Abstract
Purpose
This meta-analysis aims to compare the complication rates of discectomy/microdiscectomy (OD/MD), microendoscopic discectomy (MED), percutaneous endoscopic lumbar discectomy (PELD), percutaneous laser disc decompression (PLDD), and tubular discectomy for symptomatic lumbar disc herniation (LDH) using general classification and modified Clavien–Dindo classification (MCDC) schemes.
Methods
We searched three online databases for randomized controlled trials (RCTs) and cohort studies. Overall complication rates and complication rates per the above-mentioned classification schemes were considered as primary outcomes. Risk ratio (RR) and their 95% confidence intervals (CI) were evaluated.
Results
Seventeen RCTs and 20 cohort studies met the eligibility criteria. RCTs reporting OD/MD, MED, PELD, PLDD, and tubular discectomies had overall complication rates of 16.8% and 16.1%, 21.2%, 5.8%, 8.4%, and 25.8%, respectively. Compared with the OD/MD, there was moderate-quality evidence suggesting that PELD had a lower risk of overall complications (RR = 0.52, 95% CI 0.29–0.91) and high-quality evidence suggesting a lower risk of Type I complications per MCDC (RR = 0.37, 95% CI 0.16–0.81). Compared with the OD/MD data from cohort studies, there was low-quality evidence suggesting a higher risk of Type III complications per MCDC (RR = 10.83, 95% CI 1.29–91.18) for MED, higher risk of reherniations (RR = 1.67,95% CI 1.05–2.64) and reoperations (RR = 1.75, 95% CI 1.20–2.55) for PELD, lower risk of overall complication rates (RR = 0.42, 95% CI 0.25–0.70), post-operative complication rates (RR = 0.42, 95% CI 0.25–0.70), Type III complications per MCDC (RR = 0.39, 95% CI 0.22–0.69), reherniations (RR = 0.56, 95% CI 0.33–0.97) and reoperations (RR = 0.39, 95% CI 0.22–0.69) for PLDD.
Conclusions
Compared with the OD/MD, results of this meta-analysis suggest that PELD has a lower risk of overall complications and a lower risk of complications necessitating conservative treatment.
Graphic abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Symptomatic lumbar disc herniation (LDH) usually manifests as low back pain (LBP) and/or sciatica with a reported prevalence of 1–3% [1]. Treatment for LDH represents a significant burden on healthcare services and the economy worldwide [2, 3]. Surgical intervention is recommended for LDH patients who are non-responsive to at least six weeks of non-surgical treatment [4]. Open discectomy (OD) and microdiscectomy (MD) are surgical interventions to relieve nerve root compression and improve its function. The two are quite similar procedures with the only variation of the use of visual enhancement such as a microscope or loupes in microdiscectomy. Collectively, OD and MD are the most common surgical interventions for symptomatic LDH that produces excellent short-term clinical outcomes in the majority of patients [5, 6]. However, the rate of reherniation following OD/MD is as high as 10% [7], the incidence of LBP following surgery is almost 30% [8], and rates of revision surgery have been reported up to 20% [9].
Minimally invasive surgeries have been developed in order to reduce tissue trauma and decrease complication rates in symptomatic LDH patients [10, 11]. Percutaneous laser disc decompression (PLDD), as the first generation of minimally invasive surgery, achieved good clinical results [12,13,14]. Since then, the development of newer technologies has resulted in adapted approaches including endoscopic, tubular, cannula, and so on. The percutaneous approach, which became routine in the 1990s, includes an endoscope and cannula assembly, or use of an oval cannula. These methods comprise percutaneous endoscopic lumbar discectomy (PELD) [15, 16]. Microendoscopic discectomy (MED) techniques employ a longitudinal paramedian incision through which a sheath is placed via a transforaminal approach, extraforaminal approach, or interlaminar approach and visualization is achieved through an endoscope [17]. MED resulted in less post-operative pain and a quicker return to work compared with MD [18,19,20]. However, a significant limitation of this technique is the size of the visualized operating field. In order to obtain better visualization, the tubular retractor systems were combined with the use of the microscope in tubular microdiscectomy surgery [21].
These minimally invasive surgical interventions provide excellent clinical outcomes; however, approximately one in five cases still encounter complications [22] such as haematoma formation, durotomy, infection, and nerve root injury [23, 24]. Previous pairwise studies have not conclusively yielded that minimally invasive discectomy techniques result in lower complication rates when compared with OD/MD for symptomatic LDH patients [10, 13, 25,26,27].
The complication rates associated with different discectomy techniques may influence a surgeon’s decision to choose the most suitable surgical plan. However, there is a lack of consensus on how to define and grade complications following spine surgeries. Previous studies have shown that surgeons routinely classify complications as major and minor, intraoperative and post-operative, and into five grades following the modified Clavien–Dindo classification (MCDC) scheme [24, 28,29,30]. Although these classification schemes are commonly used for tabulating and reporting data on adverse events, surgeons often find it difficult to assign a specific complication to overlapping categories within these schemes. Standardization of the reported outcomes following discectomy for LDH will help surgeons identify, manage, and avoid intraoperative and post-operative complications.
Our previously published network meta-analysis (NMA) showed a clear ranking of different discectomy techniques on the basis of their respective complication rates using general classification and MCDC schemes [31]. However, there is a lack of information on pairwise comparisons of complication rates between different discectomy techniques. We therefore performed a systematic review and meta-analysis of all complications reported in discectomy studies to compare OD/MD with MED, PELD, PLDD, and tubular discectomy using two commonly implemented complication classification schemes (general classification that includes intraoperative and post-operative complications, and MCDC).
Methods
Search strategy
Online databases EMBASE, MEDLINE, and Cochrane Central Register of Controlled Trials were searched in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines to identify all relevant studies published between January 1977 (microdiscectomy first reported) and June 2019 [32]. The search included the following terms: “lumbar spine”, “intervertebral disc”, “herniation”, “discectomy”, “microdiscectomy”, “minimally invasive surgery”, “endoscopic”, “laser”, and “percutaneous discectomy”, with appropriate combinations of operators “AND”, “OR”, and “NOT” as described in the Electronic Supplementary Material 1 (ESM_1). The reference lists of relevant studies were evaluated for the purposes of the present study. The language of the included studies was restricted to English. The review protocols are registered on PROSPERO (International Prospective Register of Systematic Reviews number, CRD42020150582).
Inclusion criteria
-
1.
Randomized controlled trials (RCTs) and cohort studies;
-
2.
Studies which reported the comparisons between any of the minimally invasive surgeries (MED, PELD, PLDD, and tubular discectomy as comparator group) and OD/MD (as control group) for symptomatic LDH patients;
-
3.
Studies which reported at least one of the following outcomes:
-
i.
Primary outcomes including the overall complication rate and complications in two different classification schemes (general classification and MCDC).
Overall complications were defined as complications related to various discectomy surgeries.
General classification divides the complications into intraoperative and post-operative complications. Intraoperative general complications included mortality, thrombosis, and hepatitis; intraoperative specific complications included durotomy, bleeding, nerve root injury, and surgical error; post-operative general complications included urinary tract infection, miction disturbances (catheter required), pulmonary complications, and deep venous thrombosis; post-operative specific complications included infection superficial or deep, haematoma, reherniation, neurologic problems (post-operative weakness, altered sensitivity), skin problems, and psychological and coping problems.
MCDC scheme includes five types of complications:
Type I: normal recovery without intervention or pharmacologic treatment;
Type II: pharmacologic treatment needed;
Type III: invasive intervention under general anaesthesia needed;
Type IV: intensive care unit admission needed;
Type V: death.
-
ii.
The reoperation rate was included as a secondary outcome.
-
i.
Exclusion criteria
-
1.
Studies which compared discectomy procedures with other spinal surgeries, such as chemical nucleolysis, intradiscal electrothermal annuloplasty, and surgeries involving the use of an implant;
-
2.
Case reports, reviews, and conference reports;
-
3.
In vitro biomechanical studies and computational modelling studies.
Selection of studies
Two reviewers (XLC and JVC) independently reviewed all titles and abstracts that were identified in the initial online search of databases. Full-text articles and reference lists were reviewed for the relevant abstracts. When consensus could not be reached between the reviewers, a third reviewer (ADD) was consulted to resolve the disagreement.
Data extraction
Two reviewers (XLC and JVC) extracted data independently. The reviewers collected the following data: methods (study design, sample size, inclusion and exclusion criteria, study period, mean duration of follow-up), participants (number of participants, age, gender), interventions (surgical procedure), and outcomes (for each primary outcome: number of subjects and occurrence rate in general complication classification and MCDC, and revision surgery rate).
Quality assessment
The 13 criteria recommended in the Cochrane Back and Neck Group guidelines [33] were used to assess the risk of bias of RCTs that were included in this meta-analysis. “Low risk”, “high risk”, or “unclear risk” were used to score the risk of bias for individual criteria. Thereafter, for the overall risk of bias evaluation, a “low overall risk” of bias was attributed to the study when seven or more of the 13 criteria were considered low risk [33]. Studies with six or less low-risk criteria were considered as having a “high overall risk” of bias.
The Newcastle–Ottawa Scale (NOS) was used to assess the methodological quality of the included cohort studies [34]. The “star system” of NOS ranges from 0 to 9, which is judged on three broad perspectives: selection of the study, comparability, and the ascertainment of the outcome of interest. In this meta-analysis, a study awarded seven or more stars was regarded as high quality.
A sensitivity analysis was conducted to assess the impact of including studies with a high overall risk of bias. Controversial scores were resolved by the third reviewer (ADD).
Statistical analysis
We performed two separate meta-analyses (one for the RCTs and the other for the cohort studies) to examine the consistency of various studies with different potential biases.
Pooled mean complication rates were calculated by the summation of total complication events divided by the overall number of patients included in the studies reporting that specific complication. Interstudy median and interquartile range (IQR), which ranged from the first to the third quartile (Q1–Q3), were used to assess the variations in specific cross-study complication rates. The pooled estimates of risk ratio (RR) and 95% confidence intervals (CI) for direct comparisons were reported. Chi-squared (I2) statistic was used to measure heterogeneity among the trials. I2 < 50% implied homogeneity, and the analysis included a fixed-effects model by the Mantel–Haenszel method. I2 > 50% indicated heterogeneity, and consequently, a random-effects model was used according to the DerSimonian–Laird method. Meta-analyses results were also assessed using forest plots. Risk of publication bias was evaluated using the Begg–Mazumdar test. The statistical significance was set at 5% (α = 0.05).
This meta-analysis was performed according to the quality of reporting of meta-analyses group and the meta-analysis of Observational Studies in Epidemiology group recommendations for improving the quality of reporting of meta-analyses of clinical RCTs and observational studies, respectively [35, 36]. RevMan (Review Manager 5.3 version. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.) was used to evaluate the risk of bias in RCTs, and STATA software (Release 15, StataCorp LLC, TX) was used for the statistical analyses.
Evaluating the quality of evidence
The quality of the evidence informing this meta-analysis was assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) scale, which rated evidence quality as high, moderate, low, or very low using factors such as the risk of bias, inconsistency, indirectness, imprecision, and publication bias [37] (ESM_2_Table 1). The summary of findings (SoFs) table presents the endpoint of the GRADE evidence summary (ESM_2_Table 2).
Results
Study selection
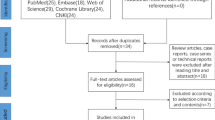
The literature search is illustrated in the PRISMA flow diagram (Fig. 1). Thirty-seven studies met the selection criteria for the purposes of the present review, which included 17 RCTs [13, 14, 25,26,27, 38,39,40,41,42,43,44,45,46,47,48,49] and 20 cohort studies [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69].
Flow chart showing the procedure and results of the literature search in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [32]. MED microendoscopic discectomy, PELD percutaneous endoscopic lumbar discectomy, PLDD percutaneous laser disc decompression
Quality assessment
The detailed risk of bias in RCTs is summarized in Fig. 2. Two of the 17 studies had a high overall risk of bias [44, 48]. Five studies were classified as having a high risk of selection bias [38, 41, 42, 46, 47]. Ten studies were deemed to have a high risk of performance bias [13, 14, 25, 26, 39, 41, 42, 46, 47, 49], and seven studies were assessed as unclear [27, 38, 40, 43,44,45, 48]. We assessed all the studies as having low attrition bias except three studies that did not clearly report [38, 40, 48]. Five studies were assessed as having a high risk of detection bias [39, 46,47,48,49]. None were assessed as having a reporting bias or other biases.
Risk of bias summary: review authors’ judgements about each risk of bias item for each randomized controlled trial included in this review. “Was the method of randomisation adequate?”, “Was the treatment allocation concealed?”, and “Were the groups similar at baseline regarding the most important prognostic indicators?” were used to assess the selection bias. “Was the patient blinded to the intervention?”, “Was the care provider blinded to the intervention?”, “Were cointerventions avoided or similar?”, and “Was the compliance acceptable in all groups?” were used to assess the performance bias. “Was the drop-out rate described and acceptable?” and “Were all randomized participants analysed in the group to which they were allocated?” were used to assess the attrition bias. “Was the outcome assessor blinded to the intervention?” and “Was the timing of the outcome assessment similar in all groups?” were used to assess the detection bias. “Are reports of the study free of suggestion of selective outcome reporting?” was used to assess the reporting bias. “Are other sources of potential bias unlikely?” was used to assess the other bias
The methodological quality of cohort studies was assessed using NOS. All cohort studies were awarded more than seven stars, which demonstrated high quality (Table 1).
Demographic data, surgical technique, and surgery-related complications from the 37 included studies are provided in Table 2. The number of pairwise studies reporting complication rates for different discectomy techniques varied: MED versus OD/MD (n = 10), PELD versus OD/MD (n = 13), PLDD versus OD/MD (n = 4), and tubular discectomy versus OD/MD (n = 10) (ESM_2_Table 3).
Meta-analysis of RCTs
Complication rates
Complications were calculated from the 17 RCTs for a total of 1967 patients with a mean follow-up duration of 24.2 months [13, 14, 25,26,27, 38,39,40,41,42,43,44,45,46,47,48,49], which included 1018 OD/MD patients with a mean follow-up duration of 33.2 months, 288 MED patients with a mean follow-up duration of 35.1 months, 258 PELD patients with a mean follow-up duration of 19.1 months, 155 PLDD patients with a mean follow-up duration of 18 months, and 248 tubular discectomy patients with a mean follow-up duration of 17.3 months (Tables 2, 3). Studies reporting OD/MD, MED, PELD, PLDD, and tubular discectomies had overall complication rates (pooled mean) of 16.8% and 16.1%, 21.2%, 5.8%, 8.4%, and 25.8%, respectively.
OD/MD, MED, PELD, PLDD, and tubular discectomy were associated with intraoperative complication rates of 6.4%, 6.8%, 7.6%, 0.0%, and 8.1%, respectively; and post-operative complications occurred in 10.2%, 11.4%, 10.4%, 6.6%, and 8.4%, respectively.
The rate of occurrence of Type 1 (per MCDC) events in OD/MD, MED, PELD, PLDD, and tubular discectomy was 10.8%, 12.2%, 13.3%, 0.0% and 3.5%, respectively. Type II complication rates were 5.5% following OD/MD, 2.4% following MED, and 0.0% following PLDD, PELD, and tubular discectomy. Type III complication rates were 7.2% following OD/MD, 7.0% following MED, 4.7% following PELD, 8.4% following PLDD, and 8.1% following tubular discectomy.
Incidence of durotomy was reported in 4.6% of OD/MD, 6.8% of MED, 0.0% of PELD, and 6.5% of tubular discectomy. OD/MD, MED, PELD, PLDD, and tubular discectomy studies reported reherniation rates of 5.5%, 4.7%, 5.8%, 8.4%, and 7.3%, respectively. Studies performing OD/MD, MED, PELD, PLDD, and tubular discectomy resulted in reoperation rates of 8.4%, 4.7%, 6.7%, 23.2%, and 11.7%, respectively (Fig. 3).
Unweighted averages of complication rates of discectomy/microdiscectomy (OD/MD), microendoscopic discectomy (MED), percutaneous endoscopic lumbar discectomy (PELD), percutaneous laser disc decompression (PLDD), and tubular discectomy for symptomatic lumbar disc herniation (LDH) using two different classification schemes (general classification and modified Clavien–Dindo classification) from randomized controlled trials (RCTs). The number of patients in each discectomy technique is mentioned in Table 3. Tub tubular discectomy, intra-op intraoperative, post-op post-operative
MED versus OD/MD
The level of evidence was of low quality due to lack of precision in the data and lack of blinding [41, 42, 46, 49]. No significant difference was found in the overall complication rates, intraoperative complication rates, post-operative complication rates, occurrence rate of Type I to Type III complications (per MCDC), durotomy rates, and incidence of reherniation and reoperation between the two procedures (Table 3).
PELD versus OD/MD
There was moderate-quality evidence of a lower risk of overall complications (RR = 0.52, 95% CI 0.29–0.91) and high-quality evidence of a lower risk of Type I complications per MCDC (RR = 0.37, 95% CI 0.16–0.81) for PELD versus OD/MD comparison (Table 3, ESM_3_Figure 1 and ESM_3_Figure 2). No significant difference was found in the intraoperative complication rates, post-operative complication rates, occurrence rates of Type I and Type III complications (per MCDC), incidence of durotomy, reherniation, and reoperation between the two procedures. We rated all the level of evidence as moderate quality due to imprecision in the reported data and lack of blinding in estimates [26, 39, 43,44,45, 47].
PLDD versus OD/MD
There was low-quality evidence and no statistically significant difference between PLDD and OD/MD for overall complication rates, post-operative complication rates, the occurrence rate of Type III complications (per MCDC), incidence of reherniation, and reoperation rates (Table 3) [13, 14]. We rated the quality of evidence as low due to the lack of precision in data and lack of blinding.
Tubular discectomy versus OD/MD
The level of evidence was of low quality for lack of precision in data and lack of blinding [25, 38, 48]. No significant difference was found in intraoperative complication rates, post-operative complication rates, occurrence rates of Type I and Type III complications (per MCDC), durotomy rates, reherniation and reoperation rates between the two procedures (Table 3). Additionally, inconsistency in findings, lack of blinding, and lack of precision in the reported data downgraded the quality of evidence for overall complication rates to very low.
Meta-analysis of cohort studies
Complication rates
Complications were calculated from 4945 patients with a mean follow-up duration of 19.9 months from the 20 cohort studies [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69], including 2530 OD/MD patients with a mean follow-up duration of 20.2 months, 999 MED patients with a mean follow-up duration of 37.8 months, 514 PELD patients with a mean follow-up duration of 19.1 months, 540 PLDD patients with a mean follow-up duration of 17 months, and 362 tubular discectomy patients with a mean follow-up duration of 10.3 months (Tables 2, 3). Studies reporting OD/MD, MED, PELD, PLDD, and tubular discectomies had overall complication rates (pooled mean) of 7.6%, 6.2%, 9.1%, 3.5%, and 11.6%, respectively.
OD/MD, MED, PELD, PLDD, and tubular discectomy were associated with intraoperative complication rates of 2.6%, 1.7%, 0.9%, 0.0%, and 7.9%, respectively. Post-operative complications occurred in 6.0%, 3.8%, 8.0%, 0.0%, and 3.5% of LDH patients who underwent OD/MD, MED, PELD, PLDD, and tubular discectomy, respectively.
The occurrence of Type I complications (per MCDC) in OD/MD, MED, PELD, PLDD, and tubular discectomies was 2.7%, 2.1%, 1.2%, 0.0%, and 7.9%, respectively. The occurrence of Type II complications was 2.7% following OD/MD, 2.3% following MED, and 0.0% following PLDD, PELD, and tubular discectomy. Similarly, Type III complications were 4.6% following OD/MD, 2.3% following MED, 4.7% following PELD, 4.4% following PLDD, and 3.2% following tubular discectomy.
Incidence of durotomy was reported in 2.6% of OD/MD, 1.7% of MED, 0.9% of PELD, 0.0% of PLDD, and 7.9% of tubular discectomy patients. OD/MD, MED, PELD, PLDD, and tubular discectomy studies reported reherniation rates of 4.2%, 0.8%, 5.6%, 3.5%, and 4.8%, respectively. Studies reporting data for OD/MD, MED, PELD, PLDD, and tubular discectomies had reoperation rates of 5.5%, 0.8%, 9.4%, 3.2%, and 3.7%, respectively (Fig. 4).
Unweighted averages of complication rates for discectomy/microdiscectomy (OD/MD), microendoscopic discectomy (MED), percutaneous endoscopic lumbar discectomy (PELD), percutaneous laser disc decompression (PLDD), and tubular discectomy for symptomatic lumbar disc herniation (LDH) using two different classification schemes (general classification and modified Clavien–Dindo classification) from cohort studies. The number of patients in each discectomy technique is mentioned in ESM_2_Table 4. Tub tubular discectomy, intra-op intraoperative, post-op post-operative
MED versus OD/MD
There was moderate-quality evidence of a higher risk of Type III complications (per MCDC) (RR = 10.83, 95% CI 1.29–91.18) (ESM_3_Figure 3) for MED versus OD/MD comparison [52, 64, 66, 68]. The large magnitude of effect upgraded the low-quality evidence from cohort studies to moderate quality. However, inconsistency in findings, high risk of bias of cohort studies, and lack of precision in the reported data downgraded the quality of no statistically significant difference between MED and OD/MD for the different complication rates, except for the occurrence rate for Type III complications, to very low.
PELD versus OD/MD
There was low-quality evidence for a higher risk of reherniation (RR = 1.67, 95% CI 1.05–2.64) (ESM_3_Figure 4) and reoperation (RR = 1.75, 95% CI 1.20–2.55) (ESM_3_Figure 5) for PELD versus OD/MD [50, 51, 58, 59, 61, 65, 69]. We rated the quality of other complication rates with no statistical significance as very low due to high risk of bias and limited precision in estimates.
PLDD versus OD/MD
There was low-quality evidence of a lower risk of overall complication rates (RR = 0.42, 95% CI 0.25–0.70) (ESM_3_Figure 6), post-operative complication rates (RR = 0.42, 95% CI 0.25–0.70) (ESM_3_Figure 7), Type III complications (per MCDC) (RR = 0.39, 95% CI 0.22–0.69) (ESM_3_Figure 8), and reoperation rates (RR = 0.39, 95% CI 0.22–0.69) (ESM_3_Figure 10) for PLDD versus OD/MD comparison [60, 67]. We rated the quality of evidence as low due to high risk of bias, inconsistency in findings, and publication bias. However, there was no large magnitude of effect to upgrade the very low-quality evidence of a lower risk of reherniation (RR = 0.56, 95% CI 0.33–0.97) (ESM_3_Figure 9) for PLDD versus OD/MD.
Tubular discectomy versus OD/MD
The quality of evidence comparing tubular discectomy versus OD/MD was very low due to imprecision in the reported data and high risk of bias. No significant difference between the complication rates per the two complication classification schemes (ESM_2_Table 4) was found between these two procedures [53,54,55,56,57, 62, 63].
Discussion
We conducted a systematic review and meta-analysis of the complication rates associated with various discectomy techniques for symptomatic LDH. Complication rates in different classification schemes and reoperation rates were extracted from 17 RCTs and 20 cohort studies.
Although safety assessment has been widely used in lumbar spine surgeries and the complication rates of a procedure are paramount to said assessment, there is no standardized way of reporting surgical complications. The general classification divides the complications into intraoperative and post-operative complications, according to the time when they become apparent [24]. It may be useful for the management of spine surgery complications to have clear guidelines for symptoms. Therapeutic consequences have been recommended as a way of classifying complications in spine surgery [28, 29]. MCDC scheme is based on the management required for each complication, which can guide clinical decision-making based on the severity of complications. We used the general classification and MCDC to evaluate the complications following discectomy surgeries for symptomatic LDH.
The hierarchy of different discectomy techniques regarding complication rates is conducive to the selection of the surgical technique. Our NMA showed a clear ranking of different discectomy techniques by their complication rates using these two classification schemes [31], which may provide a basis for deciding the surgical technique. The present systematic review and meta-analysis reports a comprehensive list of complication rates following different discectomy techniques and elucidate differences between OD/MD group and various minimally invasive discectomy techniques.
MED versus OD/MD
In our systematic review and meta-analysis, we identified a number of complications following OD/MD and MED from RCTs and cohort studies. There were differences in pooled mean complication rates following both surgical techniques (Table 3 and ESM_2_Table 4). Previous studies reported that the incidence of nerve root injury, durotomy, and reoperation in MED group was higher than that in the OD group [46, 49], which is supported by our meta-analysis results (Table 3 and ESM_2_Table 4). A possible explanation is the poor perception of depth with microendoscopic surgery and the restricting surgical field, which limit surgeons to orientate surgical instruments. However, the complications data from RCTs did not reach statistical significance. The low quality of evidence across outcomes was due to imprecision in the reported data [41, 42, 46, 49] and poor allocation (four studies were assessed as having an unclear risk) [27, 40, 46, 49] or lack of blinding to intervention (two studies were assessed as having an unclear risk [27, 40] and four studies were assessed as having a high risk [41, 42, 46, 49]). Additionally, the inconsistency in Type I complications per MCDC (I2 = 51% > 50%) downgraded the evidence to very low.
We found that MED was associated with a lower risk of Type III complications per MCDC from cohort studies (ESM_3_Figure 3). The finding indicated that a good visualization of discectomy and enhanced identification of anatomical structures through microendoscope results in a low incidence of complications requiring surgical treatment. Due to the low quality of cohort studies and large magnitude of effect, this result was assessed as moderate-quality evidence.
PELD versus OD/MD
Compared with OD/MD, PELD magnifies the operative field with a camera system so that the surgeon can identify and protect the dural sac and nerve roots. A previous meta-analysis showed a higher complication rate in the PELD group (4.69%) compared with the MD group (2.33%), but the differences were not significant [70]. There was a difference in complication rates between the two groups when data from RCTs were pooled (Table 3). We found that PELD was associated with a lower risk of overall complications (ESM_3_Figure 1) and a lower risk of Type I complications per MCDC (ESM_3_Figure 2). We also found that PELD was associated with a lower risk of reherniations (ESM_3_Figure 4) and reoperations (ESM_3_Figure 5) from cohort studies. These findings are inconsistent with previously reported data [70,71,72], which may partly be due to differences in study selection and the classification of complications. The percutaneous procedure causes less damage to surrounding tissues and obtains a good operative field through an endoscope, which are posited as the primary reasons for the lower overall complication rates. In the GRADE approach, RCTs start as high-quality evidence and cohort studies as low-quality evidence, but both can be rated down if most of the relevant evidence comes from studies that suffer from a high risk of bias [73]. The lower risk of overall complications in the PELD group was rated moderate quality due to poor allocation (one study was assessed as having high risk [47] and three studies were assessed as having unclear risk [43,44,45]) and lack of blinding (three studies were assessed as having high risk [26, 39, 47] and three studies were assessed as having unclear risk [43,44,45]) in the included studies. Additionally, a large magnitude of effect (RR = 0.37 < 0.5) upgraded the lower risk of Type I complications per MCDC for PELD versus OD/MD to high quality. The quality of all the complication rates from cohort studies is rated low or very low due to high risk of bias and/or some imprecision in estimates.
PLDD versus OD/MD
Advantages of PLDD over OD/MD are decreased tissue injury and fewer post-operative complications, such as bleeding, infection, and post-operative pain for soft tissue exposure [13], which were supported by our results (Table 3 and ESM_2_Table 4). We also found that PLDD had a lower risk of post-operative complications (low quality due to high risk of bias (cohort studies), inconsistency in findings (I2 = 55) and large magnitude of effect (RR = 0.42 < 0.5)), lower type III complications per MCDC (low quality due to high risk of bias (cohort studies), publication bias (P = 0) and large magnitude of effect (RR = 0.39 < 0.5)), lower reherniation rate (very low quality due to high risk of bias (cohort studies) and inconsistency in findings (I2 = 67)), and lower reoperation rate (low quality due to high risk of bias (cohort studies), publication bias (P = 0), and large magnitude of effect (RR = 0.39 < 0.5)). However, the limited study sample (n = 1) [67] leaves the inferences drawn open to question.
Tubular discectomy versus OD/MD
In theory, the tubular retractor with or without a microscope could help a surgeon gain better view of the operative field and result in less surgical trauma than the conventional open approach, all of which is expected to reduce intraoperative complications [19]. Compared with OD/MD, MED had a higher pooled mean intraoperative complication rate when data from cohort studies were pooled (8.4% in OD/MD group versus 8.1% in MED group). In contrast, MED had a lower complication rate when data from RCTs were pooled (6.7% in OD/MD group versus 7.9% in MED group) (Table 3 and ESM_2_Table 4). However, the differences in intraoperative complication rates between OD/MD and MED showed no statistical significance, which is consistent with previously reported data [19].
Although the results of our systematic review and meta-analysis are comprehensive, there are certain limitations which must be noted. Firstly, the small sample size of direct comparisons from RCTs may have reduced the statistical robustness of the results. Secondly, there is substantive heterogeneity in the studies due to wide variation in the duration of follow-up, and some post-operative complications may have a gestation period. Thirdly, there is a learning curve associated with the adoption of any new technology and surgical technique, and chronologically older discectomy procedures may have an advantage over newer approaches in reduced complication rates. Finally, the primary literature is varied and does not routinely discuss age and surgical levels in reporting complications, which may increase heterogeneity and reveal inherent differences associated with complications. Further, well-defined RCTs with large sample sizes are needed to improve the predictive strength of such pairwise comparisons.
Conclusion
Compared with OD/MD, results of this meta-analysis suggest that for the surgical treatment of symptomatic LDH, PELD has a lower risk of overall complications and a lower risk of complications necessitating conservative treatment. The resultant list of complication rates presented here will provide useful insights to patients and clinicians while assessing the benefits and risks associated with a specific discectomy technique.
References
Gibson JN, Cowie JG, Iprenburg M (2012) Transforaminal endoscopic spinal surgery: the future ‘gold standard’ for discectomy? A review. Surgeon 10:290–296. https://doi.org/10.1016/j.surge.2012.05.001
Postacchini F (1999) Management of herniation of the lumbar disc. J Bone Jt Surg Br 81:567–576
Luo X, Pietrobon R, Sun SX, Liu GG, Gey L (2004) Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976) 29:79–86. https://doi.org/10.1097/01.brs.0000105527.13866.0f
Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, Cho CH, DePalma MJ, Dougherty P 2nd, Fernand R, Ghiselli G, Hanna AS, Lamer T, Lisi AJ, Mazanec DJ, Meagher RJ, Nucci RC, Patel RD, Sembrano JN, Sharma AK, Summers JT, Taleghani CK, Tontz WL Jr, Toton JF, North American Spine S (2014) An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J 14:180–191. https://doi.org/10.1016/j.spinee.2013.08.003
Haines SJ, Jordan N, Boen JR, Nyman JA, Oldridge NB, Lindgren BR (2002) Discectomy strategies for lumbar disc herniation: results of the LAPDOG trial. J Clin Neurosci 9:411–417. https://doi.org/10.1054/jocn.2002.1120
Thomé C, Barth M, Scharf J, Schmiedek P (2005) Outcome after lumbar sequestrectomy compared with microdiscectomy: a prospective randomized study. J Neurosurg Spine 2:271–278. https://doi.org/10.3171/spi.2005.2.3.0271
Barrios C, Ahmed M, Arrótegui J, Björnsson A, Gillström P (1990) Microsurgery versus standard removal of the herniated lumbar disc. A 3-year comparison in 150 cases. Acta Orthop Scand 61:399–403
Silverplats K, Lind B, Zoëga B, Halldin K, Gellerstedt M, Brisby H, Rutberg L (2010) Clinical factors of importance for outcome after lumbar disc herniation surgery: long-term follow-up. Eur Spine J 19:1459–1467. https://doi.org/10.1007/s00586-010-1433-7
Virk SS, Diwan A, Phillips FM, Sandhu H, Khan SN (2017) What is the rate of revision discectomies after primary discectomy on a national scale? Clin Orthop Relat Res 475:2752–2762. https://doi.org/10.1007/s11999-017-5467-6
Rasouli MR, Rahimi-Movaghar V, Shokraneh F, Moradi-Lakeh M, Chou R (2014) Minimally invasive discectomy versus microdiscectomy/open discectomy for symptomatic lumbar disc herniation. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd010328.pub2
Deen HG, Fenton DS, Lamer TJ (2003) Minimally invasive procedures for disorders of the lumbar spine. Mayo Clin Proc 78:1249–1256
Patel N, Singh V (2018) Percutaneous lumbar laser discectomy: literature review and a retrospective analysis of 65 cases. Photomed Laser Surg 36:518–521. https://doi.org/10.1089/pho.2018.4460
Abrishamkar S, Kouchakzadeh M, Mirhosseini A, Tabesh H, Rezvani M, Moayednia A, Ganjeifar B, Mahabadi A, Yousefi E, Kooshki AM (2015) Comparison of open surgical discectomy versus plasma-laser nucleoplasty in patients with single lumbar disc herniation. J Res Med Sci 20:1133–1137. https://doi.org/10.4103/1735-1995.172979
Brouwer PA, Brand R, van den Akker-van Marle ME, Jacobs WCH, Schenk B, van den Berg-Huijsmans AA, Koes BW, Arts MA, van Buchem MA, Peul WC (2017) Percutaneous laser disc decompression versus conventional microdiscectomy for patients with sciatica: two-year results of a randomised controlled trial. Interv Neuroradiol 23:313–324. https://doi.org/10.1177/1591019917699981
Foley KT, Smith MM, Rampersaud YR (1999) Microendoscopic approach to far-lateral lumbar disc herniation. Neurosurg Focus 7:e5
Kambin P (1992) Arthroscopic microdiscectomy. Arthroscopy 8:287–295
Alvi MA, Kerezoudis P, Wahood W, Goyal A, Bydon M (2018) Operative approaches for lumbar disc herniation: a systematic review and multiple treatment meta-analysis of conventional and minimally invasive surgeries. World Neurosurg 114(391–407):e392. https://doi.org/10.1016/j.wneu.2018.02.156
Anichini G, Landi A, Caporlingua F, Beer-Furlan A, Brogna C, Delfini R, Passacantilli E (2015) Lumbar endoscopic microdiscectomy: where are we now? An updated literature review focused on clinical outcome, complications, and rate of recurrence. Biomed Res Int 2015:417801. https://doi.org/10.1155/2015/417801
Li X, Chang H, Meng X (2018) Tubular microscopes discectomy versus conventional microdiscectomy for treating lumbar disk herniation: systematic review and meta-analysis. Medicine 97:e9807. https://doi.org/10.1097/MD.0000000000009807
Riesenburger RI, David CA (2006) Lumbar microdiscectomy and microendoscopic discectomy. Minim Invasive Ther Allied Technol 15:267–270. https://doi.org/10.1080/13645700600958432
Clark AJ, Safaee MM, Khan NR, Brown MT, Foley KT (2017) Tubular microdiscectomy: techniques, complication avoidance, and review of the literature. Neurosurg Focus 43:E7. https://doi.org/10.3171/2017.5.FOCUS17202
Yorimitsu E, Chiba K, Toyama Y, Hirabayashi K (2001) Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine (Phila Pa 1976) 26:652–657
Shriver MF, Xie JJ, Tye EY, Rosenbaum BP, Kshettry VR, Benzel EC, Mroz TE (2015) Lumbar microdiscectomy complication rates: a systematic review and meta-analysis. Neurosurg Focus 39:E6. https://doi.org/10.3171/2015.7.FOCUS15281
Kraemer R, Wild A, Haak H, Herdmann J, Krauspe R, Kraemer J (2003) Classification and management of early complications in open lumbar microdiscectomy. Eur Spine J 12:239–246. https://doi.org/10.1007/s00586-002-0466-y
Arts MP, Brand R, van den Akker ME, Koes BW, Bartels RH, Tan WF, Peul WC (2011) Tubular diskectomy vs conventional microdiskectomy for the treatment of lumbar disk herniation: 2-year results of a double-blind randomized controlled trial. Neurosurgery 69:135–144. https://doi.org/10.1227/neu.0b013e318214a98c(discussion 144)
Ding ZM, Tao YQ (2017) Clinical outcomes of percutaneous transforaminal endoscopic discectomy versus fenestration discectomy in patients with lumbar disc herniation. J Int Transl Med 5:29–33. https://doi.org/10.11910/2227-6394.2017.05.01.06
Garg B, Nagraja UB, Jayaswal A (2011) Microendoscopic versus open discectomy for lumbar disc herniation: a prospective randomised study. J Orthop Surg 19:30–34
Lebude B, Yadla S, Albert T, Anderson DG, Harrop JS, Hilibrand A, Maltenfort M, Sharan A, Vaccaro AR, Ratliff JK (2010) Defining “complications” in spine surgery: neurosurgery and orthopedic spine surgeons’ survey. J Spinal Disord Tech 23:493–500. https://doi.org/10.1097/BSD.0b013e3181c11f89
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Landriel Ibanez FA, Hem S, Ajler P, Vecchi E, Ciraolo C, Baccanelli M, Tramontano R, Knezevich F, Carrizo A (2011) A new classification of complications in neurosurgery. World Neurosurg 75:709–715. https://doi.org/10.1016/j.wneu.2010.11.010(discussion 604–711)
Chen X, Chamoli U, Lapkin S, Castillo JV, Diwan AD (2019) Complication rates of different discectomy techniques for the treatment of lumbar disc herniation: a network meta-analysis. Eur Spine J 28:2588–2601. https://doi.org/10.1007/s00586-019-06142-7
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, Bronfort G, van Tulder MW (2015) 2015 Updated method guideline for systematic reviews in the cochrane back and neck group. Spine 40:1660–1673. https://doi.org/10.1097/brs.0000000000001061
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysis. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 354:1896–1900. https://doi.org/10.1016/s0140-6736(99)04149-5
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–2012. https://doi.org/10.1001/jama.283.15.2008
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64:401–406. https://doi.org/10.1016/j.jclinepi.2010.07.015
Franke J, Greiner-Perth R, Boehm H, Mahlfeld K, Grasshoff H, Allam Y, Awiszus F (2009) Comparison of a minimally invasive procedure versus standard microscopic discotomy: a prospective randomised controlled clinical trial. Eur Spine J 18:992–1000. https://doi.org/10.1007/s00586-009-0964-2
Hermantin FU, Peters T, Quartararo L, Kambin P (1999) A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am 81:958–965
Huang TJ, Hsu RW, Li YY, Cheng CC (2005) Less systemic cytokine response in patients following microendoscopic versus open lumbar discectomy. J Orthop Res 23:406–411
Hussein M (2016) Minimal incision, multifidus-sparing microendoscopic diskectomy versus conventional microdiskectomy for highly migrated intracanal lumbar disk herniations. J Am Acad Orthop Surg 24:805–813. https://doi.org/10.5435/jaaos-d-15-00588
Hussein M, Abdeldayem A, Mattar MM (2014) Surgical technique and effectiveness of microendoscopic discectomy for large uncontained lumbar disc herniations: a prospective, randomized, controlled study with 8 years of follow-up. Eur Spine J 23:1992–1999. https://doi.org/10.1007/s00586-014-3296-9
Mayer HM, Brock M (1993) Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg 78:216–225
Pan L, Zhang P, Yin Q (2014) Comparison of tissue damages caused by endoscopic lumbar discectomy and traditional lumbar discectomy: a randomised controlled trial. Int J Surg 12:534–537. https://doi.org/10.1016/j.ijsu.2014.02.015
Pan Z, Ha Y, Yi S, Cao K (2016) Efficacy of transforaminal endoscopic spine system (TESSYS) technique in treating lumbar disc herniation. Med Sci Monit 22:530–539. https://doi.org/10.12659/msm.894870
Righesso O, Falavigna A, Avanzi O (2007) Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: results of a randomized controlled trial. Neurosurgery 61:545–549. https://doi.org/10.1227/01.NEU.0000280008.72190.15
Ruetten S, Komp M, Merk H, Godolias G (2008) Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976) 33:931–939. https://doi.org/10.1097/brs.0b013e31816c8af7
Ryang YM, Oertel MF, Mayfrank L, Gilsbach JM, Rohde V (2008) Standard open microdiscectomy versus minimal access trocar microdiscectomy: results of a prospective randomized study. Neurosurgery 62:174–181. https://doi.org/10.1227/01.NEU.0000296996.00030.3F
Teli M, Lovi A, Brayda-Bruno M, Zagra A, Corriero A, Giudici F, Minoia L (2010) Higher risk of dural tears and recurrent herniation with lumbar micro-endoscopic discectomy. Eur Spine J 19:443–450. https://doi.org/10.1007/s00586-010-1290-4
Ahn SS, Kim SH, Kim DW, Lee BH (2016) Comparison of outcomes of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for young adults: a retrospective matched cohort study. World Neurosurg 86:250–258
Choi KC, Kim JS, Park CK (2016) Percutaneous endoscopic lumbar discectomy as an alternative to open lumbar microdiscectomy for large lumbar disc herniation. Pain Phys 19:E291–E300
Schizas C, Tsiridis E, Saksena J (2005) Microendoscopic discectomy compared with standard microsurgical discectomy for treatment of uncontained or large contained disc herniations. Neurosurgery 57:357–360 (discussion 357–360)
Bennis S, Scarone P, Lepeintre JF, Aldea S, Gaillard S (2009) Transtubular versus microsurgical approach for single lumbar disc herniation: a prospective study. Eur J Orthop Surg Traumatol 19:535–540
Bhatia PS, Chhabra HS, Mohapatra B, Nanda A, Sangodimath G, Kaul R (2016) Microdiscectomy or tubular discectomy: is any of them a better option for management of lumbar disc prolapse. J Craniovertebral Junct Spine 7:146–152
Cahill KS, Levi AD, Cummock MD, Liao W, Wang MY (2013) A comparison of acute hospital charges after tubular versus open microdiskectomy. World Neurosurg 80:208–212. https://doi.org/10.1016/j.wneu.2012.08.015
Choi YY, Yoon SH, Ha Y, Kim EY, Park HC, Park CO (2006) Posterior microscopic lesionectomy for lumbar disc herniation with tubular retraction using METRx(TM) system. J Korean Neurosurg Soc 40:406–411
German JW, Adamo MA, Hoppenot RG, Blossom JH, Nagle HA (2008) Perioperative results following lumbar discectomy: comparison of minimally invasive discectomy and standard microdiscectomy. Neurosurg Focus 25:E20. https://doi.org/10.3171/FOC/2008/25/8/E20
Hsu HT, Chang SJ, Yang SS, Chai CL (2013) Learning curve of full-endoscopic lumbar discectomy. Eur Spine J 22:727–733
Kim MJ, Lee SH, Jung ES, Son BG, Choi ES, Shin JH, Sung JK, Chi YC (2007) Targeted percutaneous transforaminal endoscopic diskectomy in 295 patients: comparison with results of microscopic diskectomy. Surg Neurol 68:623–631. https://doi.org/10.1016/j.surneu.2006.12.051
Kim SK, Lee SC, Park SW (2018) Trans-sacral epiduroscopic laser decompression versus the microscopic open interlaminar approach for L5-S1 disc herniation. J Spinal Cord Med 43:46–52
Kleinpeter G, Markowitsch MM, Bock F (1995) Percutaneous endoscopic lumbar discectomy: minimally invasive, but perhaps only minimally useful? Surg Neurol 43:534–541
Lau D, Han SJ, Lee JG, Lu DC, Chou D (2011) Minimally invasive compared to open microdiscectomy for lumbar disc herniation. J Clin Neurosci 18:81–84
Lee P, Liu JC, Fessler RG (2011) Perioperative results following open and minimally invasive single-level lumbar discectomy. J Clin Neurosci 18:1667–1670
Liu WG, Wu XT, Guo JH, Zhuang SY, Teng GJ (2010) Long-term outcomes of patients with lumbar disc herniation treated with percutaneous discectomy: comparative study with microendoscopic discectomy. Cardiovasc Interv Radiol 33:780–786
Liu X, Yuan S, Tian Y, Wang L, Gong L, Zheng Y, Li J (2018) Comparison of percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, and microdiscectomy for symptomatic lumbar disc herniation: minimum 2-year follow-up results. J Neurosurg Spine 28:317–325. https://doi.org/10.3171/2017.6.SPINE172
Nakagawa H, Kamimura M, Uchiyama S, Takahara K, Itsubo T, Miyasaka T (2003) Microendoscopic discectomy (MED) for lumbar disc prolapse. J Clin Neurosci 10:231–235
Tassi GP (2006) Comparison of results of 500 microdiscectomies and 500 percutaneous laser disc decompression procedures for lumbar disc herniation. Photomed Laser Surg 24:694–697
Wu X, Zhuang S, Mao Z, Chen H (2006) Microendoscopic discectomy for lumbar disc herniation: surgical technique and outcome in 873 consecutive cases. Spine (Phila Pa 1976) 31:2689–2694. https://doi.org/10.1097/01.brs.0000244615.43199.07
Yoon SM, Ahn SS, Kim KH, Kim YD, Cho JH, Kim DH (2012) Comparative study of the outcomes of percutaneous endoscopic lumbar discectomy and microscopic lumbar discectomy using the tubular retractor system based on the VAS, ODI, and SF-36. Korean J Spine 9:215–222. https://doi.org/10.14245/kjs.2012.9.3.215
Ruan W, Feng F, Liu Z, Xie J, Cai L, Ping A (2016) Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: a meta-analysis. Int J Surg 31:86–92. https://doi.org/10.1016/j.ijsu.2016.05.061
Qin R, Liu B, Hao J, Zhou P, Yao Y, Zhang F, Chen X (2018) Percutaneous endoscopic lumbar discectomy versus posterior open lumbar microdiscectomy for the treatment of symptomatic lumbar disc herniation: a systemic review and meta-analysis. World Neurosurg 120:352–362. https://doi.org/10.1016/j.wneu.2018.08.236
Kim M, Lee S, Kim HS, Park S, Shim SY, Lim DJ (2018) A comparison of percutaneous endoscopic lumbar discectomy and open lumbar microdiscectomy for lumbar disc herniation in the Korean: a meta-analysis. Biomed Res Int 2018:9073460. https://doi.org/10.1155/2018/9073460
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW Jr, Atkins D, Meerpohl J, Schunemann HJ (2011) GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 64:407–415. https://doi.org/10.1016/j.jclinepi.2010.07.017
Acknowledgements
The authors would like to thank Mark Donoghoe (Stats Central, UNSW) for his help with developing the database search strategy.
Funding
This work was supported by a Research Training Program scholarship and a University Postgraduate Award from the Australian Government and UNSW to XLC and a Scientia scholarship from UNSW to VASR. A Clinical Travelling Fellowship from the International Society for the Study of the Lumbar Spine (ISSLS) in 2018 further supported this work. JLVC was funded by an unrestricted educational and research donation by Nuvasive Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, X., Chamoli, U., Vargas Castillo, J. et al. Complication rates of different discectomy techniques for symptomatic lumbar disc herniation: a systematic review and meta-analysis. Eur Spine J 29, 1752–1770 (2020). https://doi.org/10.1007/s00586-020-06389-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06389-5