Abstract
Purpose
A two-staged posterior correction, using a temporary magnetically controlled growing rod (MCGR), was employed to gradually and safely correct severe adolescent idiopathic scoliosis (AIS). The aim of the study is illustrating the results of this procedure.
Methods
A retrospective review of a consecutive series of 17 severe AIS. The first surgery was a posterior release (multiple Ponte osteotomies) with implant of pedicle screws and MCGR on the concave side of the curve. In post-operative days, a distraction was applied with MCGR, which allowed to obtain a total mean lengthening of 2 cm in about 2 weeks, with no complications arising. In the second posterior surgery, MCGR was removed and the definitive rods were applied for final fusion. The mean pedicle screws density was 93.3% (85–100). The extension of the final posterior fusion-instrumentation was of 13.8 levels (12–15).
Results
At an average follow-up (FU) of 2.9 years, the main scoliosis curves from average pre-operative Cobb angle of 98.2° (91°–138°) bent down to 38.3° (35°–76°) after definitive fusion (p < 0.05); at last FU, the overall correction was 58.7% (50.4–71.2), with an average correction loss of 2.1° (1.5°–3.1°). At last FU, no complications were reported.
Conclusions
Gradual traction with MCGR in severe AIS proved to be a safe method to achieve progressive curve correction before posterior final fusion, with no neurologic complications associated to more aggressive one-stage surgeries. In a staged approach, MCGR appears as an alternative to halo traction, avoiding frequent traction-related complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Open thoracotomy with anterior release, followed by posterior instrumented fusion [1, 2], was one of the most commonly used surgical treatments for severe thoracic adolescent idiopathic scoliosis (AIS). Halo-traction was often used in the period between anterior release and posterior instrumentation [3].
Posterior-only correction of severe and rigid AIS was introduced by Suk et al. [4], using a vertebral column resection at the apex of the deformity. The technique was later largely adopted by Lenke et al. [5].
Staged posterior approaches were proposed for treating severe scoliosis to avoid recourse to highly demanding procedures, such as vertebral column resection, and to minimize surgical risks. Buchowski et al. [6] suggested two distraction procedures to perform a staged correction of the deformity prior to definitive posterior fusion. Hui-Min et al. [7] used temporary internal distraction as an alternative to halo traction. Recently, a two-staged posterior surgery, entailing the use of a magnetically controlled growing rod (MCGR) (MAGEC; NuVasive, Inc., San Diego, CA, USA), was proposed to gradually and safely correct a severe deformity in a girl affected by syringomyelia and Arnold-Chiari Type 1 malformation [8], as well as in another case of a girl with severe AIS [9].
This gradual distraction procedure using a temporary MCGR was adopted to treat a series of patients with severe AIS. The first surgery to be performed was a posterior aggressive release with Ponte osteotomies at many levels, with implant of pedicle screws and MCGR on the concave side of the scoliotic curve. Later, in the post-operative days, a distraction was applied with MCGR, which allowed to obtain a gradual correction of the curve. In the second posterior surgery, MCGR was removed and the definitive rods were applied for final correction and posterior fusion. The aim of the study is to illustrate the results of this procedure.
Materials and methods
A retrospective review was performed on patients who had undergone a posterior-only correction of severe scoliosis between January 2015 and February 2017 at our Spine Departments (Istituto Ortopedico Rizzoli, Bologna and Ospedale Santa Corona, Pietra Ligure, Italy). The inclusion criteria were: (1) adolescent idiopathic scoliosis, (2) main thoracic curve showing a minimum pre-operative Cobb angle of 90°, (3) age at surgery between 11 and 17 years, (4) no pre-operative treatment with halo-traction or serial corrective Risser’s plasters, (5) two-stage posterior treatment using the temporary magnetically-controlled growing rod (MCGR), (6) a minimum clinical and radiographic follow-up of 2.5 years. A total of 17 consecutive patients met our inclusion criteria. All patients and their families signed the informed consent form. The demographic data analyzed included: gender, age and body mass index (BMI) at surgery. BMI is calculated as a standard measure of body fat based on height and weight (kg/m2).
An independent highly-trained spine surgeon analyzed all X-rays and medical records. For each patient, AIS patterns were classified according to Lenke [10] and the Risser’s sign was assessed. The radiographic evaluation was performed on pre-operative standing x-rays, immediately after the two surgeries and at the latest follow-up. For every patient, the coronal and sagittal radiological parameters evaluated were: major thoracic curve (MT), minor thoracolumbar/lumbar curves (TL/L), thoracic kyphosis (T5–T12), lumbar lordosis (LL), sagittal profile at thoracolumbar junction (T10–L2), global coronal alignment (distance between the C7 plumb line and the center sacral vertical line [CSVL]), translation of the apical thoracic vertebra (AVT) to C7 plumb line, distance between CSVL and the apical lumbar vertebra (AVL) and, finally, the translation of the lowest instrumented vertebra (LIV) to CSVL. The coronal tilt of the lowest instrumented vertebra (LIV tilt) was likewise evaluated. Angles were measured using the Cobb technique [11], translations and distances in centimeters. Lordosis is expressed as a negative value, kyphosis as a positive value. Supine bending x-rays before surgery were used to determine the flexibility of the major and minor curves. MRI of full spine was performed pre-operatively to exclude congenital intramedullary anomalies.
Statistical analysis
Continuous data were reported as mean, standard deviation, minimum and maximum value, and were compared using the Student t test. A two-tailed p value of < 0.05 was considered statistically significant. Categorical variables were expressed as the number of cases or percentage.
Operative procedures
Spinal cord function was intra-operatively monitored in all patients; in fact, somatosensory evoked potentials (SEPs) and transcranial electric stimulation-motor evoked potentials (TE-MEPs) were recorded during both surgical procedures.
First posterior surgery
After exposure of the posterior elements of the spine, pedicle screws were inserted using the “anatomic” technique, such as the free-hand method [12]. The mini-laminotomy procedure [13, 14] was sometimes preferred for concave thoracic screws, as it allows palpation (with a spatula inside the canal) of the borders of the thoracic pedicles. An average of 28 screws were inserted in every patient (range 24–30), with a mean screws density of 93.3% (range 85–100). After pedicle screws placement, generous Ponte osteotomies [15] at multiple levels were performed: the gap created was of at least 6 mm. The posterior tension band of the first three upper levels of fusion was preserved to avoid later junctional problems. Before implanting the MCGR, short rods were implanted at lumbar level and in upper thoracic site in some cases to align the spine. MCGR was inserted on the concave side of the curve (Fig. 1). The rod was gently bended not close to the magnetic mechanism. The first distraction was performed intra-operatively.
Post-operative period
The scoliosis correction was performed over a series of daily distractions of the MCGR by means of an external magnet. Distraction period started 1 day after surgery. The daily planned distraction was of 2 mm, to be achieved in one or two times per day depending on the level of pain referred by the patient. CT controls at 7 and 14 days were performed and proved to be useful for monitoring the progression of scoliosis correction and the insertion of the screws. We performed the first CT scan to evaluate the progression of scoliosis correction and the insertion of the screws. The second CT scan was performed just before the second surgery so as to better evaluate the obtained lengthening of MCGR and to check no mobilization of the screws connected to MCGR. At 2 weeks post-operative follow-up, a mean 2 cm total distraction was obtained. The young patients were ambulant in the hospital ward, without recourse to a brace throughout the entire distraction procedure.
Second posterior surgery
After an average of 2 weeks (range 14–17 days), the new intervention for the final instrumented posterior fusion was performed (Figs. 2 and 3). After the removal of MGCR and other temporary rods, definitive correction started with short straight rods being applied in the apex of the deformity at the convex and/or concave sides; if correction was satisfying, longer rods were added, without removing the short ones. In other cases, the correction procedure was improved by using longer rods and removing the short ones. Posterior fusion was performed with chips of autologous local bone obtained from spinous processes and, when a thoracoplasty was performed, from the ribs’ segments that were resected.
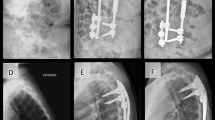
16-year old female. AIS: left proximal thoracic (40°), right main thoracic (118°) and left lumbar curve (70°), Lenke’s type 3B + curve (a). X-Rays control after distraction with MCGR: the main thoracic curve was corrected to 56° (correction of 52.5%), the lumbar curve was corrected to 32° (correction of 54.2%) (b). X-Rays (AP and LL) at last follow-up of 2.5 years: MT was 35° (70.3% of correction), the lumbar curve was corrected to 25° (correction of 64.2%) (c, d). The clinical aspect before surgery (e, f) and at last control (g, h)
15-year old female. AIS: left proximal thoracic (42°), right main thoracic (122°) and left lumbar curve (75°): Lenke’s type 3B + curve (a). X-Rays control after distraction with MCGR: the main thoracic curve was corrected to 76° (correction of 37.7%), the lumbar curve was corrected to 44° (correction of 41.3%) (b). X-Rays (AP and LL) at last FU (3 years): MT was 40° (correction of 67.2%), the lumbar curve was corrected to 25° (correction of 66.6%) (c, d). The clinical aspect before surgery (e) and at last follow-up (f)
At the end, surgery was suspended for about 20 min to check MEPs and SEPs, which remained similar to the baseline one. In two cases, however, the wake-up test was performed, due to technical difficulties in acquiring the potentials: at the end of surgery, no neurological deficit was reported.
To improve the clinical effect, surgery was finalized by performing a thoracoplasty in 12 cases (70%). The skin incision was the same of the posterior procedure. Thoracoplasty included five ribs on average for every patient, whereas the length of every rib resection was around 4 cm. No accidental pleural lesion occurred. A chest tube was inserted in every patient after thoracoplasty at the end of surgery. The chest tube had the aim to have a better control on possible pleural effusions and was removed after 5 days (median value), following evaluation of chest X-rays and clinical parameters by the thoracic surgeon.
The average operation time was a mean of 180 min (range 160–210 min) for the first procedure and a mean of 130 min (range 120–160 min) for the second surgery. Total perioperative bleeding of both surgeries was 950 ml (range 800–1300 ml), with a mean estimated blood loss of 15.4 cc/kg. No brace was used in the post-operative weeks.
Results
The entire series of 17 cases with an average follow-up of 2.9 ± 0.2 years (2.5–3.2) was reviewed. The group consisted of 12 females and 5 males, with a mean pre-operative BMI of 22.3 ± 3.1 kg/m2. The mean age at surgery was 14.5 ± 1.4 years (11–17), and the median Risser’s sign was 3.0 (1–5). The curve patterns according to the Lenke’s classification were 14 (82%) type 1 and 3 (18%) type 3.
Scoliosis major curves had an average pre-operative Cobb angle of 98.2° ± 6.9 (91°–138°), with a flexibility of 25.2% ± 2.9 (21.3–29.7), which bent down to 58.7° ± 7.2 (49°–82°) after MCGR final lengthening (p < 0.05; % of correction 41.5 ± 6.9). After second surgery, the curve was corrected to 38.3° ± 3.0 (35°–76°) in post-operative X-rays (p < 0.05); at last follow-up, the main thoracic curve was 40.1° ± 4.1 (35–79), whereas the mean overall curve correction was 58.7% ± 5.9 (50.4–71.2). The average correction loss at last FU following the post-operative period was 2.1 ± 0.6°(p > 0.05) (Table 1).
With regards to the sagittal parameters (Table 2), the mean thoracic kyphosis T5–T12 angle was 31.1° ± 4.2 (9°–36°) before surgery, 25.5° ± 8.2 (11°–35°) after definitive fusion, and 27.1° ± 7.6 (12–37°) at final follow-up evaluation. The mean thoracolumbar T10–L2 angle was − 3.5° ± 3.1 (− 9.0° to 13°) before surgery, 4.5° ± 1.2 (− 15° to 6°) after definitive fusion and 5.5° ± 1.3 on average (− 13° to 7°) at the latest follow-up.
Lumbar lordosis was − 45.9° ± 8.8 (− 55° to − 15°) on average in the pre-operative, − 39.8° ± 10.3 (− 55° to − 15°) after definitive fusion and -41.7° ± 9.9 (− 55° to − 19°) at the final follow-up evaluation. No statistically significant differences were reported for thoracic kyphosis and lumbar lordosis (p > 0.05). At thoraco-lumbar region (T10-L2) only a statistical significant improvement of regional kyphosis was reported (p < 0.05) between pre- and post-operative values.
After surgery, the coronal alignment (Table 3) showed a good trend of correction: a final better global coronal balance (C7-S1) (0.48 cm ± 0.3), a lower apical MT vertebra translation (3.1 cm ± 2.2) and a lower apical lumbar vertebra translation (1.6 cm ± 1.3) were reported. Moreover, lower coronal translation (0.9 cm ± 0.7) and coronal tilt (8.7° ± 6.5) for the lowest instrumented vertebra were noted. Statistically significant differences were reported with reference to all coronal parameters (p < 0.05).
The extension of the posterior fusion-instrumentation was 13.8 (12–15) vertebral levels. The free disc levels below the fusion area were on average of 2.48 (2–3).
Complications
One patient (5.8%) developed a pleural effusion related to the segmental ribs resections in the post-operative period, which required a longer period of chest tube insertion. The chest tube was removed 9 days later without consequences.
At latest follow-up, no fatal complications were reported. There were no cases of acute or delayed deep wound infection, pseudoarthrosis or rod breakage.
Discussion
Posterior correction of severe AIS can entail risks, mainly neurologic deficits [16]. Undergoing aggressive operations, such as vertebral column resection, may represent a risk for these patients: Suk et al. [4] obtained a relevant correction of the deformity magnitude (from 109° to 45°), but a permanent complete paraplegia in one case (6.3%) was reported. A posterior procedure with multiple less aggressive osteotomies seems to be preferable, but correction must be preceded by a period of traction. In this series, MCGR was adopted to gradually and safely correct deformities, without recourse to halo-gravity. The correction was performed with daily distractions of the device by means of a magnet applied over the skin. MCGR was introduced to correct scoliosis allowing spinal growth in patients with early-onset scoliosis [17]. The indication was extended to progressive correction of adolescent severe scoliosis, as previously described in two reports [8, 9]. Patients included in our series received a two-staged posterior surgery: after MCGR implant, a gradual distraction was applied to obtain the progressive correction of the curve by taking advantage of posterior release; in the second posterior surgery, MCGR was removed and the definitive rods were applied for final correction with posterior fusion.
The MCGR is an internal distraction device and thus offers a major advantage over halo-traction: it’s better tolerated [8, 9]. Moreover, the role of halo-traction and its safety and efficacy is actually debated. In a multicenter retrospective comparative study, Sponseller et al. [18] concluded that no statistically significant difference was noted between halo-traction and control group in coronal or sagittal curve correction, blood loss, operating time and complications; however, a vertebral body resection was less frequently performed in patients with halo-traction. Park et al. [19] and Koptan and ElMiligui [20] concluded that halo-gravity traction improved preoperative flexibility and final deformity correction. However, the following traction-related complications are frequently reported: pin loosening requiring halo ring replacement, pin-site infection needing oral antibiotics, cleaning or debridement of the pin site, cervical pain or discomfort, nystagmus, dizziness and nausea [21]. Moreover, stretching of the brachial plexus could lead to upper extremities transitory neurological deficit, which can be relieved by decreasing the weight of traction [22].
The daily gradual distractions of MCGR were applied with the patient awake and were free of complications. This procedure was performed to progressively overpower the stiffness of the curve. After a post-operative 2-week traction, correction was interrupted and the second step of surgery, i.e. the definitive correction, was carried out. The 2-week period of traction of our series was shorter than the 2-month and half performed by Cheung et al. [8] and the four-week traction by Aldeeri et al. [9], with the same MCGR applied in their patients. Different Authors used staged posterior correction with internal distraction rods (not magnetically controlled growing rod) for longer periods: Buchowski et al. [6] and Hui-Min et al. [7], for example, respectively used an approximately 24-week and a 12- to 15-week temporary internal distraction. We opted for a shorter period of traction based on the experience of Koptan and ElMiligui [20]. They used a limited period of halo-gravity traction in a series of 21 patients who underwent a three-stage correction (anterior release, 2 weeks of halo-gravity traction and posterior instrumentation). The Authors reported to have achieved near maximal curve correction after about 2 weeks of traction. Previously, Clark et al. [23] observed, using a halo-pelvic traction, a primary creep period of scoliosis correction of about 2–4 h, and a secondary creep period with a gradual reduction of spinal deformity, with the maximal curve correction being achieved after 10–12 days. The short period of traction provided in our series a simpler exposure of the posterior elements of the spine at second surgery, as a result of the previous recent intervention, thus reducing surgery duration and blood loss.
Debate exists about how to maximize curve correction in severe scoliosis. The rationale underlying the procedure reported herein is to obtain a posterior-only secure, staged and satisfying correction of the curve with the concurrent reduction of risks [8, 9]. On average, the curve correction obtained with MCGR before the second surgery was around 40%; the final curve correction obtained after second surgery was on average lower than 60% for curves of over 90°. The percentage of curve correction we obtained was lower in comparison with other more aggressive posterior procedures, such as one-stage posterior vertebral column resection (65.39% of curve reduction) [24]. In our series, no neurological complications occurred. Literature regarding vertebral column resection procedure instead reported a high rate of severe neurological complications (1.2–17.1%), including paraplegia or incomplete spinal cord injury [24].
The disadvantages of our procedure are multiple. Firstly, it is not possible to perform an MRI control after the first step of surgery for the MCGR in site. Secondly, the costs of the magnetically controlled growing rod are relatively high. Moreover, it’s a two-staged posterior surgery. Nonetheless, the advantages include the possibility of achieving a gradual correction of the curve, without using a pre-operative halo-gravity traction period, which is frequently accompanied by complications and patient discomfort [22]. The lengthening period in our case series was lower as compared to the experience reported by other Authors using the same procedure with MCGR [8, 9], internal distraction [6, 7] or halo-gravity traction [18].
The series at issue must be interpreted bearing in mind its limits: retrospective nature of the review, patients not randomized, lack of a control group and no clinical outcomes. However, the series is consecutive. This finding may be of service to reduce some of the potential selection bias associated with non-randomized studies.
Conclusions
Gradual traction with MCGR in severe AIS proved to be a safe method to achieve progressive curve correction before final fusion, without the neurologic complications described for more aggressive one-stage surgeries. In a staged approach, MCGR appears as an alternative to halo-traction, which allows frequent traction-related complications to be avoided.
References
Korovessis P (1987) Combined VDS and Harrington instrumentation for treatment of idiopathic double major curves. Spine 12:244–250. https://doi.org/10.1097/00007632-198704000-00009
Shufflebarger HL, Grimm JO, Bui V et al (1991) Anterior and posterior spinal fusion. Staged versus same-day surgery. Spine 16:930–933. https://doi.org/10.1097/00007632-199108000-00011
Savini R, Parisini P et al (1989) The surgical correction of severe vertebral deformities by combined anterior and posterior instrumentation. Prog Spinal Pathol 4:211–221
Suk SI, Chung ER, Kim JH et al (2005) Posterior vertebral column resection for severe rigid scoliosis. Spine 30:1682–1687. https://doi.org/10.1097/01.brs.0000170590.21071.c1
Lenke LG, Sides BA, Koester LA, Hensley M, Blanke KM (2010) Vertebral column resection for treatment of severe spinal deformity. Clin Orthop Relat Res 468(3):687–699. https://doi.org/10.1007/s11999-009-1037-x
Buchowski JM, Bhatnagar R, Skaggs DL et al (2006) Temporary internal distraction as an aid to correction of severe scoliosis. J Bone Joint Surg Am 88-A 9:2035–2041. https://doi.org/10.2106/JBJS.E.00823
Hui-Min H, Hua H, Hai-Ping Z et al (2012) The impact of posterior temporary internal distraction on stepwise corrective surgery for extremely severe and rigid scoliosis greater than 130°. Eur Spine J. https://doi.org/10.1007/s00586-015-4013-z
Cheung JP-Y, Samartzis D, Cheung KM-C et al (2014) A novel approach to gradual correction of severe spinal deformity in a pediatric patient using the magnetically-controlled growing rod. Spine J 14(7):e7–13. https://doi.org/10.1016/j.spinee.2014.01.046
Aldeeri R, Almansour H, Kentar Y et al (2018) Magnetically controlled growing rods for rigid scoliosis. Der Orthopade 10:867–870. https://doi.org/10.1007/s00132-018-3631-7
Lenke L, Betz R, Harms J et al (2001) Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am 83:1169–1181
Cobb JR (1948) Outline for the study of scoliosis. AAOS Instr Course Lect 5:261–275
Kim YJ, Lenke LG, Bridwell KH et al (2004) Free hand pedicle screw placement in the thoracic spine: is it safe? Spine 29:333–342. https://doi.org/10.1097/01.brs.0000109983.12113.9b
Kabins MB (2004) Point of view. Spine 29:342. https://doi.org/10.1097/01.BRS.0000109984.65138.0C
Di Silvestre M, Parisini P, Lolli F et al (2007) Complications of thoracic pedicle screws in scoliosis treatment. Spine 32:1655–1661. https://doi.org/10.1097/BRS.0b013e318074d604
Ponte A, Orlando G, Siccardi GL (2018) The true ponte osteotomy: by the one who developed it. Spine Deform 6(1):2–11. https://doi.org/10.1016/j.jspd.2017.06.006
Sucato DJ (2010) Management of severe spinal deformity: scoliosis and kyphosis. Spine 35(25):2186. https://doi.org/10.1097/BRS.0b013e3181feab19
Akbarnia BA, Cheung K, Noordeen H et al (2013) Next generation of growth-sparing techniques: preliminary clinical results of a magnetically controlled growing rod in 14 patients with early-onset scoliosis. Spine 38:665–670. https://doi.org/10.1097/BRS.0b013e3182773560
Sponseller PD, Takenaga RK, Newton P, Boachie O, Flynn J, Letko L, Betz R, Bridwell K, Gupta M, Marks M, Bastrom T (2008) The use of traction in the treatment of severe spinal deformity. Spine 33:2305–2309. https://doi.org/10.1097/BRS.0b013e318184ef79
Park DK, Braaksma B, Hammerberg KW, Sturm P (2013) The efficacy of preoperative halo-gravity traction in pediatric spinal deformity the effect of traction duration. J Spinal Disord Technol 26:146–154. https://doi.org/10.1097/bsd.0b013e318237828c
Koptan W, ElMiligui Y (2012) Three staged correction of severe rigid idiopathic scoliosis using limited halo-gravity traction. Eur Spine J 21:1091–1098. https://doi.org/10.1007/s00586-011-2111-0
Yang C, Wang H, Zheng Z et al (2017) Halo-gravity traction in the treatment of severe spinal deformity: a systematic review and meta-analysis. Eur Spine J. https://doi.org/10.1007/s00586-016-4848-y
Savini R, Parisini P, Corbascio M et al (1977) Halo-traction associated with kinesiotherapy in the corrective treatment of scoliosis with severe respiratory insufficiency. Chir Organi Mov 63(4):353–360 (PMID:872682)
Clark JA, Hsu LCS, Yau AC (1975) Viscoelastic behavior of deformed spines under correction with halo pelvic distraction. Clin Orthop 110:90–111. https://doi.org/10.1097/00003086-197507000-00014
Xie JM, Zhang Y, Wang YS et al (2014) The risk factors of neurologic deficits of one-stage posterior vertebral column resection for patients with severe and rigid spinal deformities. Eur Spine J 23(1):149–156. https://doi.org/10.1007/s00586-013-2793-6
Funding
There is no funding source for this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Di Silvestre, M., Zanirato, A., Greggi, T. et al. Severe adolescent idiopathic scoliosis: posterior staged correction using a temporary magnetically-controlled growing rod. Eur Spine J 29, 2046–2053 (2020). https://doi.org/10.1007/s00586-020-06483-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06483-8