016/j.wneu.2019.02.150
Kang T, Park SY, Lee SH, Park JH, Suh SW (2020) Assessing changes in cervical epidural pressure during biportal endoscopic lumbar discectomy. J Neurosurg Spine. https://doi.org/10.3171/2020.6.SPINE20586
Choi CM, Chung JT, Lee SJ, Choi DJ (2016) How I do it? Biportal endoscopic spinal surgery (BESS) for treatment of lumbar spinal stenosis. Acta Neurochir 158:459–463. https://doi.org/10.1007/s00701-015-2670-7
Heo DH, Kim JY, Park J-Y, Kim JS, Kim HS, Roh J, Park CK, Chung H (2022) Clinical experiences of 3-dimensional biportal endoscopic spine surgery for lumbar degenerative disease. Oper Neurosurg 22:231–238. https://doi.org/10.1227/ONS.0000000000000090
Acknowledgements
The authors thank the study investigators and staff who participated in this study.
Funding
This study was supported by the Clinical Research Project of Wu Jieping Medical Foundation (Grant no. 320.6750.15040).
Author information
Authors and Affiliations
Contributions
ZF, ZZ contributed to Methodology and Writing-original draft preparation; ZF, ZZ, WC contributed to Formal analysis and investigation; ZF, ZZ, XM contributed to Writing-review and editing; XM, YH contributed to Supervision. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Z., Zhao, Z., Cui, W. et al. Unilateral biportal endoscopic discectomy versus microdiscectomy for lumbar disc herniation: a systematic review and meta-analysis. Eur Spine J 33, 2139–2153 (2024). https://doi.org/10.1007/s00586-023-08116-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-08116-2