Abstract
Study design
A non-randomized, prospective, concurrent control cohort study.
Objective
The purpose of this study is to develop and evaluate a method to quantify the dimensions of the cone of economy (COE) and the energy expenditure associated with maintaining a balanced posture within the COE, scoliosis patients and compare them to matched non-scoliotic controls in a group of adult degenerative.
Summary of background data
Balance is defined as the ability of the human body to maintain its center of mass (COM) within the base of support with minimal postural sway. The cone of economy refers to the stable region of upright standing posture. The underlying assumption is that deviating outside one’s individual cone challenges the balance mechanisms. Adult degenerative scoliosis (ADS) patients exhibit a variety of postural changes within their COE, involving the spine, pelvis and lower extremities, in their effort to compensate for the altered posture.
Methods
Ten ADS patients and ten non-scoliotic volunteers performed a series of functional balance tests. The dimensions of the COE and the energy expenditure related to maintaining balance within the COE were measured using a human motion video capture system and dynamic surface electromyography.
Results
ADS patients presented more COM sway in the sagittal (ADS: 1.59 cm vs. H: 0.61 cm; p = 0.049) and coronal (ADS: 2.84 cm vs. H: 1.72 cm; p = 0.046) directions in comparison to the non-scoliotic control. ADS patients presented with more COM (ADS: 33.30 cm vs. H: 19.13 cm; p = 0.039) and head (ADS: 31.06 cm vs. H: 19.13 cm; p = 0.013) displacements in comparison to the non-scoliotic controls. Scoliosis patients expended more muscle activity to maintain static standing, as manifest by increased muscle activity in their erector spinae (ADS: 37.16 mV vs. H: 20.31 mV; p = 0.050), and gluteus maximus (ADS: 33.12 mV vs. H: 12.09 mV; p = 0.001) muscles.
Conclusions
We were able to develop and evaluate a method that quantifies the COE boundaries, COM displacement, and amount of sway within the COE along with the energy expenditure for a specific patient. This method of COE measurement will enable spine care practitioners to objectively evaluate their patients in an effort to determine the most appropriate treatment options, and in objectively documenting the effectiveness of their intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Balance is defined as the ability of the human body to maintain its center of mass (COM) within the base of support with minimal postural sway [1]. Sway is the movement of the COM in the horizontal plane when a person is standing in a static position. Due to small perturbations within the body (e.g., breathing, shifting body weight from one foot to the other or from forefoot to hindfoot, etc.) or from external stimuli (e.g., visual distortions, floor translations, etc.), a certain amount of sway is needed and unavoidable. Maintenance of balance requires coordination between the sensorineural and musculoskeletal systems. An increase in sway is not necessarily an indicator of dysfunctional balance, so much as it is an indicator of decreased sensorimotor control [2]. Aging, vestibular deficits, neurologic conditions, neuromuscular disease, peripheral neuropathies, and abnormal spinal curvatures, all predispose an individual to postural instability and may alter their balance.
A number of balance measurement evaluations have been developed. Evaluations based on self-reported questionnaires regarding activity levels, fall history and more, are frequently used in the clinical setting. Other functional evaluations such as the Romberg test [3], Functional Forward reaching test [3], Timed up and go test [3], Balance Evolution Systems Test (BESTest) [4], Balance Error Scoring System (BESS) [5, 6], Performance oriented Mobility Assessment (POMA) [3], and Berg balance test [7, 8], are commonly used in clinical practice as well. These evaluations are easily accessible and economically feasible in terms of time, cost, labor and equipment. However, these evaluations have a number of limitations. All of these evaluations are highly subjective and often depend on self-reported values. The validity and reliability, along with the sensitivity and specificity of these evaluations, are highly variable. In addition there can be variability in the test results depending upon the selection of the diagnostic test and selected capture endpoints.
Spinal deformities comprise a variety of conditions that affect the normal spinopelvic alignment in the coronal and or sagittal planes. Common presenting symptoms include progressive deformity, back pain, lower extremity pain and dysfunction [9]. The location of the center of balance in the sagittal plane in deformity patients is most commonly estimated using full length spinal radiographs to measure the sagittal vertical axis (SVA). The measurements are performed by dropping a plumb line from the C7 vertebra, and measuring the horizontal distance from the plumb line to the midpoint of the superior end plate of the S1 vertebra. A variety of postural changes in the spine, pelvis and lower extremities are observed in patients in their effort to compensate for the anterior shift in the gravity line. These compensatory mechanisms reported in the literature include a reduction of thoracic kyphosis, hyper-extension of spinal segments, retrolisthesis of one vertebrae upon the next, pelvic retroversion, and hip and knee flexion along with ankle dorsiflexion [10]. These mechanisms appear progressively to compensate for the increasing imbalance and bring the axis of gravity as close to a near normal biomechanical balanced position.
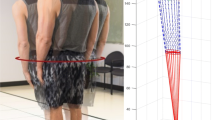
Jean Dubousset, first introduced the concept of the cone of economy and balance (COE), referring to a stable region of standing posture. The underlying assumption is that deviating outside one’s individual cone challenges the balance mechanisms (Fig. 1) [11]. The ability of the human body to maintain the COM within the COE with minimal energy expenditure results from a complex interaction of supra- and infra-pelvic alignment parameters. These parameters are influenced by the flexibility of the spine and joints of the lower extremities, neuromuscular control, muscle strength, muscle endurance, and body habitus. It becomes evident that the impact of spinal deformity on stance is multi-factorial, and thus cannot be exclusively correlated to the above described radiographic static alignment parameters.
Cone of economy as described by Dubousset [11]
Regarding the many spine and pelvic radiographic alignment parameters, multiple studies have shown that trunk imbalance especially in the sagittal plane, correlates with poor quality of life outcomes scores and progressively worsening low back pain [12, 13]. These correlations, however, do not explain symptoms for every case, and there are notable examples of patients with severe deformity and minimal functional loss, as well as others with not-so-severe deformity and severe functional loss [14]. The way an individual patient compensates for truncal imbalance may be variable and may depend on other constitutional factors such as age, neuromuscular condition, and BMI. In general though, the brain, through the righting reflex, will sacrifice focal alignment to optimize global balance within Dubousset’s “Cone of Economy”.
With the aforementioned in mind, the importance of the COE is recognized and well documented [11, 15, 16]; however, to our best knowledge, no one has attempted to quantify the dimensions of the COE and the energy expenditure associated with sway inside the COE.
The purpose of this study is to develop and evaluate a method to quantify the dimensions of the COE and the energy expenditure associated with maintaining a balanced posture within the COE, in a group of adult degenerative scoliosis patients and compare them to matched non-scoliotic controls. This method of COE measurement will enable spine care practitioners to objectively evaluate their patients in an effort to determine the most appropriate treatment options, and in objectively documenting the effectiveness of their intervention.
Methods
Subjects
To develop and evaluate our method of quantifying the COE, we collected data from ten adult degenerative scoliosis (ADS) patients (6 females; age 48.5 ± 12.9 years; height 1.65 ± 0.1 m; weight 65.98 ± 18.1 kg; Cobb angle 41.60° ± 12.1°), and ten non-scoliotic volunteers (7 females; age 49.4 ± 8.4 years; height 1.69 ± 0.1 m; weight 68.91 ± 8.33 kg). Patients were included in the study if they were between the ages of 30 and 70 years, with clinically diagnosed thoracolumbar and/or lumbo-sacro-pelvic deformity, with a coronal Cobb angle of 25° or greater, were deemed symptomatic enough to undergo surgical intervention, and were able to ambulate without assistance. Patients were excluded if they had a history of prior spine or major lower extremity surgery or previous injury that may affect standing, had a BMI higher than 35, had a primary neurological disorder, had a diabetic neuropathy or other disease that impairs the patient’s ability to ambulate or stand without assistance, and if they were pregnant. Non-scoliotic volunteers were recruited from the general population. These volunteers were between the ages of 30 and 70 years, and had no history of spinal deformity or other spinal ailment.
Preparatory procedures
All test subjects were fitted with 51 external reflective markers using locations and procedures modified from Vaughan et al. [17]. These markers were placed on the skin overlying strategic anatomic points as depicted in Fig. 2. Anthropometric measurements were taken before the test. Surface EMG electrodes were placed bilaterally on the skin overlying the external oblique (EO), multifidus (Mf) at the level of L5, erector spinae (ES) at the level of L1, gluteus maximus (GM), rectus femoris (RF), semitendinosus (ST), tibialis anterior (TA), and medial gastrocnemius (MG). The skin at the recording sites was cleaned with alcohol, shaved if necessary, and then lightly abraded to reduce impedance.
Testing procedures
Each subject performed a series of functional balance tests. The functional balance test was similar to a Romberg’s test [3] in which the patients are required to stand erect with feet together and eyes opened in their self-perceived balanced and natural position for a full minute. Each patient performed the test three times. The average of the three tests was calculated and used for further analysis.
Data acquisition
Three-dimensional (3D) kinematic data were recorded at 100 Hz using a 10 camera Vicon Video system (Vicon Nexus 2.7 Inc., Englewood, CO, USA). Electromyographic (EMG) data were recorded simultaneously at 2000 Hz. The kinematic data were low-pass filtered with a fourth-order Butterworth filter with a lower cutoff at 4 Hz. The EMG data were wirelessly transmitted to the sampling computer using an eight channel Delsys Trigno unit (Delsys, Inc, Natick, MA, USA). The Trigno bandwidth was 200–500 Hz with a signal-to-noise ratio of 1 µV root mean square baseline noise. The preset signal amplification was set to be 2000 times, with an impedance of 10 MΩ and a common-mode rejection ratio of 100 dB. The EMG data were band-pass filtered between 20 and 450 Hz with a fourth-order, no pass zero-phase-lag Butterworth filter and then was fully wave rectified.
Center of mass calculation
Center of mass was calculated based on anthropometric measurements using the body segment method and a custom algorithm software (Vicon Nexus 2.7 Inc., Englewood, CO, USA; MATLAB R2016). Since the human body consists of several segments, such as hands, forearms and upper arms, the overall mass distribution within the body is a function of the mass distribution within the individual segments and the overall posture. These parameters were historically obtained from cadaver research performed between 1950 and 1970 [18,19,20]. Research conducted in the former Soviet Union in the 1980s [21, 22] provided an alternative source for body segment parameter measures. To fully and accurately calculate the COM, we used the parameters listed from Zatsiorsky’s data which were published in 1996 [23].
Cone of economy boundaries calculation
To establish the COE boundaries, we first measured and then calculated the COM displacement (middle COE ring) and C7 marker displacement (top COE ring) in the sagittal and coronal planes during the functional balance test using a custom algorithm (MATLAB R2016). We then measured and calculated stance width and used that to set the tip of the cone (lower COE ring). This was followed by then plotting the COM displacement in the horizontal plane in the sagittal and coronal directions (Fig. 3). Finally, the minimum and maximum displacement values were calculated in both the sagittal and coronal directions. The differences between maximum and minimum values were also calculated. Those values were used to determine the boundaries of the cone of economy and sagittal and coronal sway range of motion (ROM) (Fig. 4).
Since defining the boundaries of the COE may not fully represent the true sway amount inside the COE, we further measured and calculated the overall length of the COM displacement line in the horizontal plane in all directions (Fig. 5).
Electromyography (EMG) calculation
The collected EMG data were normalized to a sub-maximum voluntary contraction. This allows us to compare muscle activity between two different groups [24]. The raw EMG data were then “full-wave rectified” (the process by which the positive and negative voltage amplitude are summed together by converting the negative value to a positive value), and then the linear envelope was calculated (the process of establishing an estimate of EMG volume of activity); this is then followed by calculating the integrated EMG activity, which is a graphic representation of the sum total EMG activity over a defined period of time (Fig. 6). In this fashion, the total accumulated muscle activity can be computed for a chosen time period [24]. Smaller values represent lesser muscle work and minimal energy expenditure.
Statistical analysis
One-way ANOVA was used to determine differences in the dimensions of the COE and the energy expenditure in ADS patients compared to non-scoliotic controls. Statistical analyses were conducted using SPSS, Version 23.0 (IBM, Inc., Chicago, IL, USA).
Results
With this newly described method to measure the COE, we were able to express the COE boundaries and specifically quantify the diameters of the COE (Fig. 4). Furthermore, our method was able to quantify the COM displacement within the COE. This result measures patients’ displacement or amount of sway inside the COE over time (Fig. 5). Moreover, our method was able to quantify muscle efficiency by calculating integrated EMG. Tables 1 and 2 outline the results of this method of COE measurement in ADS patients and non-scoliotic volunteers.
When comparing the sway, ADS patients presented more sway in the sagittal (ADS: 1.59 cm vs. H: 0.61 cm; p = 0.049) and coronal (ADS: 2.84 cm vs. H: 1.72 cm; p = 0.046) directions at the middle and top rings (Sagittal—ADS: 1.04 cm vs. H: 0.69 cm; p > 0.050 and coronal—ADS: 1.81 cm vs. H: 1.34 cm; p = 0.022) in comparison to the non-scoliotic controls. When comparing the displacement, ADS patients presented with more COM (ADS: 33.30 cm vs. H: 19.13 cm; p = 0.039) and head (ADS: 31.06 cm vs. H: 19.13 cm; p = 0.013) displacements in comparison to the non-scoliotic controls (Table 1). When comparing the size of the middle and top rings, scoliosis patients exhibited a larger diameter, particularly in the sagittal plane. We found that these scoliosis patients expended statistically significantly more muscle activity to maintain static standing, as manifest by increased muscle activity in their ES (ADS: 37.16 mV vs. H: 20.31 mV; p = 0.050), and GM (ADS: 33.12 mV vs. H: 12.09 mV; p = 0.001) muscles during 1 min standing in comparison to non-scoliotic controls (Table 2). Although they were not statistically significant, there was a trend toward greater muscle activity in the EO (ADS: 37.30 mV vs. H: 22.68 mV; p > 0.050), Mf (ADS: 31.77 mV vs. H: 23.64 mV; p > 0.050), RF (ADS: 23.97 mV vs. H: 20.03 mV; p > 0.050), ST (ADS: 29.81 mV vs. H: 21.97 mV; p > 0.050), TA (ADS: 25.45 mV vs. H: 15.39 mV; p > 0.050), and MG (ADS: 35.53 mV vs. H: 30.98 mV; p > 0.050) muscles during 1 min standing in the ADS patients in comparison to non-scoliotic controls (Table 1). With higher values in sway displacement and integrated EMG, ADS patients seem to expend more energy during a simple standing task.
Discussion
The purpose of this study was to develop and evaluate an innovative method to quantify the COE. The COE may not always be a perfect circle as presumed from the original concept described by Dubousset [11]. The COE can in fact be characterized as an individual specific ellipse, with differing sagittal and coronal diameters that can vary depending on the individual’s anatomy and any coincident spinal pathology. Moreover, defining the boundaries of the COE may not always represent the true amount of sway inside the COE, nor reflect the energy expenditure associated with maintaining balance. Therefore, much like a fingerprint, any individual can be expected to have his/her unique COE dimensions and balance parameters.
With this method, we were able to define the unique individual specific COE boundaries and quantify the diameters of the COE. In a non-scoliotic individual we demonstrated that the coronal sway is greater than the sagittal sway. In symptomatic Adult Degenerative Scoliotic patients, both the sagittal and coronal sway increased with the sagittal sway increasing proportionally more. We also demonstrated that the amount of sway inside the COE in deformity patients was greater over time when compared to the controls.
With this method, we were further able to quantify the energy expenditure as reflected by muscle activity during a simple standing task. We observed that ADS patients exhibit more muscle activity particularly in the ES and GM muscles, and thus expend more energy to maintain static standing or balance. This may be due to the abnormal spine curve structure and or to asymmetry in neuromuscular control, compounded by the biomechanical forces associated with progressive sagittal imbalance. Summing up these facts, ADS patients expend more energy during a simple standing task in an effort to maintain balance within their COE when compared to healthy controls.
Combining the above-noted findings, of increased sagittal sway and increased muscle activity in the ES and GM in symptomatic degenerative scoliosis patients, does verify the premise that “Kyphosis begets Kyphosis” as described by Benzel [25, 26], and justifies the philosophy of restoring sagittal balance as recommended by Dubousset [11].
Many published studies describe in detail the methods used to analyze a spinal deformity the sagittal plane of the spine, identify compensatory mechanisms from the hip, knee and ankle, and select patient-specific alignment targets [27,28,29]. These measures and estimates are based on static two-dimensional X-rays, with a focus on the sagittal plane measures. In one of those studies, Diebo et al. stated in their conclusions, that the dynamic aspects of alignment, along with clinical evaluation are crucial in managing spinal deformity conditions [27]. Therefore, while most of the balance research in deformity patients is done focusing on the sagittal plane and based on a static imaging, our method and this study provide the first effort to evaluate global balance and the “Cone of Economy” as a dynamic test. We fully well realize that not every spine practitioner has access to a human motion lab or the needed resources. With this in mind we would encourage those who do to think about this method and help advance the science.
Finally, using our method of measuring the COE with 3D video kinematic data and EMG data will allow spine practitioners to define for each patient’ his/her specific balance measurements. Contemporary teaching encourages surgeons to attempt to rebalance the spine to “normalized contours”. Those skilled in the art and science of deformity surgery now realize that achieving normal contours may not be ideal for every person. The use of gait analysis may help define the ideal extent of correction to achieve energy efficient mobility and posture. This may in turn lead to less invasive and symptom specific surgery.
Limitations
This method does have some inherent limitations. To use this method requires the human motion video capture capabilities and EMG measurement capabilities, as well as the expertise of a biomechanics technician, associated with a human motion lab. Additionally, we acknowledge the limitations associated with this method of kinematic modeling using the selected marker set, including; skin movement, errors in the anthropometric model, system tracking errors and data smoothing errors.
Conclusions
We were able to develop and evaluate a method that quantifies the COE measurement for a specific patient. Furthermore, we were able to quantify the patients’ COE boundaries, COM displacement and amount of sway within the COE. Moreover, we were able to quantify patients’ energy expenditure. Compared to controls, ADS patients showed wider boundaries at the top and middle rings with more sway, especially in the sagittal plane, as well as greater energy expenditure to maintain balance within their individual COE. This method of COE measurement will enable spine care practitioners to objectively evaluate their patients in an effort to determine the most appropriate treatment options, and in objectively documenting the effectiveness of their intervention.
Change history
02 February 2018
Unfortunately, in the abstract at the results section units have been published incorrectly.
References
Shumway-Cook A, Anson D, Haller S (1988) Postural sway biofeedback: its effect on reestablishing stance stability in hemiplegic patients. Arch Phys Med Rehabil 69:395–400
Davidson BS, Madigan ML, Nussbaum MA (2004) Effects of lumbar extensor fatigue and fatigue rate on postural sway. Eur J Appl Physiol 93:183–189. https://doi.org/10.1007/s00421-004-1195-1
O’Sullivan S, Schmitz T (2007) Physical rehabilitation. Davis Company, Philadelphia
Horak FB, Wrisley DM, Frank J (2009) The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther 89:484–498. https://doi.org/10.2522/ptj.20080071
Bell DR, Guskiewicz KM, Clark MA, Padua DA (2011) Systematic review of the balance error scoring system. Sports Health 3:287–295. https://doi.org/10.1177/1941738111403122
Valovich TC, Perrin DH, Gansneder BM (2003) Repeat administration elicits a practice effect with the balance error scoring system but not with the standardized assessment of concussion in high school athletes. J Athl Train 38:51–56
Schulein S (2014) Comparison of the performance-oriented mobility assessment and the Berg balance scale. Assessment tools in geriatrics and geriatric rehabilitation. Z Gerontol Geriatr 47:153–164. https://doi.org/10.1007/s00391-013-0492-x
Blum L, Korner-Bitensky N (2008) Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther 88:559–566. https://doi.org/10.2522/ptj.20070205
Aebi M (2005) The adult scoliosis. Eur Spine J 14:925–948. https://doi.org/10.1007/s00586-005-1053-9
Barrey C, Roussouly P, Perrin G, Le Huec JC (2011) Sagittal balance disorders in severe degenerative spine. Can we identify the compensatory mechanisms? Eur Spine J 20(Suppl 5):626–633. https://doi.org/10.1007/s00586-011-1930-3
Dubousset J (1994) Three-dimensional analysis of the scoliotic deformity. The pediatric spine: principles and practice. Raven Press Ltd, New York
Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F (2005) The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 30:2024–2029
Schroeder J, Schaar H, Mattes K (2013) Spinal alignment in low back pain patients and age-related side effects: a multivariate cross-sectional analysis of video rasterstereography back shape reconstruction data. Eur Spine J 22:1979–1985. https://doi.org/10.1007/s00586-013-2787-4
Glassman SD, Berven S, Bridwell K, Horton W, Dimar JR (2005) Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976) 30:682–688
Grivas TB, de Mauroy JC, Wood G, Rigo M, Hresko MT, Kotwicki T, Negrini S (2016) Brace Classification Study Group (BCSG): part one—definitions and atlas. Scoliosis Spinal Disord 11:43. https://doi.org/10.1186/s13013-016-0102-y
Savage JW, Patel AA (2014) Fixed sagittal plane imbalance. Global Spine J 4:287–296. https://doi.org/10.1055/s-0034-1394126
Vaughan CL, Davis BL, O’Conner JC (1999) Dynamics of human gait. Kiboho Publishers, Cape Town
Chandler RF, Clauser CE, McConville JT, Reynolds HM, Young JW (1975) Investigation of inertial properties of the human body (AMRL Technical Report 74–137). Aerospace Medical Research Laboratories, Wright-Patterson Air Force Base, OH
Clauser CE, McConville JT, Young JW (1969) Weight, volume, and center of mass of segments of the human body (AMRL Technical Report 69–70). Aerospace Medical Research Laboratories, Wright-Patterson Air Force Base, OH
Dempster WT (1955) Space requirements for the seated operator (WADC Technical Report 55–159). Wright Air Development Center, Wright-Patterson Air Force Base, OH
Zatsiorsky V, Seluyanov V (1983) The mass and inertia characteristics of the main segments of the human body. In: Matsui H, Kobayashi K (eds) Biomechanics VIII-B. Human Kinetics, Champaign
Zatsiorsky V, Seluyanov V (1985) Estimation of the mass and inertia characteristics of the human body by means of the best predictive regression equations. In: Winter DA, Norman RW, Wells RP, Hayes KC, Patlaa AE (eds) Biomechanics IX-B. Human Kinetics, Champaign
de Leva P (1996) Adjustments to Zatsiorsky–Seluyanov’s segment inertia parameters. J Biomech 29:1223–1230
Robertson GE, Caldwell GE, Hamill J, Kamen G, Whittlesey SN (2013) Research methods in biomechanics. Human Kinetics, Champaign
Benzel EC (2012) The cervical spine. Lippincott Williams and Wilkins, Philadelphia, pp 1083–1084
Steinmetz MP, Benzel EC (2017) Benzel’s spine surgery techniques, complication, avoidance, and management. Elsevier, Philadelphia, pp 251–253, 745–746
Diebo BG, Varghese JJ, Lafage R, Schwab FJ, Lafage V (2015) Sagittal alignment of the spine: what do you need to know? Clin Neurol Neurosurg 139:295–301
Barrey C, Roussouly P, Le Huec JC, D’Acunzi G, Perrin G (2013) Compensatory mechanisms contributing to keep the sagittal balance of the spine. Eur Spine J 22(Suppl 6):S834–S841. https://doi.org/10.1007/s00586-013-3030-z
Amabile C, Le Huec JC, Skalli W (2016) Invariance of head-pelvis alignment and compensatory mechanisms for asymptomatic adults older than 49 years. Eur Spine J. https://doi.org/10.1007/s00586-016-4830-8
Acknowledgements
The authors extend their most sincere appreciation to Professor Jean Dubousset for his review of this manuscript and for his many contributions to the art and science of spinal surgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
IRB approval
The study was approved by the Western Institutional Review Board for the Protection of Human Subjects (IRB#: 20152881).
Additional information
This original article was revised.
Rights and permissions
About this article
Cite this article
Haddas, R., Lieberman, I.H. A method to quantify the “cone of economy”. Eur Spine J 27, 1178–1187 (2018). https://doi.org/10.1007/s00586-017-5321-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5321-2