Abstract
Purpose
To determine the risk factors of neurologic deficits during PVCR correction, so as to help improve safety during and after surgery.
Methods
A consecutive series of 76 patients with severe and rigid spinal deformities who were treated with PVCR at a single institution between October 2004 and July 2011 were included in our study. Of the 76 patients, 37 were male and 39 female, with an average age of 17.5 years (range 10–48 years). There were 52 adolescent patients (with an age <18 years) and 24 adult patients (with an age ≥18 years). Preoperatively, postoperatively and 6 months after surgery, we performed systemically neurologic function evaluations of each patients through meticulous physical examination. Any new abnormality or deterioration in evaluation of neurologic function than preoperative is reckoned postoperative neurologic deficits. Ten variables that might affect the safety of neurologic deficits during PVCR procedures, including imaging factors, clinical factors and operational factors, were analyzed using univariate analysis. Then the variables with statistical difference were analyzed by using multi-factor unconditional logistic regression analysis.
Results
No patient in this series had permanent paraplegia and nerve root injury due to operation. Change of neurologic status was found in six patients after surgery. Results of single-factor comparison demonstrated that the following seven variables were statistically different (P < 0.05): location of apex at main curve (X 3), Cobb angle at the main curve at the coronal plane (X 4), scoliosis associated with thoracic hyperkyphosis (X 5), level of vertebral column resected (X 6), number of segmental vessels ligated (X 7), preexisting neurologic dysfunction (X 8), and associated with intraspinal and brain stem anomalies (X 9). The multi-factor unconditional logistic regression analysis revealed that X 8 (OR = 49.322), X 9 (OR = 18.423), X 5 (OR = 11.883), and X 6 (OR = 8.769) were independent and positively correlated with the neurologic deficit.
Conclusions
Preexisting neurologic dysfunction, associated with intraspinal and brain stem anomalies, scoliosis associated with thoracic hyperkyphosis and level of vertebral column resected are independent risk factors for neurologic deficits during PVCR procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Posterior vertebral column resection (PVCR) with pedicle instrumentation has the advantages of higher correction rate and overall balance over conventional correction techniques. In recent years, the reports on its clinical application have been gradually increasing [1–9]. In particular, to severe (the Cobb >100º), angular and rigid (flexibility <10 %) spinal deformities that are considerably decompensatory at the coronal or sagittal plane, PVCR may be the best choice [1].
Given the complexity and extraordinary technical demands associated with performing this complex technique, and paralleled satisfactory correction of spinal deformities, PVCR has been plagued with high neurologic deficits risk [2–6]. Few studies, however, have analyzed the risk factors of neurologic deficits during PVCR procedure. The purpose of this study was conducted to determine the risk factors of neurologic deficits during PVCR correction at single institution. Such data are needed to mitigate patient and surgeon expectations and may also help improve patient selection and safety during and after surgery.
Materials and methods
Patient demographics
A consecutive series of 76 patients with severe and rigid spinal deformities who were treated by PVCR at a single institution between October 2004 and July 2011 were included in our study. Approval was obtained from the institutional review board. Of the 76 patients, 37 were male and 39 female, with an average age of 17.5 years (range 10–48 years). There were 52 adolescent patients (age <18 years) and 24 adult patients (age ≥18 years). The etiology included was idiopathic spinal deformity in 31 and non-idiopathic spinal deformity in 45. Thoracic vertebral column resection was performed in 45 patients, thoracolumbar in 19 and lumbar in 12 (Table 1).
All patients underwent surgery directed and performed by the first author (J.X.).Wake-up tests were conducted after insertion of pedicle screws, completion of the deformity correction, and completion of surgery. All patients associated with patients with Chiari malformation and/or syringomyelia underwent a one-stage spinal deformity correction without a craniocervical neurological decompression beforehand. The PVCR surgical technique included implant placement above and below the planned VCR site. Then following laminectomy, provisional instrumentation was placed prior to the corpectomy and discectomy being performed. Then, closure of the defect always began with compression and shortening of the spinal canal followed by other maneuvers to optimize deformity correction. Definitive instrumentation and fusion was then performed.
Preoperatively, postoperatively and 6 months after surgery, standing anterior–posterior (AP) and lateral radiographs of the spine was performed, Cobb’s angle measured at the coronal and sagittal planes. We performed systemically neurologic function evaluations of each patient by meticulous physical examination documenting the motor, sensory, reflex function and gait assessment as well as pain at each preoperative and postoperative examination. 13 (13/76, 17.10 %) patients presented with preexisting neurologic dysfunction, including asymmetric abdominal reflex or tendon reflex in ten patients, slightly increased muscle tension and tendon hyperreflexia in lower extremities in eight, abnormal sensory in nine, positive pathological signs in lower extremities in nine and bowel or bladder incontinence in two. Any new neurological symptom related to spinal cord or nerve root compromise, evidence of abnormality or deteriorate in evaluation of neurologic function than preoperative is reckoned postoperative neurologic deficits. Six patients (6/76, 7.89 %) presented with postoperative change in neurological status, and the remaining 70 (70/76, 92.10 %) patients were normal (Table 1).
Study methods
Ten variables that might affect the neurological status during PVCR procedures were analyzed using univariate analysis (SPSS software version 13.0). The variables that were statistically different in the univariate analysis were further analyzed using multi-factor unconditional logistic regression analysis (stepwise regression method) to identify the risk factors of neurologic deficits during PVCR correction. Statistical difference was defined as P < 0.05.
Variables and identifying criteria
General factors
The general information included gender(X 1) and age (years) (X 2). The etiology (X 10) was that scoliosis with identified reasons was defined as non-idiopathic scoliosis and the remaining as idiopathic scoliosis. 13 patients with preexisting neurologic dysfunction (X 8) were detected by meticulous physical examination. 26 patients associated with intraspinal and brain stem anomalies (X 9) were identified by clinical manifestation and imaging examination before surgery, including Chiari malformation (eight patients), syringomyelia (eight patients) and tethered cord (10 patients) (Table 1).
Radiographic factors
(1) Apex of the main curve(X 3): the apex of the main curve at T2–T10 was defined as thoracic spinal deformity, thoracolumbar spinal deformity at T11–L1 and lumbar spinal deformity at L2–L5. (2) Cobb angle at the main curve at the coronal plane (X 4) was measured with Cobb method. (3) Scoliosis associated with thoracic hyperkyphosis (X 5) regarding the sagittal alignment, the Cobb angle (>40°) was detected as hyperkyphosis.
Surgical factors
The following surgical factors were assessed: (1) levels of vertebral column resected intraoperatively (X 6), and (2) number of segmental vessels ligated intraoperatively (X 7).
Results
Operation evaluation
On an average 1.5 vertebrae were removed, with mean operating time 512 min and average blood loss of 4,760 ml. Fifty-two patients were followed up for average 48.6 months (range 24–72 months). The main curve of scoliosis was corrected from average 110.4° preoperatively to 38.2° postoperatively, and kyphosis from 94.5° preoperatively to 28.6° postoperatively. The correction rate of scoliosis and kyphosis was 65.39 and 69.73 %, respectively. Loss of correction at most recent follow-up was <10 %.
Evaluation of neurologic function
No patient in this series had permanent paraplegia and nerve root injury due to operation. Change of neurologic status was found in six patients after surgery. Of the six patients, five patients presented with preexisting neurologic dysfunction before operation, including three presented with slightly more obvious pathological signs than before surgery, and two presented with more active knee and ankle hyperreflexia than before surgery. All recovered fully at a 6-month follow-up.
Of the six patients, four patients, who had scoliosis with thoracic hyperkyphosis, suffered from moderate pain of the posterior thoracic wall, radiating through the nerve root distribution region. All recovered without intervention within 3 months.
One patient without preexisting neurologic dysfunction before operation, presented with normal neurologic function immediately after surgery, but showed complete dysfunction of spinal cord 8 h later. Mechanical oppression was excluded through emergency CT and MRI examination. Methylprednisolone 30 mg/kg was administered for 30 min and maintained at a dose of 5.6 mg/kg/h until 72 h postoperative, the same dosage for acute spinal cord injury. The neurologic function of the patient gradually improved and the neurological symptoms and signs were completely eradicated in 2 weeks (Table 1).
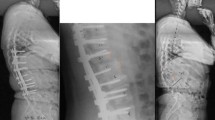
At the final follow-up, four patients with preoperative hypermyotonia of the lower extremities and tendon hyperreflexia had a return to normal function. The remaining patient with hypermyotonia demonstrated reduced muscle tension and could walk with quick steps freely. The three patients with combined spinal deformity and syringomyelia underwent MRI of cervical segments, which indicated that the diameter of the syrinx during the follow-up period was smaller than preoperatively although their preoperative asymmetrical tendon reflex was unchanged from baseline. Asymmetrical abdominal reflex or tendon reflex exhibited in patients with Chiari malformation preoperatively remained unchanged (Fig. 1).
Male, 18 years, kyphoscoliosis associated with Chiari I malformation and cervical syringomyelia. a Preoperative clinical feature. b Preoperative radiographs showed severe and rigid curves on coronal plane. c The cerebellar tonsils herniation with huge cervical syringomyelia formation. d Posterior vertebral column resection (PVCR) of T11 was performed; in the process of deformity correction, the spinal cord was unexpectedly and suddenly angled and twisted. e–f 24-month follow-up outlook radiograph. g 24-month follow-up MRI shows that the huge cervical syringomyelia had been narrowed following the spinal deformity correction
Single-factor comparison between the deficit group and the normal group
Results of univariate analysis demonstrated that the following seven variables were statistically different: location of apical vertebra at the main curve (X 3), Cobb angle of the main curve at the coronal plane (X 4), scoliosis associated with hyperkyphosis (X 5), level of vertebral column resected (X 6), number of segmental vessels ligated (X 7), preexisting neurologic dysfunction (X 8), and associated with intraspinal and brain stem anomalies (X 9) (Tables 1, 2).
Multi-factor comparison between the deficit group and the normal group
The seven variables that were statistically different were further analyzed using multi-factor unconditional logistic regression analysis (stepwise regression method). As the relationship between apex (X 3) and Cobb angle of the main curve at the coronal plane (X 4) were linear and not independent, these two variables were not included in the regression equation, whereas level of vertebral column resected (X 6) and number of segmental vessels ligated (X 7) were correlated, and level of vertebral column resected (X 6) was included in the regression analysis. Number of segmental vessels ligated (X 7) was not included in the regression equation. The results of multi-factor comparison indicated that PVCR procedures were linearly dependent on and positively correlated with the following factors with regard to the neurologic deficit: preexisting neurologic dysfunction (X 8 ) (B = 3.898, odd ratio OR = 49.322), associated with potential intraspinal and brain stem anomalies (X 9) (B = 2.914, OR = 18.423), scoliosis associated with hyperkyphosis (X 5 ) (B = 2.475, OR = 11.883), and level of vertebral column resected (X 6 ) (B = 2.171, OR = 8.769). As compared with standard partial regression coefficients (OR), it could be seen that preexisting neurologic dysfunction (X 8 ) was the most significant factor affecting neurologic deficit during PVCR (Tables 2, 3).
Discussion
So far, studies on PVCR procedures have shown that the technique has achieved significant deformity correction and overall balance as compared with conventional correction techniques [2, 5]. In our series of patients, the correction rate at the coronal and sagittal planes was 65.39 and 69.73 %, respectively. During most recent follow-up, loss of correction was <10 %. However, when achieving desirable correction, PVCR procedures are plagued with high.
Neurologic complications, which is reported to be an average of 14.3 %, ranged from 1.2 to 17.1 %, including paraplegia, incomplete spinal cord injury [2–4, 6, 10, 11]. In this article, the authors summarize their surgical techniques and evaluate patient outcomes after performing posterior vertebral column resection (PVCR) for the correction of sever and rigid spinal deformities, and investigate the risk factors to neurological neurologic deficits during this challenging procedure.
Clinical factors
In this study, preexisting neurologic dysfunction (X 8 ) was the most significant factor affecting neurologic deficit during PVCR. Studies [2–6] about PVCR have reported that most patients suffering from complete spinal cord injury have preexisting neurologic dysfunction. In our study, five of six patients (83.33 %) with postoperative neurologic deficits had preexisting neurologic dysfunction (P < 0.05). Neurologic deficit prior to the onset of treatment indicates an increased possibility of additional injury. This situation has been reported to be suggestive of brain stem and intraspinal anomalies [12–18]. In these patients, who have chronic ischemia and hypoxia of the spinal cord due to the spinal deformities, “sick spinal cord” which together with the abnormalities of the spinal cord or the canal, distraction or compression of the spinal cord following corrective maneuvers for the curve may lead to neurologic deficit secondary to either distraction of the neural elements or excessive tension on the local vasculature, leading to decreased blood flow and cord ischemia. To these patients, a circumferential decompression and shortening procedure, which would seem beneficial, caused the diseased spinal cord to migrate in multiple directions, thus reducing the tension on the spinal cord and improving blood supply, accompanied by reperfusion pathological changes.
In our study, five of six patients (83.33 %) with postoperative neurologic deficits were associated with intraspinal and brain stem anomalies (X 9). We believe that spinal deformities associated with intraspinal and brain stem anomalies (i.e., Chiari malformation, syringomyelia or tethered cord) makes the cephalic elements (cerebellar tonsillar) or the caudal elements (medullary cone or filum terminale) of the spinal cord fixed and the spinal cord tension increased, causing decrease in the blood supply to the spinal cord. With the unavoidable mechanical traction during surgery, patients with above-mentioned pathology are faced with even higher risks of neurologic injury than other patients. Reports [18, 19] on those patients who present with neurologic complications following posterior instrumentation for correction are not rare. Therefore, most authors hold that one-stage neural decompression, such as enlargement of the foramen magnum, drainage of syringomyelia and resection of filum terminale, is necessary. But recent studies [20–24] have demonstrated that shortening of the spine can improve the morphology and tension of the spinal cord, thus improving its function. Xie et al. [25] believed that, for adolescents with a main curve >90° or associated with significant neurologic deficits before surgery, PVCR was appropriate and effective alternative because it could appropriate shortening of the spinal cord improves the blood supply, decrease the tension of spinal cord and, subsequently, the function of the spinal cord. That way, the patients in our study gained desirable correction of spinal deformities and their neural elements were well protected.
Medical imaging factors
Studies [2, 6] reported a high rate of neurologic deficits in patients with kyphoscoliosis or angular kyphosis at thoracic spine. Similar result was found in our study; 4 of 6 patients (66.66 %) with postoperative neurologic changes were associated with thoracic hyperkyphosis (X 5 ). In PVCR correction for scoliosis patients with thoracic hyperkyphosis, in which the spinal cord is draped over the apical segments of the deformity and appears attenuated and under stretch at surgery, a vertebral column resection creating a segmental defect with sufficient instability, a highly risky procedure, was often employed with. In this situation, the corrective maneuvers may result in sagittal or/and coronal angulation of the proximal and distal limbs of the spinal column at the apex, dual sac kinking, and spinal instability during the surgery. In addition, the ribs connected with the vertebrae of the curve are also very small and crowded, and the neural roots and sectional vessels are close together. Therefore, interruption of several intercostal vessels and thoracic nerve roots is inevitable giving rise to the possibility of neurologic deficits.
Operational factors
Risk of neurologic deficits in multiple levels vertebral column resection during PVCR was significantly higher than in one level. Suk et al. [3, 4] reported that permanent neurologic complications only occurred to patients with 3- or 4-level vertebral column resection, and the rate of neurologic complications was 4 %. Lenke et al. [6] reported that neurologic complications occurred in 4.6 % patients with 2-level vertebral column resection. In our study, 4 of 17 (23.53 %) patients with postoperative neurologic changes had multiple-level vertebral resection. Although level of vertebral column resection contributes to the overall percent correction, and the spinal cord could bear limited shortening of the spine, it also leads to a bigger segmental defect with more sufficient instability and the greater risk of dural sac impingement or subluxation. Meanwhile, it is not easy to keep the tension on the cord caused by corrective maneuvers not higher than the initial one during the correction, all which contribute to the spinal cord injury. Fortunately, no patient in this series had complete permanent paraplegia and nerve root injury due to operation. We believed that by provisional instrumentation, in situ contouring the rod, changing the rod alternatively during correction to maintain the stabilization of the entire spinal column and avoiding sudden displacement of the spinal cord, and by proper and timely shortening the spinal cord to reducing the tension of the cord is important. Based on our intraoperative observation, for patients without preoperative neurological deficits, reverse angulation of the dural sac <20° compared with the previous status is acceptable, and shortening of not more than 3 cm or rotation within 10° of the dural sac will not affect the function of the cord [26].
In our study, level of vertebrae resected and number of segmental vessels ligated was correlated. Therefore, level of vertebrae resected was the factor included in regression analysis. Although it is a controversy, many reports hold that ligation of segmental vessels for exposure and fusion may inevitably compromise spinal cord perfusion, particularly the vessels at thoracic spine [27, 28]. Especially in patients with severe spinal deformities, the “sick” spinal cord with excessive tension on the cord increased by cord anomalies could not bear the decreased blood flow and cord ischemia anymore. There is ample evidence that hypotension can further damage a compromised spinal cord [2, 6]. So, it is important that by meticulously stripping, protecting the segmental vessels, and the blood pressure and hematocrit be maintained in a normal range, and the level of vertebrae resected be minimized (averaged 1.5 segments), secondary to decreasing segmental vessel ligation as well.
Due to the limitation of the conditions, the data of intraoperative monitoring for assessment of neurologic function are not included in our series. Instead, wake-up tests were carried out for assessing the intraoperative neurologic function. Although many studies emphasized the importance to use spinal cord monitoring during PVCR to provide early detection of damage; however, not all neurological deficits could not be monitored, and routine wake-up tests were often conducted during the correction procedure. No patient awoke with abnormal motor function in our series. The PVCR procedure provides the opportunity for continuous direct visualization around the dural sac, palpation of its tension, and making appropriate adjustments if necessary. Therefore, the lack of real-time monitoring of potentials did not result in higher neurological risks in our series. To reduce the effect on repeated wake-up tests of anesthetic, our experience showed it was effective in reducing the dose of muscle relaxant 30 min before wake-up. But the results may provide valuable reference for surgeons who have to operate without motor evoked potential devices, though we still recommend using intraoperative monitoring for optimal operation safety, if conditions permit.
Conclusion
In PVCR correction of scoliosis, the risk factors for neurologic deficits include: preexisting neurologic dysfunction, associated with potential intraspinal and brain stem anomalies, scoliosis associated with thoracic hyperkyphosis, and levels of vertebral column resected.
References
Xie J, Wang Y, Zhang Y et al (2010) Posterior vertebral column resection for correction of severe rigid spinal deformity (abstract). In: The 45th annual meeting of The Scoliosis Research Society, Kyoto
Suk SI, Kim JH, Kim WJ et al (2002) Posterior vertebral column resection for severe spinal deformities. Spine 27:2374–2382
Suk SI, Chung ER, Kim JH et al (2005) Posterior vertebral column resection for severe rigid scoliosis. Spine 30:1682–1687
Suk SI, Chung ER, Lee SM et al (2005) Posterior vertebral column resection in fixed lumbosacral deformity. Spine 30:E703–E710
Lenke LG, O’Leary PT, Bridwell KH et al (2009) Posterior vertebral column resection for severe pediatric deformity: minimum two-year follow-up of thirty-five consecutive patients. Spine 34:2213–2221
Lenke LG, Sides BA, Koester LA et al (2010) Vertebral column resection for the treatment of severe spinal deformity. Clin Orthop Relat Res 468:687–699
Zhou C, Liu L, Song Y, Liu H, Li T, Gong Q, Zeng J, Kong Q (2011) Anterior and posterior vertebral column resection for severe and rigid idiopathic scoliosis. Eur Spine J 20(10):1728–1734
Wang Y, Lenke LG (2011) Vertebral column decancellation for the management of sharp angular spinal deformity. Eur Spine J 20(10):1703–1710
Bakaloudis G, Lolli F, Di Silvestre M, Greggi T, Astolfi S, Martikos K, Vommaro F, Barbanti-Brodano G, Cioni A, Giacomini S (2011) Thoracic pedicle subtraction osteotomy in the treatment of severe pediatric deformities. Eur Spine J20(Suppl 1):S95–S104
Dorward IG, Lenke LG (2010) Osteotomies in the posterior-only treatment of complex adult spinal deformity: a comparative review. Neurosurg Focus 28:E4
Hamzaoglu A, Alanay A, Ozturk C et al (2011) Posterior vertebral column resection in severe spinal deformities. A total of 102 Cases. Spine 36:E340–E344
Musson RE, Warren DJ, Bickle I et al (2010) Imaging in childhood scoliosis: a pictorial review. Postgrad Med J 86:419–427
Inoue M, Minami S, Nakata Y et al (2005) Preoperative MRI analysis of patients with idiopathic scoliosis: a prospective study. Spine 30:108–114
Pahys JM, Samdani AF, Betz RR et al (2009) Intraspinal anomalies in infantile idiopathic scoliosis. Prevalence and role of magnetic resonance imaging. Spine 34:E434–E438
Benli IT, Uzumougil O, Aydin E et al (2006) Magnetic resonance imaging abnormalities of neural axis in Lenke type I idiopathic scoliosis. Spine 31:1828–1833
Kontio K, Davidson D, Letts M (2002) Management of scoliosis and syringomyelia in children. J Pediatr Orthop 22:771–779
Tubbs RS, McGirt MJ, Oakes WJ (2003) Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg 99:291–296
Bradlley LJ, Ratahi ED, Crawford HA et al (2007) The outcomes of scoliosis surgery in patients with syringomyelia. Spine 32:2327–2333
Ferguson RL, DeVine J, Stasikelis P et al (2002) Outcomes in surgical treatment of ‘idiopathic-like’ scoliosis associated with syringomyelia. J Spinal Disord Tech 15:301–306
Grande AW, Maher PC, Morgan CJ et al (2006) Vertebral column subtraction osteotomy for recurrent tethered cord syndrome in adults: a cadaveric study. J Neurosurg Spine 4:478–484
Kawahara N, Tomita K, Kobayashi T et al (2005) Influence of acute shortening on the spinal cord: an experimental study. Spine 30:613–620
Alemdarog ˘lu KB, Atlihan D, Cimen O et al (2007) Morphometric effects of acute shortening of the spine: the kinking and the sliding of the cord, response of the spinal nerves. Eur Spine J 16:1451–1457
Kanno H, Aizawa T, Ozawa H et al (2008) Spine-shortening vertebral osteotomy in a patient with tethered cord syndrome and a vertebral fracture case report. J Neurosurg Spine 9:62–66
Matsumoto Morio, Watanabe Kota, Tsuji Takashi et al (2009) Progressive kyphoscoliosis associated with tethered cord treated by posterior vertebral column resection. A case report. Spine 34:E965–E968
Xie J, Wang Y, Zhao Z, Zhang Y, Si Y, Yang Z et al (2011) One-stage and posterior approach for correction of moderate to severe scoliosis in adolescents associated with Chiari I malformation: is a prior suboccipital decompression always necessary? Eur Spine J 20:1106–1113
Xie J, Li T, Wang Y, Zhao Z, Zhang Y, Bi N ( (2012) Change in Cobb angle of each segment of the major curve after posterior vertebral column resection (PVCR): a preliminary discussion of correction mechanisms of PVC. Eur Spine J 21:705–710
Kato S, Kawahara N, Tomita K et al (2008) Effects on spinal cord blood flow and neurologic function secondary to interruption of bilateral segmental arteries which supply the artery of Adamkiewicz: an experimental study using a dog model. Spine 33:1533–1541
Ueda Y, Kawahara N, Tomita K et al (2005) Influence on spinal cord blood flow and function by interruption of bilateral segmental arteries up to three levels: experimental study in dogs. Spine 30:2239–2243
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, JM., Zhang, Y., Wang, YS. et al. The risk factors of neurologic deficits of one-stage posterior vertebral column resection for patients with severe and rigid spinal deformities. Eur Spine J 23, 149–156 (2014). https://doi.org/10.1007/s00586-013-2793-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-013-2793-6