Abstract
Background context
With more cement augmentation procedures done, the occurrence of serious complications is also expected to rise. Symptomatic central cement embolization is a rare but very serious complication. Moreover, the pathophysiology and treatment of intrathoracic cement embolism remain controversial.
Purpose
In this case series, we are trying to identify various presentations and suggest our emergent management scheme for symptomatic central cement embolization.
Patient sample
Retrospective case series of nine patients with symptomatic central cement embolism identified after vertebroplasty with 24 months of follow-up. Level IV.
Outcome measures
The degree of dyspnea measured by the New York Heart Association (NYHA) score and/or death related to cement embolism induced cardio/respiratory failure at the final follow-up at 24 months.
Methods
The nine patients, eight females, and one male had a mean age of 70.25 years (range 65–78 years) and were operated between January 2004 and December 2014. They had percutaneous vertebroplasty for osteoporotic non-traumatic and malignant vertebral collapse of dorsal and lumbar vertebrae. Post-vertebroplasty dyspnea and stitching chest pain were striking in the nine patients. After exclusion of cardiac ischemia and medical pulmonary causes for dyspnea, we identified radiopaque lesions on the chest X-ray. Further echocardiography and high-resolution chest CT were performed for optimal localization. Emergent heart surgery was performed in two patients: interventional therapy was conducted in one patient, while the remaining six patients were conservatively treated by anticoagulation. The management decision was taken in the setting of an interdisciplinary meeting depending on localization, fragmentation, and clinical status.
Results
All patients of this series showed gradual improvement and an uneventful hospital stay. During our 24-month follow-up phase, eight patients showed no subsequent cardiological and/or respiratory symptoms (NYHA I). However, one mortality due to advanced malignancy occurred. Preoperative anemia was the only common intersecting preoperative parameter among these nine patients.
Conclusions
After cement augmentation, close clinical monitoring is mandatory. A chest CT is pivotal in determining the interdisciplinary management approach in view of the availability of necessary expertise, facilities and the location of the cement emboli whether accessible by cardiac or vascular surgical means. The clinical presentation and its timing may vary and the patient may be seen subsequently by other health care providers obligating a wide-spread awareness for this serious entity among health care providers for this age group as spine surgeons, family and emergency room doctors, and institutional or home-care nurses. Most symptomatic central cement emboli may be treated conservatively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The frequency of local vertebral leakage of bone cement is relatively high (about 80–90%) [1]; moreover, the rate of cement leakage into the perivertebral veins seen in up to 24% of vertebral bodies treated with consequent pulmonary cement embolism varies from 4.6 to 6.8% (up to 26% in radiologic studies) [2, 3]. The prognosis of patients with bone cement cardiac embolization treated conservatively is not yet thoroughly investigated. Emergent bone cement extraction aided with cardiopulmonary bypass is the only curative solution if the bone cement lies in a surgically accessible site [4]. Distal peripheral pulmonary tree bone cement embolization is primarily a case for conservative management. As long as the cement lies in the proximal pulmonary tree, right ventricle, or right atrium, surgical removal stays the treatment of choice. However, radiological interventional approaches could also be considered, yet the rarity of this complication limits the number of interventionally managed cases in the literature [5]. A significant surgery-associated morbidity has not been reported including complications such as bleeding, endocarditis, thromboembolism, right ventricular failure, and sepsis. Therefore, the aim of our current study was to focus on the presentation and management of this complication.
Patients and methods
We report a small case series of symptomatic intrathoracic embolization of bone cement after vertebroplasty. In 11 years, we faced this remotely rare but serious complication in nine patients considering our centers in Germany and Egypt as high volume cardiac and spine surgery centers of collectively around 2700 heart and 3400 spine surgeries annually. In this period, 3941 patients and 4989 vertebral bodies have been treated by cement augmentation. These nine patients (0.23%), eight females and one male, had a mean age of 70.55 years and were operated between January 2004 and December 2014. The vertebral level of cement application and leakage, operative time, preoperative American Society of Anesthesiologist score (ASA) [6] and pre- and postoperative New York Heart Association (NYHA) class [7] was evaluated.
All patients were operated in the prone position with a high viscosity cement (Confidence Spinal Cement System®, DePuy Synthes, Raynham, MA, USA) utilizing the same operative technique. Two perpendicular arranged C-arms were utilized to identify the correct pedicular entry points and trajectories of the bilaterally transpedicular introduced Jamshidi needle as well as to carefully monitor the deployment and distribution of the cement. Ideally, the applied needle tip should reach the anterior third of the vertebral body. 3 min after component mixing at OP room temperature of 21 °C, the paste-like non-adherent cement was delivered sequentially with low-to-moderate pressure in 0.5 ml boluses under repeated image intensifier control. The amount of applied cement, which varied between 2 and 6 ml per vertebral body, depended on the clinical situation and specific cement distribution pattern. Cementing was immediately ceased when cement reached the posterior third of the body or there was any form of anterior, lateral, or intradiscal cement extravasation.
Postoperative dyspnea and/or chest pain in these nine patients warranted the initial exclusion of cardiac ischemia and other medical pulmonary causes for dyspnea and leads to the identification of radio-opaque lesions on plain chest X-rays. Further echocardiography and high-resolution chest CT were performed for optimum localization of the foreign body. Emergent heart surgery was performed in two patients; interventional therapy was conducted in one patient, while the remaining six patients were conservatively treated by anticoagulation with enoxaparin 40 mg/day for 3 months.
The management decision was founded on clinical presentation, size and location, fragmentation, and overall clinical status of the patients through an interdisciplinary meeting encompassing interventional cardiologists, radiological interventionists and cardiac, spine, and vascular surgeons. Cardiac surgery was done when the patient was severely symptomatic (NYHA III and IV) and cement was centrally embolized, and the cement embolus was mobile and floating, i.e., not wall-adherent or if there was a large peripheral embolus. Surgery or vascular intervention was not indicated in adequately perfused large central vessels. The right mini-thoracotomy approach (1 patient) was preferred except in right ventricular perforation, epicardial hematoma, or deeply indulged cement in the right ventricular myocardium, i.e., in cardiac areas not accessible through the minimal invasive right thoracotomy. Otherwise, a formal sternotomy in one patient was done. The treatment algorithm used by both study centers is depicted in Fig. 1.
Results
Seven of the patients were class II according to the American Society of Anesthesiologists (ASA), one was found to be ASA III and one was considered to be ASA class IV. This latter patient received percutaneous vertebroplasty for metastatic vertebral collapse of dorsal and lumbar vertebrae D7 and L3 and both showed venous cement leakage; the remaining eight patients obtained percutaneous cement vertebroplasty for non-traumatic osteoporotic vertebral collapse with the corresponding CT confirmed leakage at D4, two patients at D7, three patients at D10, one patient at L2, and one patient at L4 (Table 1; Fig. 2). One female patient (Fig. 3) presented with simultaneous osteoporotic fractures at D4, D5, D6 and D10, D11, D12 and L4 and L5. This patient was planned for a two-staged vertebroplasty after conservative treatment had failed to relief her severe pain. She developed cement leakage during the first stage at D4 when the upper three dorsal and the lower two lumbar vertebrae were treated. However, she did become symptomatic 3 days after discharge. The degree of dyspnea was assessed according to the NHYA score and five patients were classified as IV and the remaining four were III.
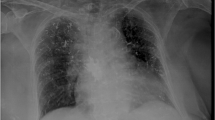
a, b A 65-year-old female presenting to us with multiple osteoporotic lumbar and thoracic vertebral fractures after failed conservative treatment. c, d We decided to augment D4–D6 and L4 and L5 in the first stage. The patient could be discharged on the third postoperative day with a marked reduction of back pain thereby obviating a second stage for the remaining D10–D12 levels. However, we overlooked at that stage the cement extrusion. e, f Patient then presented on the fifth postoperative day in the emergency unit with acute chest pain and was after routine cardiological work up diagnosed to have a large cement embolus in the right atrium and ventricle. g, h Chest X-ray after formal sternotomy and complete intracardiac cement retrieval. White arrows are directed to cement. PA pulmonary artery, RV right ventricle, TV tricuspid valve, RA right atrium, IVC inferior vena cava, LV left ventricle, PE pericardial effusion. (g) and ((f) After emergent sternotomy and open-heart surgery and removal of the embolus
After the vertebroplasty five patients immediately complained about dyspnea, one patient after 2 days, two patients presented 3 days later with dyspnea and one patient reported at the chest pain unit of our emergency room 3 days after discharge (5 days after vertebroplasty) presenting with severe pain in the chest. Note that all nine patients presented with dyspnea and three patients complained additionally of stitching chest pain.
The mean time of presentation was 2.0 days (range 1–5 days). The overall mean preoperative hemoglobin was 6.51 mmol/L (range 5.6–7.1 mmol/L). Except for controlled arterial hypertension, other relevant cardiovascular disease could only be identified in one patient with previous right-sided carotid endarterectomy.
The two patients, who were managed by cardiac surgery, had radiological (CT-) and echocardiographic evidence of cement migration into the right atrium and through the tricuspid valve. Based on the site, size, and shape and after assessment in the interdisciplinary meeting of cardiovascular, spine surgery and interventional cardiology and radiology, the decision to do emergent open-heart surgery for fear of pulmonary embolization was taken. These two patients were operated under mild hypothermia beating heart without cardioplegic arrest. After snaring the superior and inferior vena cava, right atriotomy was performed. Inspection, precise localization of the cement and then capturing and removal was conducted. Routinely, the right-sided heart chambers were completely inspected to ensure freedom of cement remnants. One of these patients was operated through a formal sternotomy and the other through a minimally invasive right thoracotomy approach. The decision of approach was based on two criteria, localization and fragmentation. These two criteria could be easily addressed through a multi-slice CT, denoting the validity of this investigation in determining the approach and hence lowering the morbidity through avoiding sternotomy in such high-risk patients. In one patient, the multi-slice CT revealed an 8-cm worm-like radiopaque structure through the tricuspid valve and minute fragmentations in the proximal pulmonary artery trunk which met the criteria of sternotomy. The other patient showed a comparable sized curved like cement structure located and coiled in the right atrium with 7 cm diameter. Luckily, this coil prevented further dislodgement through the tricuspid valve and hence supported the decision for a minimally invasive approach.
The six conservatively managed patients showed peripherally fragmented cement in the distal pulmonary tree in two patients and cement in the hemiazygos system and adherence to the wall in two other patients. The two remaining female patients showed cement in the inferior vena cava, right ventricle, truncus pulmonalis, and right pulmonary artery. These anatomically inaccessible regions led to neither severe symptoms nor to disturbances of gaseous exchange. This supported the decision of conservative management and close follow-up. In the first one, minute distal lung parenchymal infarctions with no effect on ventilation/perfusion ratio or blood gases were accidentally discovered subsequently. The mild post-vertebroplasty dyspnea in this special patient had to be respected because of her medical history and her substantiated cement embolization. Further anticoagulation by enoxaparin 40 mg subcutaneously for 3 months was recommended and a follow-up schedule was planned.
The last patient who was prone to intervention had a 5 × 1 × 0.5 cm cement plug in the inferior vena cava originating from L3 which originally was infiltrated by metastases from a non-small cell bronchial cancer. This severely ill patient presented with marked dyspnea and burning left-sided thigh pain after vertebroplasty. Immediate postoperative CT imaging revealed in addition to the above-mentioned lesion a left transpedicular cement extrusion into the spinal canal compressing the cord and left L3 root. The infracardiac anatomical region is critical. Surgically, it would need a heart–lung bypass. On the other hand, conservative treatment means a high liability of thrombus formation and consequently superior vena cava obstruction syndrome. Since both scenarios are morbid, interventional modalities may contribute successfully. This patient was subjected to light anesthesia in the hybrid room. The right femoral vein was punctured and a wire was introduced. Under angiographic guidance, the radiopaque cement was localized, captured, and then slowly withdrawn out of the femoral access. The posterior lumbar cement extrusion was extracted trough a minimally invasive microscopic-assisted approach and the leg pain recovered completely.
At 24 months, eight patients survived and had no relevant cardiac or respiratory symptoms whatsoever and all were classified to be NYHA I.
Discussion
Similar outcomes have been reported by other authors [8,9,10]. One patient as mentioned above died because of his advanced malignancy after 3 months. The mean time of appearance of symptoms began 2 days after the index procedure. Similarly, Abdul-Jalil et al. reported of dyspnea presenting in a 45-year-old female patient presenting 3 days after vertebroplasty [8].
Pathophysiologically, the bone cement passes through the basivertebral veins or the anterior external vertebral plexus followed by segmental spinal veins, the azygos or hemiazygos vein to reach the pulmonary arteries. Cement migration is eased by the valveless nature of the above-described venous system. Neoangiogenesis induced by malignancy also is believed to facilitate cement migration [9]. Cardiac embolization, cardiac perforation, and paradoxical cerebral embolization through a patent foramen ovale have been reported [11]. It remains unclear whether there is embolization of an initially large cement lump that undergoes fragmentation during the intravascular migration or if there are showers of multiple minute cement emboli from the inception.
To prevent further pulmonary infarction and activation of the coagulation cascade, simple anticoagulation for 3 months is recommended in asymptomatic accidental discovered pulmonary emboli or in symptomatic patients when the cement is small and situated in the periphery [12,13,14]. However, anticoagulation is disputed by some authors [9]. Henderson stated that there is no clear evidence that bone cement acts as a nidus for thrombogenicity and that clinically asymptomatic patients with radiologically verified central cement embolization did not receive anticoagulation without showing any adverse effect [15, 16].
Nevertheless, emergent surgical or endovascular intervention is indicated in respiratory or imminent right-sided heart failure or in the presence of large and/or mobile and floating cement fragments that may cause fatal dislodgement [17]. In the systematic literature review by Krueger et al., various treatment modalities were reported by 24 authors varying between no treatment, low-dose heparin, coumarin for 3–6 months, and surgical embolectomy [12].
The true incidence of cement embolization after vertebral cement augmentation remains unknown as the affected patients may present differently regarding chronology and intensity of the symptomatology. In the VERTOS II prospective multicenter randomized controlled trail, 80 cement-augmented vertebrae of 54 patients underwent native chest CT after a mean follow-up of 22 months (range 6–42 months) to detect possible pulmonary cement embolization. In 14 of 54 patients (26%), who were all asymptomatic, small peripherally arranged cement emboli could be identified. Cement leakage in the azygos vein was the only risk factor identified [3].
Other authors reported an incidence of perivertebral venous system cement embolization to be 16.6% [2]. However, Yeom et al. proved by post-interventional CT the presence of cement in the paravertebral veins to be more than 80% [1].
Therefore, because of the paucity of cases in the literature, there is no unified management plan for symptomatic cement emboli.
Some authors have prophylactically recommended the maintenance of increased intrathoracic pressure during the procedure, intraosseous venography before cement injection and a routine postoperative control chest X-ray [1, 18, 19]. Bhatia et al. used Gelfoam to occlude the potential basivertebral venous vessels [20]. The use of partially cured cement of high viscosity, low-pressure application, sequential PMMA application taking advantage of the temperature gradient between the body (37 °C) and ambient temperature in the OR (20 °C) [21] and continuous biplane radiological monitoring during cementing have been advocated by others [22].
In a minipig model comparing negatively surface charged biphasic calcium composite (BCC) with PMMA after experimentally inducing pulmonary embolism, Qin et al. reported a better profile in favor for BCC regarding hemodynamic changes, blood gas values, and antithrombin III and D-Dimer levels. Based on CT scanning and 3D remolding of pulmonary vessels, BCC injection caused less pulmonary embolisms. They concluded that BCC could constitute an auspicious future filler for vertebroplasty [23].
Preoperative anemia was found in all nine patients of this series, but sufficient statistical prove for its significance could not be substantiated in this study or found by us elsewhere in the relevant spine literature. Nonetheless, after having analyzed more than 5000 patients recorded in the National Surgical Quality Improvement Program (NSQIP) database who underwent aseptic revision hip and knee arthroplasties, Liodakis et al. reported preoperative anemia as the most important modifiable independent predictor for both major complications and prolonged hospital stay [24]. Similar results were reported by Aynardi et al. when they analyzed the risk factors for early postoperative mortality in 8261 total hip patients. [25] Nevertheless, the transferability of these findings to cement augmentation procedures requires larger multi-centric studies.
Limitations of this study are the small number of patients and that our facilities predominantly perform vertebroplasties calling for further multi-centric data collection.
Conclusion
Since many central embolic events in cement-augmented patients may remain undetected or run a subclinical course, our case series illustrates the need for close clinical in-hospital or outpatient monitoring and a high degree of clinical suspicion especially if patients present with post-procedural dyspnea. If clinically indicated, a chest CT would help in exact localization of the cement embolus and in planning for further management, whether operative or conservative. The further treatment path should be determined in an interdisciplinary setting and should consider the availability of necessary expertise, facilities and the location of the cement emboli whether accessible by cardiac or vascular surgical means.
The clinical presentation and its timing may vary and the patient may be seen subsequently by other health care providers obligating a wide-spread awareness for this serious entity among health care providers for this age group as spine surgeons, family, and emergency room doctors, institutional or home-care nurses. Most symptomatic central cement emboli may be treated conservatively.
References
Yeom JS, Kim WJ, Choy WS, Lee CK, Chang BS, Kang JW (2003) Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br 85:83–89
Vasconcelos C, Gailloud P, Beauchamp NJ, Heck DV, Murphy KJ (2002) Is percutaneous vertebroplasty without pretreatment venography safe? Evaluation of 205 consecutives procedures. AJNR Am J Neuroradiol 23:913–917
Venmans A, Klazen CH, Lohle PNM, Van Rooij WJ, Verhaar HJJ, De Vries J, Mali WPTM (2010) Percutaneous vertebroplasty and pulmonary cement embolism: results from VERTOS II. Am J Neuroradiol 31:1451–1453. doi:10.3174/ajnr.A2127
Lim SH, Kim H, Kim HK, Baek M-J (2008) Multiple cardiac perforations and pulmonary embolism caused by cement leakage after percutaneous vertebroplasty. Eur J Cardiothorac Surg 33:510–512. doi:10.1016/j.ejcts.2007.12.012
Gosev I, Nascimben L, Huang P-H, Mauri L, Steigner M, Mizuguchi A, Shah AM, Aranki SF (2013) Right ventricular perforation and pulmonary embolism with polymethylmethacrylate cement after percutaneous kyphoplasty. Circulation 127:1251–1253. doi:10.1161/CIRCULATIONAHA.112.144535
Owens WD, Felts JA, Spitznagel EL (1978) ASA physical status classifications: a study of consistency of ratings. Anesthesiology 49:239–243
Criteria Committee, New York Heart Association I (1964) Diseases of the heart and blood vessels. Nomenclature and criteria for diagnosis, 6th edn. Little Brown, Boston
Abdul-Jalil Y, Bartels J, Alberti O, Becker R (2007) Delayed presentation of pulmonary polymethylmethacrylate emboli after percutaneous vertebroplasty. Spine (Phila Pa 1976) 32:E589–E593. doi:10.1097/BRS.0b013e31814b84ba
Choe DH, Marom EM, Ahrar K, Truong MT, Madewell JE (2004) Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. AJR Am J Roentgenol 183:1097–1102. doi:10.2214/ajr.183.4.1831097
Wang L, Yang H, Shi Y, Jiang W, Chen L (2012) Pulmonary cement embolism associated with percutaneous vertebroplasty or kyphoplasty: a systematic review. Orthop Surg 4:182–189. doi:10.1111/j.1757-7861.2012.00193.x
Llanos RA, Viana-Tejedor A, Abella HR, Fernandez-Avilés F (2013) Pulmonary and intracardiac cement embolism after a percutaneous vertebroplasty. Clin Res Cardiol 102:395–397. doi:10.1007/s00392-013-0542-9
Krueger A, Bliemel C, Zettl R, Ruchholtz S (2009) Management of pulmonary cement embolism after percutaneous vertebroplasty and kyphoplasty: a systematic review of the literature. Eur Spine J 18:1257–1265. doi:10.1007/s00586-009-1073-y
Jang JS, Lee SH, Jung SK (2002) Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine (Phila Pa 1976) 27:E416–E418. doi:10.1097/01.BRS.0000025696.28544.96
Tozzi P, Abdelmoumene Y, Corno AF, Gersbach PA, Hoogewoud H-M, von Segesser LK (2002) Management of pulmonary embolism during acrylic vertebroplasty. Ann Thorac Surg 74:1706–1708
Henderson R (2017) Expert’s comment concerning Grand Rounds case entitled “Intracardiac bone cement embolism as a complication of vertebroplasty: management strategy” by Hatzantonis C, Czyz M, Pyzik R, Boszczyk BM. (Eur Spine J; 2016. doi:10.1007/s00586-016-4695-x). Eur Spine J. doi:10.1007/s00586-017-5089-4
Kim YJ, Lee JW, Park KW, Yeom J-S, Jeong HS, Park JM, Kang HS (2009) Pulmonary cement embolism after percutaneous vertebroplasty in osteoporotic vertebral compression fractures: incidence, characteristics, and risk factors. Radiology 251:250–259. doi:10.1148/radiol.2511080854
Baumann A, Tauss J, Baumann G, Tomka M, Hessinger M, Tiesenhausen K (2006) Cement embolization into the vena cava and pulmonal arteries after vertebroplasty: interdisciplinary management. Eur J Vasc Endovasc Surg 31:558–561. doi:10.1016/j.ejvs.2005.11.008
Peh WCG, Gilula LA (2003) Additional value of a modified method of intraosseous venography during percutaneous vertebroplasty. AJR Am J Roentgenol 180:87–91. doi:10.2214/ajr.180.1.1800087
Groen RJM, du Toit DF, Phillips FM, Hoogland PVJM, Kuizenga K, Coppes MH, Muller CJF, Grobbelaar M, Mattyssen J (2004) Anatomical and pathological considerations in percutaneous vertebroplasty and kyphoplasty: a reappraisal of the vertebral venous system. Spine (Phila Pa 1976) 29:1465–1471
Bhatia C, Barzilay Y, Krishna M, Friesem T, Pollock R (2006) Cement leakage in percutaneous vertebroplasty: effect of preinjection gelfoam embolization. Spine (Phila Pa 1976) 31:915–919. doi:10.1016/j.spinee.2005.05.035
Hoppe S, Wangler S, Aghayev E, Gantenbein B, Boger A, Benneker LM (2015) Reduction of cement leakage by sequential PMMA application in a vertebroplasty model. Eur Spine J 25:3450–3455. doi:10.1007/s00586-015-3920-3
Moreland DB, Landi MK, Grand W (2001) Vertebroplasty: techniques to avoid complications. Spine J 1:66–71. doi:10.1016/S1529-9430(01)00013-4
Qin Y, Ye J, Wang P, Gao L, Jiang J, Wang S, Shen H (2016) Evaluation of the biphasic calcium composite (BCC), a novel bone cement, in a minipig model of pulmonary embolism. J Biomater Sci Polym Ed 27:317–326. doi:10.1080/09205063.2015.1128240
Liodakis E, Bergeron SG, Zukor DJ, Huk OL, Epure LM, Antoniou J (2015) Perioperative complications and length of stay after revision total hip and knee arthroplasties: an analysis of the NSQIP database. J Arthroplasty 30:1868–1871. doi:10.1016/j.arth.2015.05.029
Aynardi M, Jacovides CL, Huang R, Mortazavi SMJ, Parvizi J (2013) Risk factors for early mortality following modern total hip arthroplasty. J Arthroplasty 28:517–520. doi:10.1016/j.arth.2012.06.040
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding this work.
Funding
There is no private or public funding source regarding this work.
Human and animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors. The surgical, as well as the conservative treatment modalities described in this manuscript, are well established and profoundly documented elsewhere in the relevant literature.
Rights and permissions
About this article
Cite this article
Barakat, A.S., Owais, T., Alhashash, M. et al. Presentation and management of symptomatic central bone cement embolization. Eur Spine J 27, 2584–2592 (2018). https://doi.org/10.1007/s00586-017-5267-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5267-4