Abstract
Background context
Laminoplasty and laminectomy with fusion are two common procedures for the treatment of cervical spondylotic myelopathy. Controversy remains regarding the superior surgical treatment.
Purpose
To compare short-term follow-up of laminoplasty to laminectomy with fusion for the treatment of cervical spondylotic myelopathy.
Study design/setting
Retrospective review comparing all patients undergoing surgical treatment for cervical spondylotic myelopathy by a single surgeon.
Patient sample
All patients undergoing laminoplasty or laminectomy with fusion by a single surgeon over a 5-year period (2007–2011).
Outcome measures
Cervical alignment and range of motion on pre- and post-operative radiographs and clinical outcome measures including Japanese Orthopaedic Association (JOA) scores, neck disability index (NDI), short form-12 mental (SF-12M) and physical (SF-12P) composite scores and visual analog pain scores for neck (VAS-N) and arm (VAS-A).
Methods
Patients undergoing laminoplasty or laminectomy with fusion by a single surgeon were reviewed. Cohorts of 41 laminoplasty patients and 31 laminectomy with fusion patients were selected based on strict criteria. The cohorts were well matched based on pre-operative clinical scores, radiographic measurements, and demographics. The average follow-up was 19.2 months for laminoplasty and 18.2 months for laminectomy with fusion. Evaluated outcomes included Japanese Orthopaedic Association (JOA) score, neck disability index (NDI), short form-12 (SF-12), visual analog pain scores (VAS), cervical sagittal alignment, cervical range of motion, length of stay, cost and complications.
Results
The improvement in JOA, SF-12 and VAS scores was similar in the two cohorts after surgery. There was no significant change in cervical sagittal alignment in either cohort. Range-of-motion decreased in both cohorts, but to a greater degree after laminectomy with fusion. C5 nerve root palsy and infection were the most common complications in both cohorts. Laminectomy with fusion was associated with a higher rate of C5 nerve root palsy and overall complications. The average hospital length of stay and cost were significantly less with laminoplasty.
Conclusions
This study provides evidence that laminoplasty may be superior to laminectomy with fusion in preserving cervical range of motion, reducing hospital stay and minimizing cost. However, the significance of these differences remains unclear, as laminoplasty clinical outcome scores were generally comparable to laminectomy with fusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical myelopathy results from compression of the spinal cord secondary to osseous or soft tissue encroachment of the spinal canal. Myelopathy can result in progressive gait disturbance, fine motor disability, weakness, and sensory dysfunction. The most common cause of myelopathy in the elderly population is spondylosis [1]. In cervical spondylotic myelopathy, the onset is often insidious and progressive [2]. Early surgical intervention has been demonstrated to halt the progression of neurological deterioration [3, 4].
Multiple surgical techniques have been described to treat cervical myelopathy [5]. Historically, laminectomy was the treatment of choice. Laminectomy affords straightforward decompression and reliable improvement in symptoms. However, a substantial percentage of patients develop complications secondary to the destabilization caused by removal of the posterior elements. Complications include post-laminectomy kyphosis, segmental instability, and neurological deterioration [6–9]. For this reason, fusions are frequently added to provide a stronger biomechanical construct. Augmentation with posterior fusion has been demonstrated to reduce post-laminectomy instability in multiple studies [10–12]. However, due to the alteration of normal cervical spine biomechanics, there is increasing concern that fusion may result in adjacent segment disease and the need for additional surgery [13, 14].
Laminoplasty was developed as an alternative to laminectomy, permitting adequate decompression while maintaining mechanical stability and motion in the cervical spine. Laminoplasty involves unilateral cuts to hinge open the lamina and increase the space available for the spinal cord. Additionally, this procedure theoretically allows for maintenance of normal cervical biomechanics and reduced risk of adjacent segment disease. However, laminoplasty has been associated with neck and shoulder pain and its use is generally restricted to patients with cervical lordosis [15–17].
There is currently no consensus in the literature concerning the superiority of laminoplasty or laminectomy with fusion in the treatment of cervical myelopathy [17–22]. A recent systematic review of the literature comparing the techniques found the majority of studies were low quality and lacking sufficient detail in the methods and results to permit pooling of the data [23]. Though several studies have demonstrated favorable outcomes using each technique [12, 14, 24–27], there is a paucity of the literature comparing the two techniques in the hands of a single surgeon. The purpose of the following study is to compare these techniques using a rigorous panel of clinical and radiographic variables in an attempt to elucidate differences treatment outcomes.
Materials and methods
Subjects
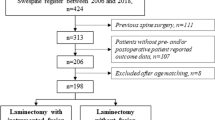
The study herein is an institutional review board-approved, retrospective review of patients treated by a single surgeon at major academic institution during a 5-year period (2007–2011). Patients with a primary diagnosis of cervical spondylotic myelopathy treated with laminoplasty or laminectomy with fusion were identified by International Classification of Diseases (ICD) and Current Procedural Terminology (CPT) codes. For study inclusion, physical exam documentation was reviewed for each patient identified in the search to confirm a diagnosis of myelopathy (gait disturbance, fine motor disability, abnormal reflexes, gross motor weakness or sensory deficits) and failure of a non-operative treatment regimen. The non-operative regimens were not standardized and varied from observation to focused neck physical therapy, injections for concurrent radiculopathy, anti-inflammatories and bracing. There was no minimum time established for duration of non-operative treatment prior to assessment of treatment failure. As this study was conducted at a large referral center, patients underwent non-standardized types and duration of non-operative management prior to referral for surgical management. Furthermore, included patients were required to have magnetic resonance imaging confirmation of cord compression involving at least three levels. Patients with any prior cervical surgery, loss of cervical lordosis, instability (>3 mm) or history of trauma were excluded from the study as these are frequently contraindications to laminoplasty. Given that laminoplasty was always completed from C3–7, only laminectomy with fusion patients undergoing surgery at those exact levels were included to control for cofounders related the magnitude of the surgery (incision length, violation of soft tissue structures, etc.). Patients undergoing supplementary procedures such as decompression of additional levels, instrumentation of additional levels or anterior procedures were excluded. Of the initial 341 patients identified during the study period from the CPT code search, 54 laminoplasty patients and 37 laminectomy with fusion patients were found to fulfill the aforementioned inclusion criteria. Removal of those with incomplete medical records or less than 12 months follow-up left a final study population of 41 laminoplasty and 31 laminectomy with fusion patients.
Surgical technique
All patients were positioned prone in a three-pronged Mayfield skull clamp with the arms adducted and held tucked to their sides.
Patients in the laminoplasty cohort underwent a C3–C7 laminoplasty using a standard midline posterior approach. At each level, an osteotomy was created at the junction of lateral mass and the lamina on the opening side. On the contralateral side, a bur was used to create a hinge. After the lamina was opened, it was fixed in a decompressed position using Medtronic Centerpiece™ laminoplasty plates (Medtronic Sofamor Danek, Memphis, TN) or NuVasive Leverage Laminoplasty System ™ plates (NuVasive, Inc., San Diego, CA) (Fig. 1). The decision to open on the left or right was determined on an individual basis by the surgeon based on the laterality of each patient’s symptoms.
The NuVasive Vuepoint (NuVasive, Inc., San Diego, CA) system was used exclusively in the laminectomy and fusion cohort. Polyaxial lateral mass screws were exclusively at all instrumented levels in combination with 3.5-mm titanium rods. A standard midline posterior approach was used, similar to the laminoplasty cohort. Lateral mass screws and rods were used for instrumentation (Fig. 1). The instrumentation was extended distally to either C7 or T1 depending upon bone quality and sagittal alignment at the cervicothoracic junction. The facet joints were decorticated and morselized local bone graft was packed into the facet joints and along the lateral masses.
The procedure selection for the patients in this study was determined principally by the date of surgery. From 2006 through August 2008, the surgeon in this study treated nearly all cervical myelopathy with C3–7 laminoplasty. However, in August 2008, the surgeon transitioned to a new cervical laminectomy and fusion system, and treated nearly all cervical myelopathy from that point forward with laminectomy and fusion. The period during which the surgeon used both techniques encompassed six total cases. These six patients were offered both options and decided which procedure they preferred resulting in three undergoing laminoplasty and three undergoing laminectomy and fusion.
All patients were given the same post-operative precautions and instructions for advancement of activities and strengthening. Cervical collars were offered for comfort only.
Outcome evaluation
Flexion, extension and neutral cervical spine plain radiographs were obtained pre-operatively and at the 6-week (±1 week) follow-up. Cervical sagittal alignment and cervical range of motion were measured using the cervical spine angle as originally described by Gore [28]. Post-operative CT studies were obtained at 1-year follow-up following laminectomy and fusion to assess fusion status. CT fusion was defined by clear interbody bony bridging on two or more consecutive sagittal CT images.
In addition to radiographic measurements, clinical outcome scores were obtained pre-operatively and at follow-up. Patient self-reported outcomes included Japanese Orthopaedic Association (JOA) scores, neck disability index (NDI), short form-12 mental (SF-12M) and physical (SF-12P) composite scores and visual analog pain scores for neck (VAS-N) and arm (VAS-A).
The JOA is a quantitative scale measuring the severity of myelopathy. The scale includes four components: (1) motor function in the arms, (2) motor function in the legs, (3) sensation and (4) bladder function. Scores range from 0 to 17, with lower scores correlating with higher disability [29]. An improvement of two points has been described as clinically important [30, 31].
The NDI is a clinical measurement of disability related specifically to the cervical spine. It has been demonstrated to have a high degree of reliability and internal consistency [32]. Scores range from 0 to 50, with a higher score correlating with higher disability. A minimal clinically important difference (MCID) of 7.5 has been established for the NDI in patients undergoing cervical fusion [33].
The SF-12 is a well-known assessment of overall health. It is composed of mental (SF-12M) and physical (SF-12P) composite scores ranging from 0 to 100, with standardized mean patient scores of 50, and higher scores indicating a better state of health. An MCID of 4.1 has been established for the SF-12P [33].
The VAS pain scale measures patient pain based on a score of 0–10. The score is measured on across a continuum, with higher scores representing a higher level of discomfort. For the purposes of this study, VAS scores for neck pain (VAS-N) and individual arms (VAS-RA and VAS-LA) were distinguished. An MCID of 2.5 has been established for neck and arm pain individually [33].
Length of stay and cost data were obtained from the hospital record and billing departments. Cost data were obtained from the medical center financial department and included all surgical, instrumentation and hospitalization fees associated with a patient’s primary hospital stay.
Complications were defined as any significant deviation from a normal post-operative course including, but not limited to, infection, new sensory or motor deficits, and reoperation. For the purposes of this study, any strength deficits relative to the pre-operative exam were considered palsies, even if present for less than 24 h.
Statistical analysis
Patient characteristics and complications were examined using Fisher’s exact tests. Within-group differences for radiographs and patient-reported outcomes were analyzed using paired t tests. Differences in change between groups were assessed using both unadjusted pre- and post-test scores as well as with adjustment for baseline outcome score and number of comorbidities. Nonparametric data (baseline comorbidities, length of stay, and cost) were assessed with the Mann–Whitney test. Odds ratios were calculated to determine the relative odds of post-operative complications between the two cohorts. Statistical analysis was carried out using R statistical software, version 3.0.2 [34], with the additional “psych [35]” and “epitools [36]” packages. Statistical significance was defined as p < 0.05; post-test results of parametric statistics are reported using the mean and 95 % confidence interval of the difference.
Results
Pre-operative comparison of cohorts
No statistically significant difference between groups was noted in any baseline demographic, radiographic or outcomes data with the exception of number of comorbidities. The median comorbidity values were 3 (IQR 1,4) in the laminoplasty group, and 4 (IQR 3,6) in the laminectomy and fusion (Table 1).
Post-operative comparison
The average follow-up was 19.2 months for the laminoplasty cohort and 18.2 months for the laminectomy with fusion cohort.
Cervical sagittal alignment
The average pre-operative cervical lordosis in the laminoplasty cohort was 12.1° and the average lordosis in the laminectomy with fusion cohort was 11.6°. All patients had a lordotic cervical sagittal alignment. There were no significant changes in sagittal alignment at follow-up and there was no significant difference between the cohorts after surgery (Table 2).
Cervical range of motion
Pre-operatively, the average cervical range of motion of the laminoplasty and laminectomy with fusion cohorts were 39.4° and 38.1°, respectively. At follow-up, both cohorts experienced a significant loss of range of motion (Table 2). However, the magnitude of loss was much larger in the laminectomy with fusion cohort (adjusted mean difference 18.04, 95 % CI 13.77–22.31). Furthermore, the laminectomy with fusion cohort experienced a significant reduction in both flexion and extension motion, while the laminoplasty cohort only experienced a reduction in flexion motion.
Fusion status
Post-operative CT studies were obtained between 6 months and 1-year to evaluate fusion. Bony fusion was documented in our review and by radiology report in 18 of 19 patients (94.7 %). The average time to post-operative CT was 10.5 months.
Patient-reported outcomes
Patient-reported outcomes are presented in Table 3. No significant differences were seen for change scores between groups on any measure for adjusted or non-adjusted data. Both groups showed statistically and clinically significant within-groups change on the JOA from pre- to post-operatively, but there was no difference in change scores between the groups post-operatively. Both groups exceeded the MCID for the physical subscale of the SF-12P. The 95 % confidence interval of the difference in neck scores between groups exceeded the MCID, indicating a potential clinical benefit of fusion over laminoplasty for neck pain. This was not seen in pain scores for either arm.
Hospital length of stay
The median length of stay for laminoplasty cohort was 3 days (IQR 2,3) while the median length of stay for the laminectomy with fusion cohort was 4 days (IQR 3,5.5). This represented a statistically significant difference (p < 0.001).
Cost
Cost data were obtained from the medical center financial department and included all surgical, instrumentation and hospitalization fees associated with a patient’s primary hospital stay. The median cost for the laminoplasty and laminectomy with fusion cohorts was 105,431 (54,718–105,184) and 128,664 (120,984–141,776), a statistically significant difference (p < 0.001).
Complications
C5 nerve root palsy was the most common peri-operative complication in both cohorts (Table 4). For the purposes of this study, any strength deficits greater than two motor grades relative to the pre-operative exam were considered palsies, even if present for less than 24 h. Laminoplasty resulted in a significantly lower rate of C5 nerve root palsy than laminectomy with fusion [32.3 versus 7.3 %, odds ratio 0.19 (95 % CI 0.03–0.66)].
The two other primary post-operative complications were infection and reoperation rate. While there was no significant difference between the two groups, the odds of occurrence were lower for the laminoplasty group for both reoperation (0.42, 95 % CI 0.08–1.95) and infection (0.19, 95 % CI 0.01–1.46). The reasons for reoperation in the laminoplasty group were infection requiring irrigation and debridement (1 patient), persistent radiculopathy treated by four-level anterior cervical discectomy and fusion (1 patient), and epidural hematoma requiring emergent evacuation (1 patient). The reasons for reoperation in the laminectomy and fusion included infection requiring irrigation and debridement (4 patients) and junctional kyphosis requiring caudal extension of the fusion construct (1 patient). Taken as a whole, laminectomy with fusion was associated with a significantly higher risk of complications than laminoplasty.
Discussion
Laminoplasty and laminectomy with fusion are the two most common surgical techniques in the treatment of cervical spondylotic myelopathy [37]. Previous studies have demonstrated success with both procedures in improving symptoms and preventing further neurological deterioration [12, 14, 21, 24–26]. However, controversy remains regarding which procedure portends superior outcomes for various surgical indications. This controversy is perpetuated by the paucity of studies directly comparing laminoplasty and laminectomy with fusion by a single surgeon [19, 20, 28, 37, 38]. The study herein addresses this void, comparing outcome measures between laminoplasty and laminectomy with fusion in two well-matched cohorts.
Multiple validated clinical outcomes scores were compared between the cohorts, including JOA, NDI, SF-12M, SF-12P, VAS-N and VAS-A. In most measured outcomes, the cohorts demonstrated improvement of similar magnitude which is consistent with a recent meta-analysis comparing the techniques [22]. Given the specific inclusion criteria, the final sample size limited the power to detect a clinically significant difference between groups. Observation of the confidence intervals in Tables 3 and 4 may assist in developing hypotheses about potential clinically meaningful differences that were not identified as statistically significant in our study, and could be examined in a larger prospective trial.
The improvement in myelopathic symptoms found in this study were similar to previous reports by Highsmith et al. [19] and Heller et al. [20]. In the present study, both groups exceeded a commonly accepted MCID, indicating important clinical benefit on a scale specifically developed for this population. There was, however, no significant difference in the improvement in JOA scores between the two cohorts. Highsmith et al. [19] reported no significant difference in JOA scores while Heller et al. [20] reported no difference in Nurick scores.
Although the principal goal of laminoplasty and laminectomy with fusion is to alleviate myelopathic symptoms, improvement in neck pain is a secondary objective. In the past, laminoplasty has been associated with shoulder and neck pain [15] while laminectomy with fusion has been associated with an improvement in neck pain [19]. In their study directly comparing VAS neck pain in laminoplasty and laminectomy with fusion, Highsmith et al. showed that laminectomy with fusion significantly improves neck pain while laminoplasty did not [19]. While no significant difference was found between groups in this study, the 95 % confidence interval of the difference in neck scores between groups exceeded the established MCID of 2.5 points for neck pain, indicating a potential clinical indication for fusion.
The authors are unaware of any other studies in the literature directly comparing NDI, SF-12M, SF-12P or VAS-A pain between laminoplasty and laminectomy with fusion. The results of the present study demonstrate that laminoplasty and laminectomy with fusion result in similar improvements in these outcome scores. The fact that the SF-12 scores were comparable between the two cohorts is of particular interest, as the SF-12 is a validated assessment of overall health. This study demonstrates that even though there may be differences in focused outcome scores (e.g., neck pain, cervical range of motion), such differences do not necessarily translate into on overall better state of physical or mental well-being.
In addition to clinical outcome scores, radiographic measurements were made. At follow-up, there was no significant change in the sagittal alignment of either cohort. The fact that sagittal alignment is maintained is specifically relevant to the laminoplasty cohort. With laminoplasty, there is concern that the posterior elements may be compromised, leading to destabilization and progressive kyphosis. The data from this study do not suggest that laminoplasty results in significant destabilization. However, it is important to note that the inclusion criteria of this study required a lordotic cervical alignment before surgery. In patients with loss of cervical lordosis, it is indeed possible that laminoplasty may result in progressive kyphosis.
Radiographs were also used to assess cervical range of motion. At follow-up, both cohorts experienced a significant loss of motion. However, the magnitude of loss was much larger in the laminectomy with fusion cohort. This result is intuitive and laminoplasty obviously provides the benefit of preserving maximum cervical motion. However, the clinical significance of this retained motion remains unclear. As noted before, laminectomy with fusion trended towards a larger improvement in neck pain (VAS-N), so it is possible that the retained motion with laminoplasty also results in increased neck pain.
The complications reported in this study were consistent with those previously reported in the literature [20, 37]. The most common complication in both cohorts was C5 nerve root palsy, followed by infection. Laminectomy with fusion resulted in a markedly elevated risk of transient C5 nerve root palsy and overall complication rate. Again, all palsies were temporary and resolved completely during follow-up. The findings of this study are consistent with prior studies which report a higher rate of nerve palsy after laminectomy with fusion than laminoplasty [39]. Recent literature has increasingly implicated foraminal stenosis in the development of post-operative C5 palsy in both anterior and posterior cervical fusion surgery [40–42]. It is important to note that the laminectomy and fusion cohort had significantly more comorbidities and a higher rate of diabetes mellitus. In a 2015 study surveying members of AOSpine, Tetreault et al. found that comorbidities were the most important clinical predictor of complications in cervical surgery for myelopathy [43].
The hospital length of stay and overall cost were less with laminoplasty than laminectomy with fusion. These differences have been attributed to shorter operative time, less soft tissue disruption and post-operative pain, and less instrumentation during the laminoplasty procedure [19]. As pain control is a primary determinant of hospital length of stay, we believe the 1-day difference between the laminoplasty and laminectomy groups represents a clinically significant difference in peri-operative pain control. Although length of stay may not influence long-term pain or functional outcome scores, it undoubtedly influences total treatment cost.
The strengths of this study are the relatively large sample sizes and the well-matched cohorts. There was no significant difference between the cohorts in any demographic, clinical or radiographic variable before surgery with the exception of number of comorbidities. Adjusting for this difference had no effect on any outcomes assessed. It is noteworthy that the cohorts were matched based on the levels treated (exclusively C3–C7). This matching of levels resulted in the exclusion of the majority of patients initially identified in the CPT code search. To date, there is no other study in the literature that compares laminoplasty and laminectomy with fusion patients treated at identical levels. Variance in the number of treated levels would likely significantly affect tissue dissection, range of motion, outcome scores and complications.
This study has several important limitations. Though the cohorts were well matched, inequalities inevitably existed. There is a historical bias in the data whereby all patients treated in the beginning of the study period were treated with laminoplasty and a switch was made in the middle of the study to laminectomy and fusion. Conversely, the fact only one operative technique was used at any given time leads to unbiased selection of patients. It is possible that the increased experience attained by the surgeon over time positively biased results with laminectomy and fusion and negatively biased results with laminoplasty. Additionally, although cross-sectional imaging was available for many in the laminectomy and fusion cohort in this study, cross-sectional or oblique plain film imaging was not routinely obtained in the laminoplasty cohort and, thus, the role of foraminal stenosis in differential rate of C5 palsy between the cohorts could not be assessed.
A prospective, randomized study would likely result in higher quality comparisons. The sample sizes are modest. Furthermore, this study is limited to patients without cervical sagittal deformity. In patients with significant loss of cervical lordosis or instability, laminectomy with fusion may be superior to laminoplasty. The length of follow-up in this study is insufficient to make any comments regarding the development of adjacent segment disease or other long-term outcomes. Finally, the selection of procedure in this study was determined by surgeon preference. During the study period, the surgeon transitioned from strictly laminoplasty to laminectomy and fusion. Although fewer than ten patients were operated on during a period when the surgeon was using both treatments, this overlap does introduce selection bias for the procedures chosen for those patients.
Overall, this study provides evidence that, when matched for patient status and operative levels, laminoplasty may be superior to laminectomy with fusion in preserving cervical range of motion, reducing hospital stay and minimizing cost. While the difference in improvement did not reach statistical significance, there was greater improvement in neck pain in the laminectomy and fusion group. However, the clinical significance of these differences remains unclear as there was no significant difference in any patient-reported outcome measure and nearly identical improvement in SF-12, JOA, and NDI scores.
References
Rao RD, Gourab K, David KS (2006) Operative treatment of cervical spondylotic myelopathy. J Bone Joint Surg Am 88:1619–1640. doi:10.2106/JBJS.F.00014
Clarke E, Robinson PK (1956) Cervical myelopathy: a complication of cervical spondylosis. Brain 79:483–510
Fujiwara K, Yonenobu K, Ebara S, Yamashita K, Ono K (1989) The prognosis of surgery for cervical compression myelopathy. An analysis of the factors involved. J Bone Joint Surg Br 71:393–398
Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM (2003) Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J 3:33–45
Traynelis VC, Arnold PM, Fourney DR, Bransford RJ, Fischer DJ, Skelly AC (2013) Alternative procedures for the treatment of cervical spondylotic myelopathy: arthroplasty, oblique corpectomy, skip laminectomy: evaluation of comparative effectiveness and safety. Spine 38:S210–S231. doi:10.1097/brs.0000000000000009
Lonstein JE (1977) Post-laminectomy kyphosis. Clin Orthop Relat Res 128:93–100
Guigui P, Benoist M, Deburge A (1998) Spinal deformity and instability after multilevel cervical laminectomy for spondylotic myelopathy. Spine 23:440–447
Dai L, Ni B, Yuan W, Jia L (1998) Radiculopathy after laminectomy for cervical compression myelopathy. J Bone Joint Surg Br 80:846–849
Kaye ID, Marascalchi BJ, Macagno AE, Lafage VA, Bendo JA, Passias PG (2015) Predictors of morbidity and mortality among patients with cervical spondylotic myelopathy treated surgically. Eur Spine J 24:2910–2917. doi:10.1007/s00586-015-4010-2
Gonzalez-Feria L, Peraita-Peraita P (1975) Cervical spondylotic myelopathy: a cooperative study. Clin Neurol Neurosurg 78:19–33
Maurer PK, Ellenbogen RG, Ecklund J, Simonds GR, van Dam B, Ondra SL (1991) Cervical spondylotic myelopathy: treatment with posterior decompression and Luque rectangle bone fusion. Neurosurgery 28:680–683 (discussion 683–684)
Huang RC, Girardi FP, Poynton AR, Cammisa FP Jr (2003) Treatment of multilevel cervical spondylotic myeloradiculopathy with posterior decompression and fusion with lateral mass plate fixation and local bone graft. J Spinal Disord Tech 16:123–129
Cherubino P, Benazzo F, Borromeo U, Perle S (1990) Degenerative arthritis of the adjacent spinal joints following anterior cervical spinal fusion: clinicoradiologic and statistical correlations. Ital J Orthop Traumatol 16:533–543
Kato Y, Iwasaki M, Fuji T, Yonenobu K, Ochi T (1998) Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg 89:217–223. doi:10.3171/jns.1998.89.2.0217
Hosono N, Yonenobu K, Ono K (1996) Neck and shoulder pain after laminoplasty. A noticeable complication. Spine 21:1969–1973
Ratliff JK, Cooper PR (2003) Cervical laminoplasty: a critical review. J Neurosurg 98:230–238
Nurboja B, Kachramanoglou C, Choi D (2011) Cervical laminectomy versus laminoplasty: is there a difference in outcome and post-operative pain? Neurosurgery. doi:10.1227/NEU.0b013e31823cf16b
Manzano GR, Casella G, Wang MY, Vanni S, Levi AD (2012) A prospective, randomized trial comparing expansile cervical laminoplasty and cervical laminectomy and fusion for multilevel cervical myelopathy. Neurosurg 70:264–277. doi:10.1227/NEU.0b013e3182305669
Highsmith JM, Dhall SS, Haid RW Jr, Rodts GE Jr, Mummaneni PV (2011) Treatment of cervical stenotic myelopathy: a cost and outcome comparison of laminoplasty versus laminectomy and lateral mass fusion. J Neurosurg Spine 14:619–625. doi:10.3171/2011.1.SPINE10206
Heller JG, Edwards CC 2nd, Murakami H, Rodts GE (2001) Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine 26:1330–1336
Hukuda S, Ogata M, Mochizuki T, Shichikawa K (1988) Laminectomy versus laminoplasty for cervical myelopathy: brief report. J Bone Joint Surg Br 70:325–326
Lee CH, Lee J, Kang JD, Hyun SJ, Kim KJ, Jahng TA, Kim HJ (2015) Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: a meta-analysis of clinical and radiological outcomes. J Neurosurg Spine 22:589–595. doi:10.3171/2014.10.spine1498
Bartels RH, van Tulder MW, Moojen WA, Arts MP, Peul WC (2015) Laminoplasty and laminectomy for cervical spondylotic myelopathy: a systematic review. Eur Spine J 24(Suppl 2):160–167. doi:10.1007/s00586-013-2771-z
Gok B, McLoughlin GS, Sciubba DM, McGirt MJ, Chaichana KL, Wolinsky JP, Bydon A, Gokaslan ZL, Witham TF (2009) Surgical management of cervical spondylotic myelopathy with laminectomy and instrumented fusion. Neurol Res 31:1097–1101. doi:10.1179/174313209X383277
Houten JK, Cooper PR (2003) Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery 52:1081–1087 (discussion 1087–1088)
Kumar VG, Rea GL, Mervis LJ, McGregor JM (1999) Cervical spondylotic myelopathy: functional and radiographic long-term outcome after laminectomy and posterior fusion. Neurosurgery 44:771–777 (discussion 777–778)
Epstein NE (2015) Commentary on article: laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: a meta-analysis of clinical and radiological outcomes by Chang-Hyun Lee et al. Surg Neurol Int 6:S379–S382. doi:10.4103/2152-7806.163957
Manzano GR, Casella G, Wang MY, Vanni S, Levi AD (2012) A prospective, randomized trial comparing expansile cervical laminoplasty and cervical laminectomy and fusion for multilevel cervical myelopathy. Neurosurgery 70:264–277. doi:10.1227/NEU.0b013e3182305669
Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y (1985) Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br 67:609–615
Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG (2011) Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine 14:348–355. doi:10.3171/2010.10.spine091029
Bartels RH, Verbeek AL, Grotenhuis JA (2007) Design of Lamifuse: a randomised, multi-centre controlled trial comparing laminectomy without or with dorsal fusion for cervical myeloradiculopathy. BMC Musculoskelet disorders 8:111. doi:10.1186/1471-2474-8-111
Vernon H, Mior S (1991) The neck disability index: a study of reliability and validity. J Manip Physiol Ther 14:409–415
Carreon LY, Glassman SD, Campbell MJ, Anderson PA (2010) Neck disability index, short form-36 physical component summary, and pain scales for neck and arm pain: the minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J 10:469–474. doi:10.1016/j.spinee.2010.02.007
Team RDC (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Revelle W (2013) Psych: procedures for personality and psychological research, Northwestern University, Evanston, Illinois, USA. http://cran.r-projectorg/package=psych. Version = 142. Accessed 01 Feb 2015
Developer TJA (2012) Epitools: epidemiology tools. R package version 0.5-7. http://cran.r-projectorg/package=epitools. Accessed 01 Feb 2015
Woods BI, Hohl J, Lee J, Donaldson W 3rd, Kang J (2011) Laminoplasty versus laminectomy and fusion for multilevel cervical spondylotic myelopathy. Clin Orthop Relat Res 469:688–695. doi:10.1007/s11999-010-1653-5
Nurboja B, Kachramanoglou C, Choi D (2012) Cervical laminectomy vs laminoplasty: is there a difference in outcome and postoperative pain? Neurosurgery 70:965–970. doi:10.1227/NEU.0b013e31823cf16b (discussion 970)
Nassr A, Eck JC, Ponnappan RK, Zanoun RR, Donaldson WF 3rd, Kang JD (2012) The incidence of C5 palsy after multilevel cervical decompression procedures: a review of 750 consecutive cases. Spine 37:174–178. doi:10.1097/BRS.0b013e318219cfe9
Bydon M, Macki M, Kaloostian P, Sciubba DM, Wolinsky JP, Gokaslan ZL, Belzberg AJ, Bydon A, Witham TF (2014) Incidence and prognostic factors of c5 palsy: a clinical study of 1001 cases and review of the literature. Neurosurgery 74:595–604. doi:10.1227/neu.0000000000000322 (discussion 604-595)
Wu FL, Sun Y, Pan SF, Zhang L, Liu ZJ (2014) Risk factors associated with upper extremity palsy after expansive open-door laminoplasty for cervical myelopathy. Spine J 14:909–915. doi:10.1016/j.spinee.2013.07.445
Yamanaka K, Tachibana T, Moriyama T, Okada F, Maruo K, Inoue S, Horinouchi Y, Yoshiya S (2014) C-5 palsy after cervical laminoplasty with instrumented posterior fusion. J Neurosurg Spine 20:1–4. doi:10.3171/2013.9.spine12952
Tetreault L, Singh A, Fawcett M, Nater A, Fehlings MG (2015) An assessment of the key predictors of perioperative complications in patients with cervical spondylotic myelopathy undergoing surgical treatment: results from a survey of 916 AOSpine international members. World Neurosurg. doi:10.1016/j.wneu.2015.01.021
Financial and material support
No authors received financial support for any of the work reported herein.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflict of interest.
Rights and permissions
About this article
Cite this article
Blizzard, D.J., Caputo, A.M., Sheets, C.Z. et al. Laminoplasty versus laminectomy with fusion for the treatment of spondylotic cervical myelopathy: short-term follow-up. Eur Spine J 26, 85–93 (2017). https://doi.org/10.1007/s00586-016-4746-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4746-3