Abstract
Purpose
Patient blood management has been recently emphasized to avoid perioperative blood transfusion in AIS surgery. Hydroxyapatite charged collagen sponge (HCS) is a bone substitute material made of collagen and ceramized hydroxyapatite, with associated haemostatic properties. The goal of this study was to assess the impact of HCS in the perioperative blood loss in AIS surgery.
Methods
After IRB approval, all AIS patients undergoing primary correction were prospectively included over a 15-month period. Patients receiving HCS at the end of the procedure were compared to a control group (matched for age, gender, and fusion levels) without any haemostatic agent or bone substitute. The same perioperative blood saving strategies were used in both groups. Two subfascial drains were used for 48 h in all patients. Perioperative blood loss and transfusion rates were analysed.
Results
A total of 34 patients were included in each group. No difference in drainage volume was observed at day 1, but the reduction was statistically different at day 3 (1135 mL [800–1640] versus 930 [480–1510], p = 0.028, 0.63 ml/Kg/h [0.4–0.92] versus 0.46 [0.29–0.7], p = 0.042). Multivariate analysis found that the use of HCS was associated with a decrease in the postoperative blood loss (OR = 1.17 [1.10–1.25]). The transfusion rate was lower in the HCS group [0 (0% vs. 3(8.8%), p = 0.076)]. No infection occurred, and no complication was reported.
Conclusion
With 27% reduction in drain volume, hydroxyapatite charged collagen sponge can be considered as a blood salving strategy in AIS surgery. The role of the biomaterial in fusion rate still needs to be further assessed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patient blood management techniques have been recently developed to decrease the risk of blood transfusion in long and complex surgeries [2, 6]. Indeed, blood heterologous transfusion has been proved to be positively correlated with increased postoperative morbidity, such as early surgical site infections, and with immune and viral complications [4].Thus, in major orthopaedic adult or paediatric procedures, such as multi-level spine surgery, salve saving strategies are commonly used, associating preoperative, perioperative and postoperative measures to reduce blood loss [7, 21]. Adolescent idiopathic scoliosis (AIS) correction remains one of the most haemorrhagic procedures, for which blood saving strategy improvement is still ongoing [9]. Optimizing the haemoglobin rate (iron, erythropoietin) is key preoperatively, while reducing blood loss (installation, blood pressure, tranexamic acid and cell salvage) remains the main goal during surgery. These measures have helped reduce transfusion rates in AIS from 25 to 2%, but the goal should be to make allotransfusion a non-existing event in such young patients [18]. Recent studies have reported that 50% of the perioperative bleeding during AIS posterior fusion occurred at the end of the procedure, when the surgeon performed decortication and postoperatively with the use of close suction drainage. In fact, the blood volume obtained in the drains has also shown positive correlation with transfusion rate to be in AIS [17]. At the end of surgery, bone graft is added after correction to obtain solid fusion [5]. Iliac crest bone harvesting has been considered the gold standard for years, but it has been associated with increased operative time, postoperative pain and more importantly increased blood loss [16]. Local autograft is now the most commonly used, but the adjunction of allograft or bone substitutes has recently gained popularity [11, 25]. However, the advantage in terms of fusion rates and cost/benefit of the various products available remains debatable, since pseudarthrosis is only reported in less than 5% in AIS treated with modern instrumentation [23]. Hydroxyapatite charged collagen sponge (HCS) is an interesting material, presented as a sponge made of bovine collagen and ceramized hydroxyapatite, that combines both osteoconductive and haemostatic properties [15]. Hence, despite a theoretical risk for prion infection, this product could be considered to reduce postoperative blood loss. Encouraging preliminary clinical experience has already been reported in orthopaedic (foot, cervical spine, hip and knee) and maxillo-facial surgery, but no clinical series has been published to date. The aim of this study was therefore to evaluate the efficacy of HCS adjunction in the reduction of perioperative blood loss in AIS surgery.
Materials and methods
Patients and study design
After IRB approval, all consecutive patients under 18 years old operated for AIS posterior fusion between September 2020 and December 2021 were included. A minimum 4-month follow-up was required to look for early complications. Exclusion criteria were revision surgery, prior anterior release, associated thoracoplasty and fixation below L4. All patients with associated bleeding disorder were also excluded. Data were collected prospectively and retrospectively analysed.
Operative procedure
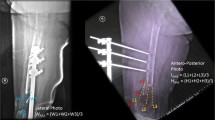
All patients underwent posterior spinal fusion using hybrid constructs, combining bilateral pedicle screws (below the inflection point, from T11 to L4) [Solera (Medtronic, Minneapolis, USA)], concave thoracic sublaminar bands (above the inflection point, from T5 to T11) [Jazz systems (Implanet, Bordeaux, France), convex thoracic uniplanar pedicle screws and proximal supralaminar angled hooks on the upper instrumented vertebra (UIV) with 5.5-mm CoCr diameter rods. All the surgeries in both groups were performed by the same surgeon. No selective thoracic fusion was performed, and no patient underwent 3-column osteotomy. Bone scalpel (Misonix, Farmingdale, NY, USA) was used in all cases to perform facetectomies and periapical (3–4 levels) posterior column osteotomies. Posteromedial translation was the main technique used for thoracic correction, combined with segmental derotation and in situ bending. Local autograft from the spinous processes and lamina decortication was used in all cases. No bone substitute was used in group 1 (control), while in group 2 (HCS) group] 2 sponges of 7 × 11 cm per patient were cut (1–1.5 cm large pieces) and placed in posterolateral position (lateral to the rods) as well as medially between the rods, above the autograft (Fig. 1). Closed suction drainage (2 subfascial 8 mm drains, opened 6 h after closure) was used for 48 h in all patients.
Perioperative anaesthesia
Perioperative protocols were similar in both groups during the study period. Anaesthesia was performed with intravenous agents (with concentration objective) using propofol and remifentanil and a muscle relaxant (atracurium 0.5 mg kg−1). Dexamethasone was administered at the beginning of surgery (0.15 mg kg−1 after induction), and intrathecal morphine (5 µg kg−1) was performed by the surgeon at the end of the surgical approach. Body temperature was controlled during the whole procedure and maintained between 36.5° and 37 °C. Muscle relaxant was infused continuously during surgery and reversed at the end of the procedure. All patients were monitored using the bispectral index and an oesophageal Doppler. After obtaining the best Doppler signal, indexed stroke volume (iSV) was optimized and maximized (boluses of 10 ml kg−1 of normal saline).
The “patient blood management” strategy was standardized in both groups. Preoperatively, iron and vitamin B9 implementation started 6 weeks prior to surgery. Erythropoietin injections (600UI/Kg/week, maximum 4 injections) were also performed on a weekly basis, until the haemoglobin rate reached 15 g dL−1. During surgery, tranexamic acid was used, with a loading dose of 10 mg kg−1followed by a continuous infusion (5 mg Kg h−1) until the end of the procedure. Cell salvage was used systematically and blood reinfused if the quantity was above 3 mL/kg. Postoperative multimodal analgesia included morphine (patient controlled analgesia, PCA), paracetamol, NSAIDs, gabapentin and nefopam. All patients benefited from enhanced recovery after surgery (ERAS) protocols, as previously described [13]. Red cell transfusion indications during the perioperative period were an haemoglobin rate under 7 g/dL or under 10 g/dL in case of cardiac comorbidity.
Data
Demographic data (age, weight), ASA grade, haemoglobin levels (preoperative on the day of surgery and at postoperative days 1 and 3), duration of surgery, duration of anaesthesia, fusion levels, intraoperative and perioperative blood transfusions, volume collected in the closed suction drains (until day 2) and length of hospital stays were collected. Using the weight-related total blood volume formula previously published to estimate the bleeding, the red cell deficit was estimated, including autologous or heterologous blood transfusion when required during the perioperative period [19]. Thus, the intraoperative blood loss was calculated by red cell deficit (Table 2) and the perioperative operative bleeding was estimated at day 1 and day 3.
Statistical analysis
Power calculation was based on a previously published study exploring the use of a procedure to decrease the bleeding of 25%. Based on this estimated rate, the necessary sample size in each group was set to be 32 patients, with an alpha value of 0.05 and a power analysis of 90%. For that reason, 34 consecutive patients were included in each group. Descriptive statistics were reported using median and range for continuous variables and percentages (%) for discrete ones. Statistical comparisons between the two cohorts used the Mann–Whitney nonparametric tests for continuous variables and the Chi2 (or Fisher’s exact test) for discrete variables. Nonparametric tests were performed instead of parametric ones because of their ability to detect strongly significant differences. In order to determine factors associated with HCS-related decrease in blood transfusion, a univariate analysis was performed for factors that might impact perioperative transfusion. All factors exhibiting a p-value < 0.2 were entered in a stepwise multivariable analysis logistic regression. Statistical analysis was carried out on SPSS 26.0 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, version 26.0. Armonk, NY: IBM Corp). The level of significance was set at p < 0.05.
Results
Demographic and operative data are reported in Table 1. No significant difference was found between groups. Mean follow-up was 18 months.
No difference was found in the drain volume on the first postoperative day, but the reduction was significant in the HCS group at day 3 (19%, 930 mL [480–1510] versus 1135 mL [800–1640], p = 0.028). Significant difference was also found in the total postoperative drain volume, measured hourly and adjusted to patient’s weight (27% reduction, 0.46 mL/Kg/h [0.29–0.7] versus 0.63 mL/Kg/h [0.4–0.92], p = 0.042). However, no difference was found regarding postoperative haematocrits and estimated red cell deficit, which is an estimation of the bleeding (Table 2). The transfusion rate was lower in the HCS group [0 (0%) vs. 3 (8.8%), p = 0.076] but the difference did not reach significance. The multivariate analysis confirmed that the adjunction of HCS at the end of the procedure was associated with a decreased postoperative drain volume (drains (OR = 1.17 [1.10–1.25])) (Table 3). No surgical site infection (SSI) and no unplanned return to the operating room (UROR) was reported. Radiological data with preoperative, early postoperative and latest follow-up are reported in Table 4.
Discussion
Results of the current study showed that the use of an HCS was associated with a significant reduction in postoperative drain volume after AIS surgery and that it could be considered an additional blood saving measure. No patient received heterologous transfusion in the study, and no complication was reported.
The main objective in AIS surgery is to obtain the best 3D correction possible, stable over time, while reducing perioperative morbidity. In the last decades, family burden has been dramatically reduced with shorter hospitalization stay (3 to 5 days) and earlier return to daily activities (school and recreations) thanks to enhanced recovery protocols [14]. Blood saving strategies are part of the “patient blood management” concept, described 10 years ago to avoid anaemia and blood transfusion in the perioperative period, responsible for increased morbidity [3]. Limiting blood transfusion to a minimum rate should therefore be a constant effort, especially in adolescents or young adults, to reduce postoperative complications such as early SSI but also red cell alloimmunization.
Three distinct periods can be distinguished during AIS care. The first one is preoperative, and the efficiency of iron supplementation and erythropoietin have already been reported [1, 20]. The second period is the surgery itself, during which tranexamic acid is essential [7, 9]. Cell saver can also be installed, but the volume obtained after treatment remains usually moderated (from 100 to 300 mL with variable haematocrits), so the benefit/cost balance is still controversial in primary AIS fusion without osteotomy. Topical haemostatic agents can also be used during the procedure, such as thrombin-gelatine matrix, but massive and uncontrolled bleeding remains rare in AIS patients without inherited bleeding disorders. The benefit of adding intraoperatively gelatine matrix with human thrombin to the standard surgical methods to reduce blood loss has already been proven in a randomized study, but the product used by Helenius and al had no osteoinductive properties [10]. HCS is an interesting material, made of bovine collagen and ceramized hydroxyapatite, combining both osteoconductive and haemostatic properties (Figs. 2 and 3). Presented as a sponge, it can be cut in different sizes and shapes and placed in the “dead spaces” lateral to the rods, but also on the lamina after decortication, with the objective of reducing postoperative blood loss. Although no in vitro exists regarding the haemostatic properties of the product. Preliminary encouraging experience has been reported in maxillo-facial and orthopaedic surgery (foot, knee, hip and lumbar spine), but no clinical series has been published to date. The use of drains remains debatable in AIS surgery, as it might increase blood loss in the postoperatively (last period), but it is also recommended by some authors to reduce the risk of hematoma and pain level [12, 24]. In our department, subfascial drains (8 mm) were systematic after surgery, but placed in closed suction 6 h after closure and only kept for 48 h (based on incision time). These drains were useful in the present study to evaluate postoperative blood loss and showed that HCS had a significant influence on drain volume (27% reduction when adjusted to patient’s weight) and showed in situ local haemostatic properties. In addition, the heterologous transfusion rate was reduced from 8 to 0%, but this trend will need to be confirmed in larger series. Despite these findings, no significant difference was observed in the overall estimated blood loss, calculated on day 1 and day 3 after surgery, but this might be due to the small sample sizes. In terms of cost, economic study is required to evaluate the benefit of this biomaterial. The price of each sponge represents a significant amount of extra cost for an AIS procedure. However, this price needs to be compared with the one of 1 red cell unit, taking into account hospital paramedical cost and potential complications, which can be spared if blood loss is decreased.
If reducing perioperative morbidity is a major concern in AIS surgery, the final goal remains the long-term stability of the correction. Bone graft is still mandatory, but the gold standard has switched from iliac crest bone graft (ICBG) to local bone graft (LBG), taken from the spinous processes and lamina decortication, which has been associated with reduced operative time, postoperative pain and blood loss [16]. The adjunction of allograft or bone substitute is often reported, but the differences in terms of outcomes (clinical and radiological) are unclear, and LBG alone has recently proven to achieve successful fusion in 99% of patients undergoing posterior fusion with modern instrumentation [8, 22, 25]. The use of HCS should therefore be more driven in AIS by the efficient haemostatic properties reported in the series, but the material could also be interesting as a bone substitute in other indications (bone cysts, revision, adult scoliosis and degenerative spine). However, the aim of the current study was not to determine whether HCS could influence fusion rate in AIS, and further investigation remains necessary with long-term follow-up. However, no infection, no inflammatory reaction and no other adverse effect was reported during the study period, so HCS can be considered as a safe alternative to other bone substitutes with additional haemostatic properties reinforcing blood saving strategies.
This study has some limitations. First, this is not a controlled trial, but a retrospective analysis of data collected prospectively in a consecutive series of patients. Second, the additional cost and operative time induced by the product adjunction need to be investigated and balanced with patient’s benefice. However, no overall operative time difference was reported between groups (p = 0.981, Table 1), but the scrub nurses were asked to cut the sponges while the surgeon was performing the laminae decortication and placing the bone autograft. Finally, the study was monocentric, with a single senior spine surgeon involved, and these preliminary results will need to be further confirmed on larger cohorts with different surgical correction techniques in terms of blood sparing efficiency.
Availability of data and materials
Not applicable.
References
Colomina MJ, Bagó J, Pellisé F, Godet C, Villanueva C (2004) Preoperative erythropoietin in spine surgery. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 13(Suppl 1):S40-49
Desai N, Schofield N, Richards T (2018) Perioperative patient blood management to improve outcomes. Anesth Analg 127(5):1211–1220
Fisahn C, Jeyamohan S, Norvell DC et al (2017) Association between allogeneic blood transfusion and postoperative infection in major spine surgery. Clin Spine Surg 30(7):E988–E992
Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM (2015) Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 102(11):1314–1324
Giorgi P, Capitani D, Sprio S et al (2017) A new bioinspired collagen-hydroxyapatite bone graft substitute in adult scoliosis surgery: results at 3-year follow-up. J Appl Biomater Funct Mater 15(3):e262–e270
Gombotz H, Hofmann A, Rehak P, Kurz J (2011) Patient blood management (part 1)-patient-specific concept to reduce and avoid anemia, blood loss and transfusion. Anasthesiol Intensivmed Notfallmedizin Schmerzther AINS 46(6):396–401
Goobie SM, Zurakowski D, Glotzbecker MP et al (2018) Tranexamic acid is efficacious at decreasing the rate of blood loss in adolescent scoliosis surgery: a randomized placebo-controlled trial. J Bone Joint Surg Am 100(23):2024–2032
Harshavardhana NS, Noordeen MHH (2015) Surgical results with the use of silicated calcium phosphate (SiCaP) as bone graft substitute in posterior spinal fusion (PSF) for adolescent idiopathic scoliosis (AIS). Scoliosis 10:27
Hasan MS, Yunus SN, Ng CC, Chan CYW, Chiu CK, Kwan MK (2021) Tranexamic acid in pediatric scoliosis surgery (TRIPSS): a prospective randomized trial comparing high dose and low dose tranexamic acid in adolescent idiopathic scoliosis (AIS) undergoing posterior spinal fusion surgery. Spine 46:E1170–E1177
Helenius I, Keskinen H, Syvänen J et al (2016) Gelatine matrix with human thrombin decreases blood loss in adolescents undergoing posterior spinal fusion for idiopathic scoliosis: a multicentre, randomised clinical trial. Bone Jt J 98-B(3):395–401
Ilharreborde B, Morel E, Fitoussi F et al (2008) Bioactive glass as a bone substitute for spinal fusion in adolescent idiopathic scoliosis: a comparative study with iliac crest autograft. J Pediatr Orthop 28(3):347–351
Jang H-D, Park SS, Kim K et al (2021) Is routine use of drain really necessary for posterior lumbar interbody fusion surgery? A retrospective case series with a historical control group. Glob Spine J. https://doi.org/10.1177/21925682211001801
Julien-Marsollier F, Michelet D, Assaker R et al (2020) Enhanced recovery after surgical correction of adolescent idiopathic scoliosis. Paediatr Anaesth 30(10):1068–1076
Julien-Marsollier F, Michelet D, Assaker R et al (2021) Enhanced recovery after surgery: many ways for the same destination. Paediatr Anaesth 31(3):375–376
Katthagen BD, Mittelmeier H (1984) Experimental animal investigation of bone regeneration with collagen-apatite. Arch Orthop Trauma Surg Arch Orthop Unf Chir 103(5):291–302
Kirzner N, Hilliard L, Martin C, Quan G, Liew S, Humadi A (2018) Bone graft in posterior spine fusion for adolescent idiopathic scoliosis: a meta-analysis. ANZ J Surg 88(12):1247–1252
Kochai A, Erkorkmaz Ü (2019) The role of drains in adolescent idiopathic scoliosis surgery: Is it necessary? Medicine Baltimore 98(51):e18061
Mange TR, Sucato DJ, Poppino KF, Jo C-H, Ramo BR (2020) The incidence and risk factors for perioperative allogeneic blood transfusion in primary idiopathic scoliosis surgery. Spine Deform 8(4):695–702
Michelet D, Julien-Marsollier F, Hilly J, Diallo T, Vidal C, Dahmani S (2018) Predictive factors of intraoperative cell salvage during pediatric scoliosis surgery. Cell saver during scoliosis surgery in children. Anaesth Crit Care Pain Med 37(2):141–146
Mikhail C, Pennington Z, Arnold PM et al (2020) Minimizing blood loss in spine surgery. Glob Spine J 10(1 Suppl):71S-83S
Nazareth A, Shymon SJ, Andras L, Goldstein RY, Kay RM (2019) Impact of tranexamic acid use on blood loss and transfusion rates following femoral varus derotational osteotomy in children with cerebral palsy. J Child Orthop 13(2):190–195
Pesenti S, Ghailane S, Varghese JJ et al (2017) Bone substitutes in adolescent idiopathic scoliosis surgery using sublaminar bands: is it useful? Case control Study Int Orthop 41(10):2083–2090
Theologis AA, Tabaraee E, Lin T, Lubicky J, Diab M (2015) Spinal deformity study group type of bone graft or substitute does not affect outcome of spine fusion with instrumentation for adolescent idiopathic scoliosis. Spine 40(17):1345–1351
von Eckardstein KL, Dohmes JE, Rohde V (2016) Use of closed suction devices and other drains in spinal surgery: results of an online, Germany-wide questionnaire. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 25(3):708–715
Yataganbaba A, Gahukamble A, Antoniou G, Freeman BJC, Cundy PJ (2021) Local bone grafting is sufficient for instrumented adolescent idiopathic scoliosis surgery: a preliminary study. J Pediatr Orthop 41(8):e641–e645
Funding
No grant for this study.
Author information
Authors and Affiliations
Contributions
FJ-M contributed to study design, statistical analysis and performed measurements and manuscript preparation. Consent to participate and to publish was obtained from OK; LP and AH contributed to study design and performed measurements and manuscript preparation; BI contributed to study design and performed measurements, statistical analysis and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
Brice Ilharreborde reports work as a consultant for Implanet, ZimmerBiomet and Medtronics.
Ethical approval
Yes (comite d evaluationd’ethique des projets de l’hopital ROBERT Debre).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Julien-Marsollier, F., Penisson, L., Happiette, A. et al. Can hydroxyapatite charged collagen sponge help reduce perioperative blood loss in adolescent idiopathic scoliosis surgery? Preliminary results in 68 patients. Eur Spine J 32, 883–888 (2023). https://doi.org/10.1007/s00586-022-07512-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07512-4