Abstract
Purpose
Use gait analysis to establish and detail the clinically relevant components of normal human gait, analyze the gait characteristics for those afflicted with spinal pathology, and identify those aspects of human gait that correlate with pre- and postoperative patient function and outcomes.
Methods
Twenty patients with adult degenerative scoliosis (ADS), 20 patients with cervical spondylotic myelopathy (CSM), and 15 healthy volunteers performed over-ground gait trials with a comfortable self-selected speed using video cameras to measure patient motion, surface electromyography (EMG) to record muscle activity, and force plates to record ground reaction force (GRF). Gait distance and temporal parameters, ankle, knee, hip, pelvic, and trunk range of motion (ROM), duration of lower extremity EMG activity and peak vertical GRF were measured.
Results
Patients with ADS and CSM exhibited a significantly slower gait speed, decrease in step length, cadence, longer stride time, stance time, double support time, and an increase in step width compared to those in the control group. These patients also exhibited a significantly different ankle, knee, pelvic, and trunk ROM. Moreover, spinal disorder patients exhibited a significantly longer duration of rectus femoris, semitendinosus, tibialis anterior and medial gastrocnemius muscle activity along with an altered vertical GRF pattern.
Conclusions
Gait analysis provides an objective measure of functional gait in healthy controls as well as those with ADS and CSM. This study established and detailed some of the important kinematic and kinetic variables of gait in patients with spinal disorders. We recommend that spine care providers use gait analysis as part of their clinical evaluation to provide an objective measure of function.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human gait is defined by reciprocal, bipedal locomotion and influences an individual’s participation and interaction with society. Gait is a repetitive and cyclical action that is constantly being modulated based on the individual’s desired speed [1, 2]. Human gait can be conceptualized as a series of incomplete falls where by the lower limbs act as inverted pendula in an effort to minimize the energy cost of locomotion by reducing the vertical displacement of the body’s center of mass [3, 4]. Impairments in mobility are a frequent complaint of individuals seeking orthopedic/rehabilitation services and are often the focus of the patient’s goals for treatment [2]. Many musculoskeletal and neurological disorders result in an altered gait pattern [5]. Not surprising, those with spinal disorders present with an altered gait pattern and objectively evaluating the gait will help to fully understand how the spinal pathology results in disability, reduced quality of life, and/or impairment of daily activities [5,6,7,8,9,10,11,12,13,14]. Rehabilitation experts have relied on a firm understanding of the basic mechanics of normal locomotion to determine the links between impairments of discrete segments of the musculoskeletal system and the patient’s abnormal gait patterns, likewise spine practitioners should also be familiar with these same principles [1].
Clinical gait analysis is the process by which quantitative information is collected to aid in understanding the etiology of gait abnormalities. [8, 10, 15,16,17]. This process is facilitated through the use of specialized technology such as, computer-interfaced video cameras to measure patient motion, electrodes placed on the surface of the skin to record muscle activity [18], and force plates embedded in the floor to monitor the forces that an ambulatory patient exerts on the ground [19]. Essential to this process is the interpretation of the data by an experienced, interdisciplinary team with knowledge of normal and pathological gait [15,16,17, 20].
Despite normal variations in gait, there are characteristic step and stride patterns that can be used to distinguish between individuals with and without impairments [10, 11, 15, 17, 19,20,21]. The typical distance and temporal parameters of gait are defined in Table 1. These parameters are commonly altered in gait disorders, typically manifesting with decreased gait speed and stride length. In the case of unilateral disorders, there is often a disturbance in swing and stance times, resulting in an abnormal swing-stance ratio [2]. Such factors serve as a useful, objective outcome measures that can be monitored over the course of treatment.
Surgical success has traditionally been judged by two primary measures: static imaging and the patient’s self-reported outcome measures (PROMs). Despite the dependence on PROMs, they are frequently misleading and inaccurate due to recall bias, or even worse someone other than the patient completing the questionnaire [22, 23]. In addition, static imaging provides valuable structural information, yet does not provide functional information and can likewise be misleading by virtue of the imaging technique. Several published studies describe in detail the methods used to analyze spinal deformity in the sagittal plane and measure select patient-specific alignment targets [24,25,26]. In one of those studies, Diebo et al. concluded, that the dynamic aspects of alignment, along with clinical evaluation are crucial in managing spinal deformity conditions [24]. Gait analysis is able to quantitatively measure this dynamic component of alignment and provide objective outcomes measures. These issues justify the need for a truly objective outcome measure that documents function. Therefore, the purpose of this study is to use formal gait analysis to establish and detail the clinically relevant components of normal human gait, analyze the gait characteristics for those afflicted with spinal pathology, and identify those aspects of human gait that correlate with pre- and postoperative patient function and outcomes.
Materials and methods
After receiving institutional review board committee approval, we prospectively recruited patients with spinal pathology who presented to our offices and were deemed surgical candidates. We also recruited normal volunteers to undergo gait analysis to validate and compare our results to normal gait data in the literature [19,20,21, 27,28,29,30,31,32] and also to serve as normal controls to which the surgical patients will be compared.
Subjects
We collected data from 20 patients with adult degenerative scoliosis (ADS; Age 62.71 ± 6.9 years; Height 1.64 ± 0.1 m; Weight 79.37 ± 22.2 kg), 20 patients with cervical spondylotic myelopathy (CSM; Age 60.90 ± 6.8 years; Height 1.66 ± 0.1 m; Weight 85.16 ± 15.6 kg), and 15 healthy volunteers (Age 55.01 ± 6.1 years; Height 1.71 ± 0.1 m; Weight 72.14 ± 15.7 kg). Patients were included in the study if they were between the ages of 30 and 70 years old, diagnosed with ADS or CSM by the following clinical and radiographic criteria. The inclusion criteria for ADS was defined as patients with a progressive and symptomatic degenerative scoliosis with Cobb angle greater than 25° complaining of axial back pain, and or radicular or stenotic symptoms. The inclusion criteria for CSM was defined as patients who had confirmed spinal cord compression on imaging and concordant myelopathic signs or symptoms of spinal cord dysfunction (e.g. hand clumsiness, gait disturbance, hyperreflexia, long-track signs, etc.). Patients were excluded if they had a history of prior spine or major lower extremity surgery, BMI greater than 35, primary neurological disorder other than the cervical myelopathy, diabetic neuropathy or other disease that impairs the patient’s ability to ambulate or stand without assistance, and those that may be pregnant. Healthy volunteers were recruited from the general population.
Preparatory procedures
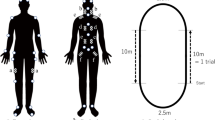
All test subjects were fitted with full-body external reflective markers placed according to procedures modified from Vaughan et al. [33]. These markers were placed on the skin overlying strategic anatomic points as depicted in Fig. 1. A static video trial was recorded with subjects positioned in a neutral standing posture to create a reference for defining neutral joint angles. Surface EMG electrodes were placed bilaterally on the skin overlying the rectus femoris (RF), semitendinosus (ST), tibialis anterior (TA), and medial gastrocnemius (MG). The skin at the recording sites was cleaned with alcohol, shaved if necessary, and then lightly abraded to reduce impedance. After electrode placement, the subjects then performed sub-maximum voluntary contraction (sMVC) of each muscle group. The sMVC tests are simple functional tests that assess the individual muscle groups via efforts such as plantarflexion and dorsiflexion of the feet (for TA—dorsiflexion and the MG complex—plantarflexion). These maneuvers establish a baseline measure for subsequent normalization during functional activities.
Testing procedures
Each subject performed a series of over-ground (in contrast to treadmill) gait trials at a comfortable self-selected speed. Subjects walked a total of 10 m, stepping on three sequential force platforms along the way. Subjects repeated the series of gait trials until five acceptable trials were obtained.
Data acquisition
Three-dimensional (3D) kinematic data was recorded at 200 Hz using a 10 camera Vicon Video system (Vicon Nexus 2.0 Inc., Englewood, CO). Ground reaction force (GRF) (AMTI Corp, Watertown, MA.) and electromyographic (EMG) (Delsys, Inc, Natick, MA) data were recorded simultaneously at 2000 Hz. The kinematic data was low pass filteredFootnote 1 with a 4th order Butterworth filter with a lower cutoff at 4 Hz. The GRF data was also low pass filtered with a similar filtering technique. The EMG data was wirelessly transmitted to the sampling computer using a 16 channel Delsys Trigno unit. The Trigno bandwidth was 200–500 Hz with a signal-to-noise ratio of 1 µV root mean square baseline noise. The preset signal amplification was set to be 2000 times, with an impedance of 10 MΩ and a common-mode rejection ratio of 100 dB. The EMG data was band-pass filtered between 20 and 450 Hz with a fourth-order, no pass zero-phase-lag Butterworth filter and then was fully wave rectified. The filtered EMG data was normalized to the sMVC previously described and further analyzed to identify muscle magnitude and muscle timing onset. The mean normalized amplitude of each brust of muscle activity, was detected by double-threshold method algorithm as a period of at least 50 ms while muscle was active [30, 35]. For the purposes of the study, only the right side was considered the reference side to be analyzed during the gait cycle.
Statistical analyses
One-way ANOVA with Bonferroni Post Hoc analyses was used to determine differences in gait patterns in adult degenerative scoliosis and cervical spondylotic myelopathy patients compared to healthy controls. Statistical analyses were conducted using SPSS, Version 23.0 (IBM, Inc., Chicago, IL).
Results
The collected gait data for the three groups and the literature normal data are included in Table 1. There were no significant differences between the results we measured in the healthy volunteers compared to normal gait data cited in the literature [19,20,21, 27,28,29,30,31,32]. This finding validates our equipment and data collection methodology. On the other hand, patients with spinal disorders exhibited significant differences in their gait patterns when compared to the healthy controls.
Patients with adult degenerative scoliosis exhibited a significantly slower gait speed (0.27 m/sec; p = 0.001), shorter step length (0.09 m; p = 0.020), shorter stride length (0.19 m; p = 0.011), and increased step width (0.03 m; p = 0.05). Furthermore, ADS patients had a decrease in cadence (14 step/min; p = 0.025), longer stride time (0.17 s; p = 0.031), stance time (0.15 s; p = 0.009), and double support time (0.12 s; p = 0.044). Swing time, swing-stance ratio, and single support time were not found to be statistically different (Table 1). These ADS patients also exhibited a significantly smaller knee ROM in the sagittal plane (14°; p = 0.046). In the frontal plane, they exhibited a larger knee (14°; p = 0.020) ROM, lesser pelvic (3°; p = 0.005) and trunk (2°; p = 0.050) ROM (Table 2, Fig. 2). In addition, these patients exhibited a significantly longer duration of activity in RF (15%; p = 0.001), ST (14%; p = 0.001), TA (9%; p = 0.014), and MG (11%; p = 0.001) as percentage of their gait cycle in comparison to healthy controls (Table 3, Fig. 3). Furthermore, ADS patients had a higher vertical GRF valley (0.07% of BW; p = 0.050) in comparison to the healthy group (Table 4, Fig. 4).
a Normalization and extraction of amplidute over brusts of rectus femoris muscle activation over a gait cycle from a representative health adult. b Brusts of rectus femoris, c semitendinosus, d tibialis anterior, e medial gastrocnemius muscle activation over a gait cycle in representative patients with scoliosis, cervical myelopathy, and healthy adult controls
Cervical spondylotic myelopathy patients exhibited a significantly slower gait speed (0.24 m/sec; p = 0.037), decreased step length (0.11 m; p = 0.014), stride length (0.20 m; p = 0.019) and increased step width (0.05 m; p = 0.001) compared to controls. Cadence, stride and stance times, swing-stance ratio, and single support time were not found to be statistically different (Table 1). Furthermore, CSM patients showed a significantly larger ankle ROM (5°; p = 0.024) and smaller knee ROM (15°; p = 0.050) in the sagittal plane, along with bigger ankle (2°; p = 0.050) ROM in the coronal plane (Table 2, Fig. 2). Cervical spondylotic myelopathy patients presented with a significantly longer duration of activity in RF (16%; p = 0.001), ST (17%; p = 0.001), TA (20%; p = 0.001), and MG (14%; p = 0.001) as a percentage of their gait cycle in comparison to healthy volunteers (Table 3, Fig. 3). Furthermore, CSM patients presented lower vertical GRF 2nd peaks (0.09% of BW; p = 0.050), later 1st peaks (3% of gait cycle; p = 0.048) and valleys (3% of gait cycle; p = 0.049) in comparison to controls (Table 4, Fig. 4).
Discussion
Gait is a repetitive and cyclical process that is constantly being modulated based on an individual’s desired speed [1, 2]. The gait cycle consists of two phases: the period where the reference foot is on the ground (stance phase) and the period it is off the ground (swing phase) [36]. In healthy adults, the stance phase of gait accounts for approximately 60% of the gait cycle, and the remaining 40% consists of the swing phase [2]. The stance phase accomplishes three objectives in locomotion: it provides adequate support to avoid falling, it acts to absorb the shock of impact between the limb and the ground, and it provides adequate forward and backward forces to perpetuate gait. The swing phase is meant to provide limb clearance, appropriate limb placement for heel-strike, and transfer of forward momentum [2]. By recognizing these individual components and overall goals, clinicians can examine the specific sequence of muscle activity and precise movement of limb segments to gain an appreciation for how specific musculoskeletal pathology affects a patent’s mobility and gait.
Clinical gait analysis is a process whereby gait characteristics are measured, abnormalities are identified, causes are postulated, and treatments are proposed. The approach used most often involves the placement of external markers on strategic anatomic locations on the trunk and lower extremities of the patient. These markers are then monitored by special video cameras as the patient walks along a straight, level pathway. The camera images are analyzed by a central computer with biomechanical programs to quantify the movement of specific body segments within space [36]. This motion analysis is then combined with electromyography data and ground reaction force measurements when the patient’s foot strikes the ground to provide a comprehensive assessment of the biomechanics of locomotion. Through the thoughtful use of technology, quantitative gait analysis provides an opportunity to appreciate the details of complex movement patterns that simultaneously involve the motion of various lower and upper extremity segments around multiple joints in several motion planes [12,13,14]. It is important to appreciate that while treatment decisions can be formed using clinical gait analysis measurements, these decisions are ultimately derived from a combination of the available objective clinical data, along with the clinician’s experience in managing the particular diagnosis [12,13,14]. Quantitative gait analysis does not dictate clinical treatment, but rather quantifies the effect of a disease process and its treatment on gait.
Studies that examine the electrical activity of muscles during locomotion are important in defining the role of muscles in producing and controlling human gait. First, the duration of large bursts of activity for most muscles is quite brief, and most of these bursts occur at the transitions between the swing and stance phases of gait. There is also considerable variability in the timing or amount in muscle activity across individuals [2]. Reviewing the EMG data of these large muscle groups demonstrate that most of the activity is actually characterized by eccentric contraction followed by concentric contraction. Furthermore, the motion occurring during concentric contraction actually continues after the contraction ceases. Thus, the chief function of lower extremity muscles during locomotion are to modulate one’s motion by providing an initial burst, or push, in the opposite direction to which the individual wishes to move. The results we present verify that patients afflicted with spinal disorders present with longer muscle activity duration and an altered muscle activity pattern during the gait cycle in the RF, ST, TA and MG muscles (Table 3 and Fig. 3). This altered muscle effort results in an inefficient gait pattern with higher energy demands. These findings imply that affected patients are trying to protect or splint their backs and/or lower extremities in an effort to optimize overall function and reduce joint loading.
With every step, the foot applies a load to the ground and the ground pushes back, imparting a GRF to each foot. The magnitude and direction of this GRF changes throughout the stance phase of each foot and is directly related to the acceleration of the body’s center of mass [1]. Vertical GRFs contribute significantly to joint reaction forces, and exaggerated or abnormal joint reaction forces contribute to pain in patients with joint pathology. The GRF typically is described by a vertical force as well as anterior–posterior and medial–lateral shear forces. The vertical GRF under each foot is characterized by an initial spike followed by a double-peaked curve (Fig. 4). The first brief rise in GRF reflects the impact of heel-strike [2]. The two subsequent peaks are greater than 100% of body weight (BW) and occur when the body accelerates upward. The first peak appears at heel contact, showing a rapid rise to a value in excess of body weight as full weight bearing takes place and the body’s downward velocity is being arrested. Then, as the knee flexes during midstance, the ground is partially unloaded and vertical GRF drops below body weight. The valley between the peaks is less than 100% of BW and occurs during single limb support. At push-off the plantar flexors are active, causing a second peak greater than body weight, which demonstrates that the body’s center of mass is being accelerated upwards to increase its upward velocity [2]. Our data established that vertical GRFs are altered in patients with symptomatic ADS and CSM. These patients produced higher double peaks as different from 100% of BW (Table 4). Moreover, these patients with spinal disorders demonstrated a different vertical GRF pattern in comparison to the healthy group (Fig. 4) which implies more energy waste and a less efficient gait cycle.
The available literature regarding head and trunk ROM reveal that these segments undergo systematic translation and rotation in three dimensions and exhibit both intra-subject and inter-subject variability. The trunk exhibits slight flexion and extension during gait, adopting a more erect or extended position during single support and more flexed position during double limb support. Coronal plane motion of the trunk is dictated by the need to keep the center of mass over the stance foot. Therefore, the trunk leans slightly to the stance limb at each step. In the axial plane, the rotation of the trunk is opposite the rotation of the pelvis, with the trunk rotating forward on the side in which the arm is swinging forward. The motion coupling between the trunk and pelvis contributes to the efficiency and stability of gait [2]. Patients who lose the ability to rotate the trunk separately from the pelvis, such as scoliosis patients, may lose gait efficiency and require more energy to walk. Our results verified that patients with ADS presented with significantly less pelvic and trunk ROM in the frontal and coronal planes, consistent with the above described clinical findings.
The most common symptom in CSM is gait disturbance [13]. Our results verify that CSM patients walk slower, with reduced trunk and lower extremity function and efficiency in comparison to asymptomatic controls. CSM patients also have decreased stride length, along with a wider stride width compared to healthy controls. Additionally, CSM patients have diminished toe-off and increased heel-strike forces, which reflects their difficulty with both propelling themselves forward and also with catching their center of mass during heel-strike to avoid falling over. Furthermore, they spend a greater proportion of their gait cycle in the stance phase, reflective of their difficulties with balance.
With this initial study, we were able to validate the equipment and testing protocols for our lab. The gait data generated from the normal volunteers match published normative values. The kinematic variables presented in this paper include the discrete displacement patterns of joints, the activity pattern of lower extremity muscles, and the GRF associated with gait. Although all of these variables are subject to intra- and inter-subject variability, representative values are presented to provide the clinician with a frame of reference for gait in those afflicted by ADS and CSM.
We established that joint excursion is largest in the sagittal plane and exhibits stereotypical patterns and sequences. We also characterized the activity of the major muscle groups of the lower extremity. In most cases, joint movement continues after muscle activity has ceased. This both confirms and quantifies the balance and gait difficulties that patients with CSM experience. It is beneficial to have objective, quantitative data to describe changes in subjective clinical findings such as gait. Identification of these gait parameters (i.e., walking speed, stride length, and stride width) can help tailor and monitor the progress during post-operative rehabilitation protocols.
While using all three modalities (EMG, GRF, motion tracking) provides the most complete picture of a patient’s gait biomechanics, each of the individual tests offers unique data that can be used for different purposes. Each of the modalities above (i.e. EMG, GRF, motion tracking) can potentially be used by itself. EMG directly measures the activity of specific muscles, and thus can be a good tool to monitor the recovery of specific muscles postoperatively or to track a patient’s strength during rehabilitation. The use of force plates can provide us numerical information related to the GRF magnitude and timing. Those are very valuable when analyzing weight acceptance and push-off phases of gait, shock absorption of the impact of the foot striking the ground, and forward progress with each. Human motion capture can provide 3D kinematic and spatiotemporal parameters during walking. Specific joint angles and ROM can be determine and compared to healthy controls. Having the ability to quantify and objectively compare our patients’ function to healthy controls offers a unique ability to demonstrate the success of spinal surgery. Having stated the above, the use of all three modalities in combination provides the most comprehensive assessment of gait in both the normal and pathologic states. However, one must appreciate that gait analysis can be very sensitive to gait alterations, but it may not be specific to each pathological entity as many individual and overlapping musculoskeletal factors can contribute to the gait alteration.
This study has taken the first step towards applying formal gait analysis to analyzing the global impact that certain spinal disorders have on whole-body biomechanics and gait. By formally characterizing normal human gait in a group of asymptomatic controls, this study was able to identify specific gait parameters that deteriorate with ADS and CSM. This test can be used on any spinal pathology, including but not limited to artificial disc replacement, adolescences scoliosis, sacroiliac fusion, chronic low back patients.
This study does have its limitations. The data demonstrates a large inter-subject variability, therefore, uncertainty around our estimates is a limitation that raises the risk of chance findings. Additionally, we acknowledge the limitations associated with kinematic modeling using the selected marker set, including skin movement, errors in the anthropometric model, system tracking errors and data smoothing errors. Furthermore, for the purposes of the study, we considered only the right side as the reference side, however, bilateral motion and muscle activity should be examined especially in patients with degenerative scoliosis, to fully assess side to side asymmetries.
Gait analysis provides an opportunity to appreciate the details of complex movement patterns, including movements that occur concurrently in multiple planes of motion. It is crucial to have objective, quantitative data to describe changes in subjective clinical findings such as gait [12,13,14]. It also allows clinicians to understand and correlate the associated muscle activity to the patient’s spinal disorders. Identification of these gait parameters (i.e. gait speed, step length and width, stride length, cadence, stride and stance times, ankle, knee, hip and pelvic ROM, muscle activity duration and pattern, and vertical GRF pattern and values) can help tailor and monitor the progress of post-operative rehabilitation protocols. Spine care providers can benefit from paying attention to these outcome measures as part of their clinical functional evaluation. Contemporary research has focused heavily on the use of patient reported outcome measures (PROMs) such as the Oswestry Disability Index (ODI), the Visual Analogue Pain Scale (VAS), and the Short Form 36 (SF-36) generic health questionnaires. With the results of this study, we suggest that gait analysis provides a reliable and objective method to correlate patient function to PROMs and enrich the discussion of how spinal disorders affect patient gait, function, and outcomes pre- and post-operatively. Using gait analysis studies before and after intervention for spinal pathology will help justify and validate various treatment options by quantifying the effects of the disease and its treatment on gait.
Conclusions
Gait analysis provides an objective measure of functional gait in healthy controls as well as those with ADS and CSM. This study established and detailed some of the important kinematic and kinetic variables of gait in patients with spinal disorders. When compared to the controls, patients with spinal disorders walked slower and had abnormal duration and patterns of muscle activity and GRF. A decrease in walking speed, with the resultant reduction in acceleration, may be a protective mechanism to reduce joint loads and consequently, joint pain, in an effort to optimize overall function. Furthermore, spine patients have diminished balance and motion and potentially cannot react as quickly and safely to the constantly changing center of mass of normal walking. We recommend that spine care providers use gait analysis as part of their clinical evaluation to provide an objective measure of function and to better understand the effects of the disease and its treatment on their patients’ gait, function, and, ultimately, quality of life.
Notes
Low pass filter is often used to remove high frequencies from digitized kinematic data and as a digital antialiasing filter. The cutoff is selected so that low frequencies are unchanged but higher frequencies are attenuated. This is the most common filter type [34].
References
Winter DA (2009) Biomechanics and motor control of human movement. Wiley, Hoboken
Oatis CA (2004) Kinesiology—the mechanics and pathomechanics of human movement. Lippincott Williams & Wilkins, Philadelphia
Kuo AD (2007) The six determinants of gait and the inverted pendulum analogy: a dynamic walking perspective. Hum Mov Sci 26:617–656. https://doi.org/10.1016/j.humov.2007.04.003
Kuo AD, Donelan JM (2010) Dynamic principles of gait and their clinical implications. Phys Ther 90:157–174. https://doi.org/10.2522/ptj.20090125
Kramers-de Quervain IA, Muller R, Stacoff A, Grob D, Stussi E (2004) Gait analysis in patients with idiopathic scoliosis. Eur Spine J 13:449–456. https://doi.org/10.1007/s00586-003-0588-x
Yagi M, Ohne H, Konomi T, Fujiyoshi K, Kaneko S, Takemitsu M, Machida M, Yato Y, Asazuma T (2017) Walking balance and compensatory gait mechanisms in surgically treated patients with adult spinal deformity. Spine J 17:409–417. https://doi.org/10.1016/j.spinee.2016.10.014
Baskwill AJ, Belli P, Kelleher L (2017) Evaluation of a gait assessment module using 3D motion capture technology. Int J Ther Massage Bodyw 10:3–9
Engsberg JRBK, Wagner JM, Uhrich ML, Blanke K, Lenke LG (2003) Gait changes as the result of deformity reconstruction surgery in a group of adults with lumbar scoliosis. Spine 28:1836–1843
Malone A, Meldrum D, Bolger C (2012) Gait impairment in cervical spondylotic myelopathy: comparison with age- and gender-matched healthy controls. Eur Spine J 21:2456–2466
Malone A, Meldrum D, Bolger C (2015) Three-dimensional gait analysis outcomes at 1 year following decompressive surgery for cervical spondylotic myelopathy. Eur Spine J 24:48–56
Nishimura H, Endo K, Suzuki H, Tanaka H, Shishido T, Yamamoto K (2015) Gait analysis in cervical spondylotic myelopathy. Asian Spine J 9:321–326. https://doi.org/10.4184/asj.2015.9.3.321
Haddas R, Belanger T (2017) Clinical gait analysis on a patient undergoing surgical correction of kyphosis from severe ankylosing spondylitis. Int J Spine Surg 11:138–144. https://doi.org/10.14444/4018
Siasios ID, Spanos SL, Kanellopoulos AK, Fotiadou A, Pollina J, Schneider D, Becker A, Dimopoulos VG, Fountas KN (2017) The role of gait analysis in the evaluation of patients with cervical myelopathy: a literature review study. World Neurosurg 101:275–282. https://doi.org/10.1016/j.wneu.2017.01.122
Shiba Y, Taneichi H, Inami S, Moridaira H, Takeuchi D, Nohara Y (2016) Dynamic global sagittal alignment evaluated by three-dimensional gait analysis in patients with degenerative lumbar kyphoscoliosis. Eur Spine J 25:2572–2579. https://doi.org/10.1007/s00586-016-4648-4
DeLuca PA, Davis RB 3rd, Ounpuu S, Rose S, Sirkin R (1997) Alterations in surgical decision making in patients with cerebral palsy based on three-dimensional gait analysis. J Pediatr Orthop 17:608–614
Ounpuu S, Davis R, DeLuca P (1996) Joint kinetics: methods, interpretation and treatment decision-making in children with cerebral palsy and myelomeningocele. Gait and Posture 4:62–78
Õunpuu S, DeLuca PA, Davis RB (1997) The role of hip flexor and hamstring surgery on pelvic and hip motion in persons with cerebral palsy: an examination of the pre and post operative kinematics and kinetics. Gait and Posture 5:152
Ounpuu S, Winter DA (1989) Bilateral electromyographical analysis of the lower limbs during walking in normal adults. Electroencephalogr Clin Neurophysiol 72:429–438
Hollman JH, McDade EM, Petersen RC (2011) Normative spatiotemporal gait parameters in older adults. Gait Posture 34:111–118. https://doi.org/10.1016/j.gaitpost.2011.03.024
Ko SU, Ling SM, Schreiber C, Nesbitt M, Ferrucci L (2011) Gait patterns during different walking conditions in older adults with and without knee osteoarthritis–results from the Baltimore longitudinal study of aging. Gait Posture 33:205–210. https://doi.org/10.1016/j.gaitpost.2010.11.006
Chung CY, Park MS, Lee SH, Kong SJ, Lee KM (2010) Kinematic aspects of trunk motion and gender effect in normal adults. J Neuroeng Rehabil 7:9. https://doi.org/10.1186/1743-0003-7-9
McCormick JD, Werner BC, Shimer AL (2013) Patient-reported outcome measures in spine surgery. J Am Acad Orthop Surg 21:99–107. https://doi.org/10.5435/JAAOS-21-02-99
Johnston BC, Patrick DL, Busse JW, Schunemann HJ, Agarwal A, Guyatt GH (2013) Patient-reported outcomes in meta-analyses–Part 1: assessing risk of bias and combining outcomes. Health Qual Life Outcomes 11:109. https://doi.org/10.1186/1477-7525-11-109
Diebo BG, Varghese JJ, Lafage R, Schwab FJ, Lafage V (2015) Sagittal alignment of the spine: what do you need to know? Clin Neurol Neurosurg 139:295–301
Barrey C, Roussouly P, Le Huec JC, D’Acunzi G, Perrin G (2013) Compensatory mechanisms contributing to keep the sagittal balance of the spine. Eur Spine J 22(Suppl 6):S834–S841. https://doi.org/10.1007/s00586-013-3030-z
Amabile C, Le Huec JC, Skalli W (2016) Invariance of head-pelvis alignment and compensatory mechanisms for asymptomatic adults older than 49 years. Eur Spine J. https://doi.org/10.1007/s00586-016-4830-8
Cimolin V, Galli M, Grugni G, Vismara L, Albertini G, Rigoldi C, Capodaglio P (2010) Gait patterns in Prader–Willi and Down syndrome patients. J Neuroeng Rehabil 7:28. https://doi.org/10.1186/1743-0003-7-28
Sawacha Z, Cristoferi G, Guarneri G, Corazza S, Dona G, Denti P, Facchinetti A, Avogaro A, Cobelli C (2009) Characterizing multisegment foot kinematics during gait in diabetic foot patients. J Neuroeng Rehabil 6:37. https://doi.org/10.1186/1743-0003-6-37
Renaud A, Fuentes A, Hagemeister N, Lavigne M, Vendittoli PA (2016) Clinical and biomechanical evaluations of staged bilateral total knee arthroplasty patients with two different implant designs. Open Orthop J 10:155–165. https://doi.org/10.2174/1874325001610010155
Malone A, Meldrum D, Gleeson J, Bolger C (2013) Electromyographic characteristics of gait impairment in cervical spondylotic myelopathy. Eur Spine J 22:2538–2544. https://doi.org/10.1007/s00586-013-2928-9
Sacco IC, Akashi PM, Hennig EM (2010) A comparison of lower limb EMG and ground reaction forces between barefoot and shod gait in participants with diabetic neuropathic and healthy controls. BMC Musculoskelet Disord 11:24. https://doi.org/10.1186/1471-2474-11-24
Bovonsunthonchai S, Khobkhun F, Vachalathiti R (2015) Ground reaction forces of the lead and trail limbs when stepping over an obstacle. Med Sci Monit 21:2041–2049. https://doi.org/10.12659/MSM.893965
Vaughan CL, Davis BL, O’Conner JC (1999) Dynamics of human gait. Kiboho Publishers, Cape Town
Robertson GE, Caldwell GE, Hamill J, Kamen G, Whittlesey SN (2013) Research methods in biomechanics. Human Kinetics, Champaign
Malone A, Meldrum D, Gleeson J, Bolger C (2011) Reliability of surface electromyography timing parameters in gait in cervical spondylotic myelopathy. J Electromyogr Kinesiol 21:1004–1010. https://doi.org/10.1016/j.jelekin.2011.09.003
Ferrari A, Benedetti MG, Pavan E, Frigo C, Bettinelli D, Rabuffetti M, Crenna P, Leardini A (2008) Quantitative comparison of five current protocols in gait analysis. Gait Posture 28:207–216. https://doi.org/10.1016/j.gaitpost.2007.11.009
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflict of interest.
IRB approval
The study was approved by the Western Institutional Review Board for the Protection of Human Subjects (IRB#: 20152881).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haddas, R., Ju, K.L., Belanger, T. et al. The use of gait analysis in the assessment of patients afflicted with spinal disorders. Eur Spine J 27, 1712–1723 (2018). https://doi.org/10.1007/s00586-018-5569-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5569-1