Abstract
Purpose
Thoracic inlet angle (TIA) is a sagittal radiographic parameter with a constant value regardless of posture and is significantly correlated with the sagittal balance of the cervical spine. However, the practical use of TIA has not been studied. This study aimed to investigate the usefulness of the preoperative TIA for predicting the development of kyphotic deformity after cervical laminoplasty in comparison to the preoperative T1 slope (T1S).
Methods
A total of 98 patients who underwent cervical laminoplasty without preoperative kyphotic alignment were included (mean age, 73.7 years; 41.8% female). Radiography was evaluated before surgery and at the 2-year follow-up examination. The cervical sagittal parameters were measured on standing radiographs, and the TIA was measured on T2-weighted MRI in a supine position. Cervical alignment with a C2–C7 angle of ≥ 0° was defined as lordosis, and that with an angle of < 0° was defined as kyphosis.
Results
Postoperative kyphosis occurred in 11 patients (11.2%). Preoperatively, the kyphosis group showed significantly lower values in the T1S (23.5° vs. 30.3°, p = 0.034) and TIA (76.1° vs. 81.8°, p = 0.042). We performed ROC curve analysis to clarify the impact of the preoperative TIA and T1S on kyphotic deformity after laminoplasty. The optimal cutoff angles for TIA and T1S were 68° and 19°, respectively, with similar diagnostic accuracy.
Conclusion
This study demonstrated the clinical utility of the preoperative TIA for predicting the risk of postoperative kyphotic deformity after cervical laminoplasty. These findings suggest the importance of the preoperative assessment of thoracic inlet alignment in cervical spine surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The thoracic inlet angle (TIA), proposed by Lee in 2012, is a morphologic parameter that correlates with cervical sagittal alignment. Owing to the rigidity of the rib cage, TIA is considered a constant value specific to each patient regardless of his/her posture [1]. Prior studies have shown concordance between TIA values measured on radiographs in a standing position and magnetic resonance imaging (MRI) in a supine position [2, 3]. Although TIA has been considered an important parameter of cervical alignment, similar to the pelvic incidence in lumbo-pelvic alignment [4], the practical utility of the TIA has not been well characterized.
Kyphotic deformity is a common complication after cervical laminoplasty with a reported incidence of up to 10% [5]. Kyphotic deformity may result in severe neck pain after surgery, neurological dysfunction, and even reoperation [6,7,8]. The preoperative T1 slope (T1S) in a standing position or its substitute C7 slope (C7S) are the radiographic parameters that are most frequently investigated in relation to the prediction of postoperative kyphotic deformity [9]; however, these parameters are often difficult to evaluate because of poor visibility of the endplates with overlying anatomical structures such as shoulder blades or the presence of severe myelopathy that precludes assessment in a standing position. Therefore, more practical parameters that are not affected by patients’ physique and neurological status are needed.
In this study, we evaluated the preoperative TIA on MRI in a supine position in patients who underwent cervical laminoplasty. The purpose of this study was to investigate the usefulness of the TIA for predicting the development of kyphotic deformity after cervical laminoplasty in comparison to other radiographic parameters.
Materials and methods
Study design and settings
This retrospective, single-center study included patients who underwent cervical laminoplasty for cervical spine myelopathy (CSM) and ossification of the posterior longitudinal ligament (OPLL) between January 2013 and December 2018. All patients were enrolled if they met the following inclusion criteria for this study: (1) received both MRI and standing radiography, (2) preoperative lordotic or straight sagittal alignment (C2–C7 angle ≥ 0°), (3) open-door expansive laminoplasty without posterior fusion and (4) a minimum follow-up period of 2 years. The exclusion criteria were as follows: (1) the presence of rheumatoid arthritis, neuromuscular diseases, mental disorders, electrolyte abnormalities, or hemodialysis, (2) loss to follow-up or incomplete data, and (3) inability to undergo radiographic evaluation in a standing position due to difficulty standing without any support (Fig. 1).
This study was designed and conducted according to the ethical principles of the Declaration of Helsinki. The research protocol was approved by our institutional review board (approval number: HS2019-117), and informed consent was obtained from all of the patients.
Demographic data collection
Demographic data were collected, including age, sex and Japanese Orthopaedic Association cervical myelopathy (JOA) score.
Radiographic outcomes
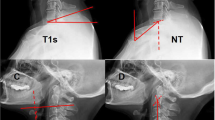
The sagittal radiographic parameters included the C2–C7 cervical lordotic angle (C2–C7A), C2–C7 sagittal vertical axis (cSVA), neck tilt (NT) and T1S in a standing position. TIA was defined as the angle formed by a line from the center of the T1 upper endplate (T1UEP) vertical to the T1UEP and a line connecting the center of the T1UEP and the upper end of the sternum [1]. TIA was also measured on T2-weighted MRI in a supine position. The global sagittal balance included the sagittal vertical axis (SVA) and the T1 pelvic angle (TPA) on whole-spine radiographs in a standing position. The measurements of the parameters are shown in Fig. 2. In the present study, cervical alignment with a C2–C7 angle ≥ 0° was defined as lordosis, and that with an angle of < 0° was defined as kyphosis [10, 11].
Measurement of sagittal parameters. A The sagittal radiographic parameters in a standing position. C2–C7 cervical lordotic angle (C2–C7A) was measured by the Cobb method, it was defined as the angle formed between the horizontal line of the C2 lower endplate and the horizontal line of the C7 lower endplate. T1 Slope (T1S) was defined as the angle formed between the horizontal plane and the T1 upper endplate (T1UEP). Neck Tilt (NT) was defined as the angle formed between a vertical line of the sternum tip and a line drawn from the sternum tip through the center of the T1UEP. C2–C7 sagittal vertical axis (cSVA) was defined as the distance between the plumb line through the C2 center and the plumb line of the posterior of C7 upper endplate. B T2-weighted MRI in a supine position. Thoracic inlet angle (TIA) was defined as the angle formed between a line from the center of the T1UEP vertical to the T1UEP and a line connecting the center of the T1UEP and the upper end of the sternum. C Whole-spine radiographs in a standing position. Sagittal vertical axis (SVA) was defined as the distance between the plumb line through the C7 center and the plumb line of the posterior of S1 upper endplate. T1 pelvic angle (TPA) was defined the angle formed between a line from the center of T1 to the femoral heads and a line from the femoral heads to the center of S1 upper endplate
The primary outcome of this study was the development of postoperative kyphosis, which was determined based on a lateral radiograph obtained through two years of postoperative follow-up.
Surgical technique and management of the posterior tension band for open-door expansive laminoplasty
Cervical laminoplasty, clinically characterized by multilevel simultaneous decompression, has been widely used and has resulted in favorable outcomes for the treatment of cervical myelopathy [12]. Open-door expansive laminoplasty was performed for all patients in this study. The C4-6 laminae received open en-bloc with C7 rostral-side and C3 caudal-side laminotomy or C3 laminectomy. The insertions of the deep extensor musculature and the nuchal ligament into the C2 and C7 spinous processes were meticulously preserved to maintain cervical lordotic alignment after surgery [13]. Mini-plates were used to maintain the opening of the laminae. The mini-plates were anchored with screws at the lateral mass and opened lamina. Incisions were closed with absorbable sutures in the skin layer after repairing the nuchal ligament, and a sterile dressing was placed. The duration of cervical collar placement was one week after surgery.
Statistical analyses
The Mann‒Whitney U test was used for the comparison of continuous variables. Fisher’s exact probability test was used for the comparison of categorical variables. Receiver operating characteristic (ROC) curves of the candidate parameters were generated, and areas under the curve (AUCs) were calculated to identify the accuracy and reliability of these parameters for predicting the development of postoperative kyphosis. High, moderate and low accuracy was defined as AUCs of > 0.9, 0.7–0.9, and 0.5–0.7, respectively, while an AUC of < 0.5 was defined as a chance result.
All statistical analyses were conducted using the EZR software program (version 1.41.1, Saitama Medical Center, Jichi Medical University, Saitama, Japan) [14]. P values of < 0.05 were considered to indicate statistical significance, and the variance was the standard deviation.
Results
Patients
A total of 98 patients without preoperative kyphotic alignment were included in this study (mean age, 73.7 [range, 65–90] years; 41.8% female) (Table 1). Postoperative kyphosis occurred in 11 patients (11.2%). The age, sex, and JOA score were comparable between the two groups.
Radiographic parameters at baseline
Preoperatively, the cSVA and NT were comparable between the kyphosis and nonkyphosis groups (cSVA, 24.2 mm vs. 25.2 mm; NT, 51.3° vs. 53.2°) (P > 0.05) (Table 2). The kyphosis group showed significantly lower values in the C2–C7A (4.2° vs. 16.5°, p < 0.001), T1S (23.5° vs. 30.3°, p = 0.034) and TIA (76.1° vs. 81.8°, p = 0.042) than the nonkyphosis group. The global sagittal balance, including the SVA and TPA, was comparable between the two groups (SVA, 29.1 mm vs. 43.5 mm; TPA, 17.3° vs. 18.3°) (P > 0.05).
Correlations of parameters
We performed a correlation coefficient test to explore the correlation between TIA and other parameters (Fig. 3). There were positive correlations between TIA and other parameters, and the correlation coefficients of T1S, C2–C7A, NT and cSVA were 0.43, 0.24, 0.52 and 0.44, respectively.
Correlation between the TIA and other parameters. There were weak or moderate positive correlations between TIA and other parameters. A The correlation coefficient between TIA and T1A was 0.43. B The correlation coefficient between TIA and NT was 0.52. C The correlation coefficient between TIA and C2–C7 A was 0.24. D The correlation coefficient between TIA and cSVA was 0.44
Risk factor analyses
We compared the ROC curves to evaluate the predictive ability of the TIA and T1S as preoperative radiographic parameters (Fig. 4). The preoperative TIA and T1S had similar diagnostic accuracy as predictors of postoperative kyphosis (TIA, AUC = 0.68, 95% CI = 0.49 to 0.86; T1S, AUC = 0.70, 95% CI = 0.49 to 0.91, DeLong test: P = 0.83).
The ROC curve for predicting postoperative kyphosis. A The ROC curve of T1S for predicting postoperative kyphosis (AUC = 0.70, 95% CI = 0.49 to 0.91). The optimal cutoff angle was 19° (sensitivity = 0.5 and specificity = 0.89). B The ROC curve of TIA for predicting postoperative kyphosis (AUC = 0.68, 95% CI = 0.49 to 0.86). The optimal cutoff angle was 68° (sensitivity = 0.33 and specificity = 0.97)
Next, we performed an ROC curve analysis to clarify the impact of the preoperative TIA and T1S on kyphotic deformity after laminoplasty. The optimal cutoff angles of the TIA and T1S were 68° (sensitivity = 0.33 and specificity = 0.97) and 19° (sensitivity = 0.5 and specificity = 0.89), respectively.
Discussion
This study revealed the clinical utility of preoperative TIA for predicting the risk of postoperative kyphotic deformity after cervical laminoplasty in comparison to the T1S. A positive correlation was found between TIA measured by MRI in the supine position and other sagittal parameters measured on a standing radiograph, with the parameters showing similar accuracy in predicting postoperative kyphosis. The optimal cutoff angles of the TIA and T1S were 68° and 19°, respectively.
Postoperative kyphotic deformity and radiographic parameters
Postoperative kyphotic deformity is one of the major complications after cervical laminoplasty. Postoperative cervical sagittal imbalance, including kyphotic deformity, is associated with neck pain, neurological deterioration and functional disability, such as difficulty in forward gazing [7, 8, 15, 16]. Previous studies reported that postoperative cervical kyphotic deformity occurred in approximately 5–10% of patients after cervical laminoplasty [5]. Although there are various factors that cause postoperative cervical kyphotic deformity, several studies have reported parameters for predicting postoperative kyphosis. The optimal cutoff value of preoperative C2–C7A for predicting postlaminoplasty kyphosis has been reported to be 7–10° in patients without preoperative kyphotic alignment [10, 17]. Sakai et al. [18] reported that the center of gravity of the head to the C7 SVA ≥ 42 mm was a risk factor for postoperative kyphosis. The T1S was also a useful parameter for predicting postoperative kyphosis [19]. When postoperative kyphosis was defined as the loss of the cervical lordotic angle, a high T1S was associated with a higher risk of postoperative kyphosis [20]. Furthermore, the T1S is not only a predictor of postoperative kyphosis but also an important factor in determining the sagittal balance of the cervical spine [4, 21]. However, T1UEP, an element of the T1S, can be difficult to visualize on radiographs due to overlying anatomical structures [22]. Tamai et al. [9] reported that 62.2% of T1UEPs on radiographs were invisible. They also reported that the C7S could potentially be substituted for the T1S, but that 6.7% of C7 upper endplates were similarly invisible.
Clinical utility of the TIA in spine surgery
In this study, we proposed the alternative use of the TIA when the T1S or C7S was difficult to evaluate. The advantages of TIA is that it is an anatomical parameter that is unaffected by posture. This characteristic is similar to pelvic incidence (PI) in spinal-pelvic parameters, suggesting that TIA could be used to predict the innate cervical lordotic alignment [1, 4]. The TIA is constant, irrespective of the patient's position and can be beneficial in cases where the evaluation of T1S is challenging due to overlap of the upper arm and scapula. Changes in position can significantly affect most sagittal parameters, but the TIA was the only parameter that did not change with different positions [2, 3]. In addition, several studies have reported associations between the TIA and other parameters [4, 23].Yang et al. [24] reported that the TIA minus the C0–C7 angle may significantly impact cervical alignment, and a greater value of TIA minus the C0–C7 angle was related to a greater degree of C2–C7 SVA. However, the practical use and potential utility of the TIA has not been studied and applied in spine surgery.
The present study demonstrated the clinical utility of the TIA for predicting the risk of postoperative cervical kyphotic deformity after cervical laminoplasty. It could be presumed that a low TIA decreases the T1S and that a low T1S requires low cervical lordosis to obtain balanced cervical sagittal alignment. As a result, patients with a low preoperative TIA had a smaller preoperative C2–C7A, and a small C2–C7A was associated with the risk of postoperative kyphosis, as previously reported [10]. TIA can be beneficial in cases where the evaluation of T1S is challenging due to overlap of the upper arm and scapula. Conversely, T1S may be a more precise parameter for evaluating sagittal alignment in a standing position, taking into account the impact of current thoracolumbar alignment on the cervical posture. The combination of both parameters has the potential to synergize their respective strengths and weaknesses.
Limitations
The present study is associated with several limitations. First, this was a small retrospective cohort study, so larger validation studies should be performed to confirm the findings of the present study. Second, we investigated the utility of the TIA alone, without validating its formulae with other sagittal parameters. The TIA was conceptualized as an analogy to PI in lumbar-pelvic alignment, so the TIA might be more effective when combined with other cervical sagittal parameters. Third, our study did not assess the risk of an excessively large TIA. Previous research has reported that postoperative cervical kyphotic alignment changes were increased in patients with a large T1S [20]. Therefore, the T1S is a parameter that is not suitable if it is too small or too large [25], with an optimal range reported to be between 13° and 25° [19]. It is anticipated that the TIA may exhibit similar characteristics, and future research should aim to investigate the optimal range of the TIA.
Despite these limitations, we have elucidated the clinical implications of the preoperative TIA regarding the risk of postoperative kyphotic deformity. While the current study validated the use of the TIA alone, future research is anticipated to explore its application in combination with other sagittal spine parameters.
Conclusions
This study demonstrated the clinical utility of the preoperative TIA for predicting the risk of postoperative kyphotic deformity after cervical laminoplasty in comparison to the T1S. These findings suggest the importance of the preoperative assessment of the thoracic inlet alignment in cervical spine surgery, as the TIA is a feasible and measurable parameter independent of the patient position and anatomical structures.
References
Lee SH, Kim KT, Seo EM et al (2012) The influence of thoracic inlet alignment on the craniocervical sagittal balance in asymptomatic adults. J Spinal Disord Tech 25(2):E41–E47. https://doi.org/10.1097/BSD.0b013e3182396301
Xing R, Zhou G, Chen Q et al (2017) MRI to measure cervical sagittal parameters: a comparison with plain radiographs. Arch Orthop Trauma Surg 137(4):451–455. https://doi.org/10.1007/s00402-017-2639-5
Cheng J, Liu P, Sun D et al (2019) Correlation of cervical and thoracic inlet sagittal parameters by MRI and radiography in patients with cervical spondylosis. Medicine 98(7):e14393. https://doi.org/10.1097/MD.0000000000014393
Lee SH, Son ES, Seo EM et al (2015) Factors determining cervical spine sagittal balance in asymptomatic adults: correlation with spinopelvic balance and thoracic inlet alignment. Spine J 15(4):705–712. https://doi.org/10.1016/j.spinee.2013.06.059
Suk KS, Kim KT, Lee JH et al (2007) Sagittal alignment of the cervical spine after the laminoplasty. Spine 32(23):E656–E660. https://doi.org/10.1097/BRS.0b013e318158c573
Suda K, Abumi K, Ito M et al (2003) Local kyphosis reduces surgical outcomes of expansive open-door laminoplasty for cervical spondylotic myelopathy. Spine 28(12):1258–1262. https://doi.org/10.1097/01.BRS.0000065487.82469.D9
Scheer JK, Tang JA, Smith JS et al (2013) Cervical spine alignment, sagittal deformity, and clinical implications: a review. J Neurosurg Spine 19(2):141–159. https://doi.org/10.3171/2013.4.SPINE12838
Roguski M, Benzel EC, Curran JN et al (2014) Postoperative cervical sagittal imbalance negatively affects outcomes after surgery for cervical spondylotic myelopathy. Spine 39(25):2070–2077. https://doi.org/10.1097/BRS.0000000000000641
Tamai K, Buser Z, Paholpak P et al (2018) Can C7 slope substitute the t1 slope? An analysis using cervical radiographs and kinematic MRIs. Spine 43(7):520–525. https://doi.org/10.1097/BRS.0000000000002371
Machino M, Ando K, Kobayashi K et al (2020) Postoperative kyphosis in cervical spondylotic myelopathy: cut-off preoperative angle for predicting the postlaminoplasty kyphosis. Spine 45(10):641–648. https://doi.org/10.1097/BRS.0000000000003345
Choi I, Roh SW, Rhim SC, Jeon SR (2018) The time course of cervical alignment after cervical expansive laminoplasty: determining optimal cut-off preoperative angle for predicting postoperative kyphosis. Medicine 97(47):e13335. https://doi.org/10.1097/MD.0000000000013335
Duetzmann S, Cole T, Ratliff JK (2015) Cervical laminoplasty developments and trends, 2003–2013: a systematic review. J Neurosurg Spine 23(1):24–34. https://doi.org/10.3171/2014.11.SPINE14427
Takasawa E, Sorimachi Y, Iizuka Y et al (2019) Risk factors for rapidly progressive neurological deterioration in cervical spondylotic myelopathy. Spine 44(12):E723–E730. https://doi.org/10.1097/BRS.0000000000002969
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Ames CP, Blondel B, Scheer JK et al (2013) Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine 38(22 Suppl 1):S149–S160. https://doi.org/10.1097/BRS.0b013e3182a7f449
Iyer S, Nemani VM, Nguyen J et al (2016) Impact of cervical sagittal alignment parameters on neck disability. Spine 41(5):371–377. https://doi.org/10.1097/BRS.0000000000001221
Takasawa E, Iizuka Y, Ishiwata S et al (2023) Impact of the preoperative nutritional status on postoperative kyphosis in geriatric patients undergoing cervical laminoplasty. Eur Spine J 32(1):374–381. https://doi.org/10.1007/s00586-022-07481-8
Sakai K, Yoshii T, Hirai T et al (2016) Cervical sagittal imbalance is a predictor of kyphotic deformity after laminoplasty in cervical spondylotic myelopathy patients without preoperative kyphotic alignment. Spine 41(4):299–305. https://doi.org/10.1097/BRS.0000000000001206
Knott PT, Mardjetko SM, Techy F (2010) The use of the T1 sagittal angle in predicting overall sagittal balance of the spine. Spine J 10(11):994–998. https://doi.org/10.1016/j.spinee.2010.08.031
Kim TH, Lee SY, Kim YC et al (2013) T1 slope as a predictor of kyphotic alignment change after laminoplasty in patients with cervical myelopathy. Spine 38(16):E992–E997. https://doi.org/10.1097/BRS.0b013e3182972e1b
Hyun SJ, Kim KJ, Jahng TA et al (2016) Relationship between t1 slope and cervical alignment following multilevel posterior cervical fusion surgery: impact of T1 slope minus cervical lordosis. Spine 41(7):E396–E402. https://doi.org/10.1097/BRS.0000000000001264
Park BJ, Gold CJ, Woodroffe RW et al (2021) What is the most accurate substitute for an invisible T1 slope in cervical radiographs? A comparative study of a novel method with previously reported substitutes. J Neurosurg Spine. https://doi.org/10.3171/2021.8.SPINE21901
Lee SH, Hyun SJ, Jain A (2020) Cervical sagittal alignment: literature review and future directions. Neurospine 17(3):478–496. https://doi.org/10.14245/ns.2040392.196
Yang K, Li XY, Wang Y et al (2022) Relationship between TIA minus C0–7 angle and C2–7 SVA: analysis of 113 symptomatic patients. BMC Musculoskelet Disord 23(1):338. https://doi.org/10.1186/s12891-022-05301-0
Ling FP, Chevillotte T, Leglise A et al (2018) Which parameters are relevant in sagittal balance analysis of the cervical spine? A Lit Rev Eur Spine J 27(Suppl 1):8–15. https://doi.org/10.1007/s00586-018-5462-y
Acknowledgement
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takakura, K., Takasawa, E., Mieda, T. et al. Usefulness of the preoperative thoracic inlet angle in comparison to the T1 slope for predicting cervical kyphosis after laminoplasty. Eur Spine J 33, 1179–1186 (2024). https://doi.org/10.1007/s00586-023-08095-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-08095-4