Abstract
Purpose
In this research, we investigated the coordination pattern and consistency of coordination between the thorax and pelvis during gait in patients with idiopathic scoliosis.
Methods
Across the study, 69 adolescent girls (controls: 30, patients: 39) participated. All participants were asked to walk 10 m barefoot at a self-selected speed. The walking speed, stride length, and range of motion of the pelvic and thoracic angles were collected using a three-dimensional optical motion analysis system, and the thorax–pelvis coordination was quantified using a vector coding technique. The frequency of four different patterns of coordination (in-phase, anti-phase, pelvis only, and thorax only) and the consistency of coordination including direction and magnitude during the gait cycle of the two groups were investigated. Independent-sample t tests were performed to examine differences between the two groups with regard to coordination patterns and consistency.
Results
The patients with idiopathic scoliosis showed significantly higher in-phase and relatively lower anti-phase in the transverse plane compared to controls. Additionally, the pelvis only in the transverse, frontal, and sagittal planes was significantly lower in patients. The consistency of coordination in patients was significantly lower than in controls in direction and magnitude on the transverse and frontal planes.
Conclusion
From viewpoint of the thorax–pelvis coordination, patients with IS had less gait stability in the trunk than controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic scoliosis (IS) is the most frequent type of scoliosis. It involves three-dimensional deformation of the spine and is known to alter postural orientation and cause a pathological gait [1–5]. Patients with IS have an asymmetric postural orientation of the shoulder and pelvis in the transverse and frontal planes in static condition and asymmetric movement of the trunk [3], a smaller range of motion (RoM) of the pelvis and spine in the transverse and frontal planes [1], and greater stiffness of the quadratus lumborum and erector spinae muscles in dynamic condition [6, 7]. The asymmetrical, restricted movement and muscle stiffness induce abnormalities of the thorax–pelvis coordination needed to maintain stability of the trunk [8, 9].
Coordination is defined as the ability to maintain a proper cyclic relationship between different segments or joints [10]. In particular, thorax–pelvis coordination has the important role of maintaining the stability of the entire body in a normal gait because the thorax and pelvis minimize the angular momentum of the trunk by counter-rotating toward each other [11–14]. Also, the coordination can be quantitatively analyzed by pattern and consistency [8, 12, 15, 16]. The thorax–pelvis coordination pattern can be categorized as in-phase if the two segments tend to rotate in the same direction; anti-phase if the two segments tend to rotate in opposite directions; pelvis only if only the pelvis rotates; and thorax only if only the thorax rotates [17]. Those categories allow us to quantify which type of coordination pattern dominates in stable movement [18]. On the other hand, in periodic repeated movements such as walking, the consistency of coordination represents maintenance of stable coordination and can be quantified as the direction and magnitude for the relative movement of the two segments [16]. Generally, healthy individuals have a higher anti-phase coordination pattern and a greater consistency of coordination between the thorax and pelvis than patients with pathology. Those are the fundamental characteristics of trunk movement for gait stability [15, 16, 19, 20].
Several studies have investigated the thorax–pelvis coordination of patients with trunk-related diseases to evaluate gait stability. During walking or running, patients with low back pain showed a higher in-phase coordination pattern in the frontal or transverse plane than normal. That pattern is characteristic of coordination that increases the stability of lumbar movement by restraining the counter-rotation of the thorax and pelvis to reduce strain on the soft tissues surrounding the lumbar column [21]. Also, patients with ankylosing spondylitis were shown to have in-phase thorax–pelvis coordination in the transverse plane during a loading response (sub-phase of the gait cycle) because of trunk axial stiffness. Those results indicate that ankylosing spondylitis patients have a less stable gait than normal [19]. Field-Fote and Tepavac [15] demonstrated that patients with spinal cord injury have lower consistency of coordination in the sagittal plane than normal and that training can improve the consistency of coordination to stabilize the gait for patients. Analysis of coordination is useful for assessment of gait stability and has been widely used for other diseases; however, the coordination of patients with IS has not yet been investigated.
Because spinal deformity alters the center of mass, it causes development of asymmetric trunk movement and a decline in gait stability [1, 2]. Also, IS patients have problems with dynamic balance control caused by their asymmetry of trunk rotation in the transverse plane [2, 22], smaller trunk RoM because of muscle stiffness (such as in the quadratus lumborum and erector spinae) [6], vestibular dysfunction [23], and an impaired somatosensory system [24]. All of those symptoms can cause unstable gait. To understand how IS patients maintain a stable gait, researchers commonly use RoM or asymmetric kinematic variables, which are suitable for analyzing movement changes in a specific segment. On the other hand, those variables provide limited information about the coordination of adjacent segments in ensuring a stable gait. Therefore, analysis of how coordination between two different segments maintains stability in the trunk is required additionally to investigate the gait stability for IS patients.
The purpose of our study is to investigate the characteristics of thorax–pelvis coordination in IS patients to evaluate gait stability. We quantitatively compared the pattern and consistency of thorax–pelvis coordination during gait between patients with IS and normal participants. We have hypothesized that IS patients have (1) a higher in-phase coordination pattern, (2) lower consistency of coordination than normal controls during gait.
Materials and methods
Subjects
A total of 69 adolescent girls participated in the study. We fully explained the purpose and process of the experiments and obtained consent from all participants prior to their participation. All experimental processes were carried out under the approval of the Institutional Review Board of Korea University Guro Hospital. A group of 39 patients (age = 15.1 ± 2.1 years, height = 155.2 ± 8.2 cm, weight = 45.6 ± 9.5 kg) were interviewed to examine the clinical history such as birth history and normal growth history, and were diagnosed with IS by an orthopedic clinician. All patients were selected who had no sign of a motor grade, sensory response of upper and lower extremities and pathological neurological responses which were evaluated through physical examination; and had Cobb’s angle greater than 10° with no history of any treatment or surgery. Among the patients, 23 had a main curvature at the thoracic level and 16 at the thoracolumbar level. The average Cobb’s angle was 33.6° with standard deviation ±11.8° and range 13.0°–65.0°. IS patients with leg length discrepancies higher than 1 cm with low back pain were excluded from this study. The control group was composed of 30 age-matched adolescent girls (age = 14.8 ± 2.7 years, height = 154.9 ± 5.6 cm, weight = 44.7 ± 6.3 kg) with no history of musculoskeletal disease and no spinal deformation in the frontal or sagittal planes.
Protocol and data collection
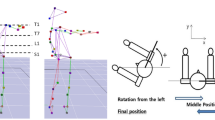
To obtain motion data for each participant, we attached a total of 14 reflective markers, each with a diameter of 14 mm, to the thorax, pelvis and feet of the subjects using double-sided adhesive tape according to a modified Helen Hayes marker protocol [25]. The locations of the reflective markers were selected by palpation on anatomical landmarks with agreement from a skilled operator and a clinician to minimize inter-experimentalist error. To obtain the thoracic segment angle, a local coordination system was created using four reflective markers attached to the seventh cervical vertebrae, tenth thoracic vertebrae, jugular notch where the clavicles meet the sternum, and xiphoid process of the sternum. The relative rotation angle was calculated with respect to the laboratory coordinate system. The sequence used to obtain the thoracic segment angle was also used to determine the pelvic segment angle with reflective markers attached to the left anterior superior, right anterior superior, left posterior superior, and right posterior superior iliac spine (Fig. 1a). To obtain the spatiotemporal variables, a total of six reflective markers were attached to the lateral malleolus, calcaneus and second metatarsal head of each foot. Vicon motion analysis system (six-camera system 460, 120 Hz, Vicon Motion Systems, Oxford, UK) and SB-InScan foot pressure measurement system (SWING BANK Ltd., Republic of Korea) were used to calculate the spatiotemporal variables and kinematic data on the thorax and pelvis [26]. Participants underwent sufficient walking practice in the laboratory, and we recorded six repetitive walking trials of a 10-m straight line, walked barefoot at a self-selected speed. Kinematic data sets were low-pass filtered with a fourth-order Butterworth filter using a cut-off frequency of 6 Hz to remove noise [27]. All walking trials were normalized through cubic spline interpolation to the 100 % gait cycle measured from one heel strike to the following heel strike of same foot. The experimental protocols to data collection were conducted by a single skilled operator and clinician.
Marker placement, coordination pattern, and consistency of the thorax and pelvis. a Marker placement of the thorax and pelvis used for the present study. b Calculation of coupling angle in the angle–angle plot. c Definition of coordination pattern. d Sample representation of coordination patterns between the thorax and pelvis in frontal plane motion. e Conceptual description of the consistency of coordination. The values of a and m indicate the consistency of coordination. Assuming an angle–angle plot with six trials, with motion expressed from the nth to the n + 1th frame as a vector, a approached 1 as the six vector directions converged, and m approached 1 as the six vector magnitudes converged. When direction and magnitude were similar, a and m both approached 1
Spatiotemporal variables and range of motion of the pelvic angle
Spatiotemporal variables, such as walking speed, stride length, stance phase, and the RoM of the pelvic angle were calculated to assess the fundamental characteristics of gait in controls and patients [1, 6, 7]. Walking speed and stride length were normalized by leg length into a dimensionless value to eliminate influences of body size [28]. The average of the spatiotemporal variables and RoM of the pelvic angle from six repetitive trials was used as a representative value for each participant.
Vector coding technique
Vector coding technique (VCT) was used to quantify the coordination between the thorax and pelvis of each subject during gait [16]. VCT is a common non-linear technique for quantifying the pattern and consistency of coordination by calculating the coupling angle in an angle–angle plot. This technique has the advantage of not losing any spatial information for segment movement because it does not require normalization of data [29]. It was designed to give the direction and magnitude of coordination using segmental or joint angle for easy access to clinicians [15]. To identify the coordination pattern, an angle–angle plot was constructed with a horizontal axis of the pelvic angle and a vertical axis of the thoracic angle. The coupling angle (γ) was defined as the positive direction angle between a horizontal line and the vector constructed from the nth point toward the n + 1th point of a normalized gait cycle, shown as the angle–angle plot, with a range of 0°–360° (Fig. 1b). Coordination patterns were categorized following the method proposed by Chang et al. [17] as described in the introduction (Fig. 1c, d). Each coordination pattern showed a frequency during one gait cycle that was normalized to 100 %. For instance, if the in-phase coordination pattern was observed for 25 % of one normalized gait cycle, it is expressed as in-phase = 25. The average coordination pattern from six repetitive kinematic data sets was used as a representative value for each participant, and gait data on all six data sets were used for coordination consistency calculations.
The consistency of coordination was represented as direction a and magnitude m calculated from the VCT [16]. The value a represents a direction component of consistency for the vector constructed to calculate the coupling angle at the same instance period from several gait cycles (Fig. 1e). The value m represents a magnitude component of consistency for the vector with horizontal axis (\( x_{n,n + 1} \)) and vertical axis (\( y_{n, n + 1} \)). The magnitude of the vector is expressed as \( l_{n, n + 1} \). The cosine and sine of \( \gamma_{n, n + 1} \) can be calculated using the formulas below.
Repetition of six trials of this process allowed us to calculate the mean cosine (\( \overline{{\cos \;\gamma_{n,n + 1} }} \)) and mean sine (\( \overline{{\sin \;\gamma_{n,n + 1} }} \)) for each value from each trial. Using formula below, the value of a from the nth to the n + 1th frame can be calculated.
Given the average value of \( a_{n, n + 1} \) for all normalized gait cycles from the formula above, the final value of a can be calculated. Let the standard deviation of \( l_{n, n + 1} \) for six trials be represented as \( \sigma_{n,n + 1} \), and let the largest value from \( \sigma_{1, 2} \) to \( \sigma_{99,100} \) be represented as σ max, \( m_{n, n + 1} \) from the interval of the nth to the n + 1th frame can be calculated using the formula below.
With the calculated \( m_{n, n + 1} \) from the process above, the final value m can be calculated by averaging the values of \( m_{n, n + 1} \) for all frames of the normalized gait cycle. The values of a and m range from 0 to 1. When those values are close to 1, it indicates high consistency of coordination between the thorax and pelvis (Fig. 1e).
Statistical analysis
Independent-sample t tests were performed to compare controls and patients for spatiotemporal variables, RoM of the pelvic angle, coordination pattern, and consistency of coordination. Data normality of all variables was verified using the Shapiro–Wilk test. Significance level was 0.05, and all analyses used SPSS 21.0 software (SPSS Inc., Chicago, IL, USA).
Results
Spatiotemporal variables and range of motion of the pelvic angle
Table 1 shows the spatiotemporal variables and RoM of the pelvic angle of controls and patients. The results for controls measured within the normal limits are reported in previous studies [11, 30–33]. Significant differences were observed in walking speed, stride length, and RoM of the pelvic angle in the transverse and frontal planes between patients and controls (p < 0.05). Stance phase and the RoM of the pelvic angle in the sagittal plane showed no significant differences between groups.
Coordination pattern
Figure 2 shows the coordination patterns between the thorax and pelvis in controls and patients. In the transverse plane, patients had significantly higher in-phase coordination (patients, 35.09 ± 12.22, controls, 22.22 ± 10.35, p < 0.05) and significantly lower anti-phase coordination (patients, 11.85 ± 5.84, controls, 16.10 ± 4.72, p < 0.05) than controls. The pelvis only in the patients was significantly lower in all three anatomical planes (transverse plane, patients, 39.93 ± 11.62, controls, 49.99 ± 12.63, p < 0.05; frontal plane, patients, 56.44 ± 13.00, controls, 66.40 ± 14.63, p < 0.05; sagittal plane, patients, 11.53 ± 5.59, controls, 13.70 ± 3.04, p < 0.05). Other coordination patterns were similar in controls and patients.
Consistency of coordination
The value a, which indicates consistency in the direction component of coordination, and the value m, which indicates consistency in the magnitude component, were significantly lower (p < 0.05) in the patients than in the controls in the transverse and frontal planes (Table 2). However, in the sagittal plane, no significant differences in a or m were observed.
Discussion
In this study, the thorax–pelvis coordination of patients with IS during gait was investigated by comparing the coordination patterns and consistency with those of controls. The coordination patterns in all three anatomical planes differed between patients with IS and controls. In particular, in patients, the in-phase in the transverse plane was significantly higher than in controls, and the anti-phase was lower. The changes in thorax–pelvis coordination pattern could be related to the kinematics of those two segments because the coordination pattern was calculated from the segmental angles of the thorax and pelvis. Thus, the coordination pattern might be changed if at least one segment of thorax or pelvis has the changes of movement pattern [21]. Actually, the RoM of the pelvic angle of patients was significantly smaller than that of controls in the transverse and frontal planes (Table 1; Fig. 3a, b), which agreed with the results from Chen et al. [1] and Mahaudens et al. [6]. In those results, the smaller RoM in patients was explained as a mechanism to compensate for trunk imbalance caused by spinal deformity. Mahaudens et al. [6], in particular, explained the smaller RoM of pelvic angle as a result of prolonged activation of muscles in the lumbar spine and pelvis. Although an electromyographic study was not performed, patients from our study were thought to stabilize their trunk motion by lessening the RoM of their pelvic angle. Lessening the RoM of pelvic angle might cause changes in the coordination between the thorax and pelvis in patients with IS. Walking speed and stride length were lower in patients than in controls (Table 1). Those changes also lessened the RoM of the pelvic angle during walking [34] and induced in-phase coordination between the thorax and pelvis in the transverse plane [35, 36]. In summary, the lower RoM of pelvic angle, walking speed, and stride length seen in patients induce the higher in-phase coordination in the transverse plane of the thorax–pelvis coordination pattern. This suggests that patients with IS have a less stable gait than controls.
The pelvis only coordination in patients was significantly lower than in controls in all three planes. However, the thorax only coordination did not show a significant difference between patients and controls (Fig. 2). Because the thoracic angular momentum contributes more to whole-body angular momentum than does the pelvic angular momentum in a normal gait [13], changing the thoracic movement would be the most efficient way to achieve a stable gait. However, changes in pelvic movement, not thoracic movement, were observed in patients. Thus, the movements of the thorax with spinal deformation function normally; patients could self-regulate the thorax–pelvis coordination by adjusting pelvic movement. Although, pelvic movements in the sagittal plane in patients had a similar RoM to controls (Fig. 3c), the pelvis only coordination in patients was lower. This result arose from the small RoM of the pelvic angle in the sagittal plane compared to the RoM of the frontal and transverse planes. Thus, RoM in the sagittal plane is sensitive to slight pelvic movement and seems to affect coordination between the thorax and pelvis. A significant difference in coordination patterns in the sagittal plane was observed; however, those results should be interpreted with care.
The consistency of coordination between the thorax and pelvis was significantly lower in direction and magnitude in the transverse and frontal planes in the patients. These results indicate that the stability of control mechanisms underlying coordination behavior was lower in patients than in controls. According to previous studies on IS, patients have poor postural control related to trunk imbalance from spine deformation [1, 6, 22], somatosensory dysfunction [24], visual deficiency [37], proprioceptive deficiency [38], and vestibular disorders [23, 39]. These kinds of defects might affect the comprehensive motion control mechanism in a complex manner, and they could lower the consistency of coordination in patients with IS. Lastly, the direction component (the value a) had high reproducibility with regard to consistency of coordination, but magnitude component (the value m) had relatively low reproducibility. The counter-rotation (rotation in the reverse direction) of the thorax and pelvis on the vertical axis reduces the total body angular momentum [40], and the reduced angular momentum indicates that whole-body stability can be maintained in a normal gait [11–14]. Considering the main findings of previous studies, the direction component of coordination could have more contribution to the gait stability than the magnitude component of that, and it may be more reliable for researchers to apply the direction component when they assess gait stability based on coordination between the thorax and pelvis.
Conclusion
This study compared the coordination of patients with IS and controls during gait. Patients were found to have higher in-phase and lower anti-phase coordination in the transverse plane and also to have less consistency of coordination in the transverse and frontal planes than controls. Thus, from the viewpoint of the thorax–pelvis coordination, patients with IS had less gait stability in the trunk than controls. A limitation of this study was reproducibility of an everyday gait in a laboratory setting because of a lack of consecutive periodic kinematic data. Using an optical motion capture system and six infrared cameras, the capable measurement range during continuous walking was limited. The location of the reflective markers attached to subjects is very sensitive even for a skilled operator and can cause calculation errors as a result of small discrepancies; and, there would be soft tissue artifacts. Although we assumed that those errors could be ignored, they might have been included in the movement of the thorax and pelvis.
Despite those limitations, the results of this study contribute to the understanding of characteristics of coordination between the thorax and pelvis of IS patients. Thus, our results might be valuable in evaluating the gait stability of IS patients and in designing conservative treatments for maintenance of stable trunk movement. Also, IS can be classified in more detail using methods such as Cobb’s angle, King type, and Lenke classification. However, the experimental design of the present study was unsuitable to analyze differences in patients classified with different types or degrees of IS. Additional research for the IS patients subdivided by classification method is required in the future.
References
Chen P, Wang J, Tsuang Y, Liao T, Huang P, Hang Y (1998) The postural stability control and gait pattern of idiopathic scoliosis adolescents. Clin Biomech 13(Suppl 1):S52–S58. doi:10.1016/S0268-0033(97)00075-2
Kramers-de Quervain IA, Müller R, Stacoff A, Grob D, Stüssi E (2004) Gait analysis in patients with idiopathic scoliosis. Eur Spine J 13(5):449–456. doi:10.1007/s00586-003-0588-x
Nault ML, Allard P, Hinse S, Le Blanc R, Caron O, Labelle H, Sadeghi H (2002) Relations between standing stability and body posture parameters in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 27(17):1911–1917. doi:10.1097/00007632-200209010-00018
Stokes IA (1994) Three-dimensional terminology of spinal deformity: a report presented to the Scoliosis Research Society by the Scoliosis Research Society Working Group on 3-D terminology of spinal deformity. Spine (Phila Pa 1976) 19(2):236–248
Stokes IA (1989) Axial rotation component of thoracic scoliosis. J Orthop Res 7(5):702–708
Mahaudens P, Banse X, Mousny M, Detrembleur C (2009) Gait in adolescent idiopathic scoliosis: kinematics and electromyographic analysis. Eur Spine J 18(4):512–521. doi:10.1007/s00586-009-0899-7
Mahaudens P, Thonnard J, Detrembleur C (2005) Influence of structural pelvic disorders during standing and walking in adolescents with idiopathic scoliosis. Spine J 5(4):427–433. doi:10.1016/j.spinee.2004.11.014
Seay JF, Van Emmerik RE, Hamill J (2011) Low back pain status affects pelvis-trunk coordination and variability during walking and running. Clin Biomech 26(6):572–578. doi:10.1016/j.clinbiomech.2010.11.012
Wu WH, Lin XC, Meijer OG, Gao JT, Hu H, Prins MR, Liang BW, Zhang LQ, Van Dieën JH, Bruijn SM (2014) Effects of experimentally increased trunk stiffness on thorax and pelvis rotations during walking. Hum Mov Sci 33(1):194–202. doi:10.1016/j.humov.2013.09.002
Krasovsky T, Levin MF (2010) Review: toward a better understanding of coordination in healthy and poststroke gait. Neurorehabil Neural Repair 24(3):213–224. doi:10.1177/1545968309348509
Whittle MW, Levine D (1999) Three-dimensional relationships between the movements of the pelvis and lumbar spine during normal gait. Hum Mov Sci 18(5):681–692. doi:10.1016/S0167-9457(99)00032-9
Lamoth C, Beek P, Meijer O (2002) Pelvis–thorax coordination in the transverse plane during gait. Gait Posture 16(2):101–114. doi:10.1016/S0966-6362(01)00146-1
Bruijn SM, Meijer OG, Van Dieen JH, Kingma I, Lamoth CJ (2008) Coordination of leg swing, thorax rotations, and pelvis rotations during gait: the organisation of total body angular momentum. Gait Posture 27(3):455–462. doi:10.1016/j.gaitpost.2007.05.017
Roemmich RT, Field AM, Elrod JM, Stegemöller EL, Okun MS, Hass CJ (2013) Interlimb coordination is impaired during walking in persons with Parkinson’s disease. Clin Biomech 28(1):93–97. doi:10.1016/j.clinbiomech.2012.09.005
Field-Fote EC, Tepavac D (2002) Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther 82(7):707–715
Tepavac D, Field-Fote EC (2001) Vector coding: a technique for quantification of intersegmental coupling in multicyclic behaviors. J Appl Biomech 17(3):259–270
Chang R, Van Emmerik R, Hamill J (2008) Quantifying rearfoot–forefoot coordination in human walking. J Biomech 41(14):3101–3105. doi:10.1016/j.jbiomech.2008.07.024
Needham R, Naemi R, Chockalingam N (2014) Quantifying lumbar–pelvis coordination during gait using a modified vector coding technique. J Biomech 47(5):1020–1026. doi:10.1016/j.jbiomech.2013.12.032
Mangone M, Scettri P, Paoloni M, Procaccianti R, Spadaro A, Santilli V (2011) Pelvis–shoulder coordination during level walking in patients with ankylosing spondylitis. Gait Posture 34(1):1–5. doi:10.1016/j.gaitpost.2011.02.002
Selles RW, Wagenaar RC, Smit TH, Wuisman PI (2001) Disorders in trunk rotation during walking in patients with low back pain: a dynamical systems approach. Clin Biomech 16(3):175–181. doi:10.1016/S0268-0033(00)00080-2
Seay JF, Van Emmerik RE, Hamill J (2011) Influence of low back pain status on pelvis-trunk coordination during walking and running. Spine (Phila Pa 1976) 36(16):E1070–E1079. doi:10.1097/BRS.0b013e3182015f7c
Yang JH, Suh S, Sung PS, Park W (2013) Asymmetrical gait in adolescents with idiopathic scoliosis. Eur Spine J 22(11):2407–2413. doi:10.1007/s00586-013-2845-y
Mallau S, Bollini G, Jouve JL, Assaiante C (2007) Locomotor skills and balance strategies in adolescents idiopathic scoliosis. Spine (Phila Pa 1976) 32(1):E14–E22. doi:10.1097/01.brs.0000251069.58498.eb
Lao ML, Chow DH, Guo X, Cheng JC, Holmes AD (2008) Impaired dynamic balance control in adolescents with idiopathic scoliosis and abnormal somatosensory evoked potentials. J Pediatr Orthop 28(8):846–849. doi:10.1097/BPO.0b013e31818e1bc9
Kadaba MP, Ramakrishnan H, Wootten M (1990) Measurement of lower extremity kinematics during level walking. J Orthop Res 8(3):383–392. doi:10.1002/jor.1100080310
Beak S, Choi A, Choi S, Oh SE, Mun JH, Yang H, Sim T, Song H (2013) Upper torso and pelvis linear velocity during the downswing of elite golfers. Biomed Eng Online 12:13. doi:10.1186/1475-925X-12-13
Winter DA (2009) Biomechanics and motor control of human movement. Wiley, New York
Kubo M, Ulrich B (2006) Coordination of pelvis-HAT (head, arms and trunk) in anterior–posterior and medio-lateral directions during treadmill gait in preadolescents with/without Down syndrome. Gait Posture 23(4):512–518. doi:10.1016/j.gaitpost.2005.06.007
Hamill J, McDermott WJ, Haddad JM (2000) Issues in quantifying variability from a dynamical systems perspective. J Appl Biomech 16(4):407–418
Schwartz MH, Rozumalski A, Trost JP (2008) The effect of walking speed on the gait of typically developing children. J Biomech 41(8):1639–1650. doi:10.1016/j.jbiomech.2008.03.015
Lay AN, Hass CJ, Gregor RJ (2006) The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. J Biomech 39(9):1621–1628. doi:10.1016/j.jbiomech.2005.05.005
Perry J, Burnfield JM (2010) Gait analysis: normal and pathological function, 2nd edn. Slack Incorporated, New Jersey
Syczewska M, Lukaszewska A, Górak B, Graff K (2006) Changes in gait pattern in patients with scoliosis. Med Rehabil 10(4):12–21
Murray MP, Kory RC, Clarkson BH (1969) Walking patterns in healthy old men. J Gerontol 24(2):169–178
Huang Y, Meijer OG, Lin J, Bruijn SM, Wu W, Lin X, Hu H, Huang C, Shi L, van Dieën JH (2010) The effects of stride length and stride frequency on trunk coordination in human walking. Gait Posture 31(4):444–449. doi:10.1016/j.gaitpost.2010.01.019
van Emmerik REA, Wagenaar R (1996) Effects of walking velocity on relative phase dynamics in the trunk in human walking. J Biomech 29(9):1175–1184. doi:10.1016/0021-9290(95)00128-X
Catanzariti JF, Salomez E, Bruandet JM, Thevenon A (2001) Visual deficiency and scoliosis. Spine (Phila Pa 1976) 26(1):48–52. doi:10.1097/00007632-200101010-00010
Simoneau M, Richer N, Mercier P, Allard P, Teasdale N (2006) Sensory deprivation and balance control in idiopathic scoliosis adolescent. Exp Brain Res 170(4):576–582. doi:10.1007/s00221-005-0246-0
Gauchard GC, Lascombes P, Kuhnast M, Perrin PP (2001) Influence of different types of progressive idiopathic scoliosis on static and dynamic postural control. Spine (Phila Pa 1976) 26(9):1052–1058. doi:10.1097/00007632-200105010-00014
Gracovetsky S (1985) An hypothesis for the role of the spine in human locomotion: a challenge to current thinking. J Biomed Eng 7(3):205–216. doi:10.1016/0141-5425(85)90021-4
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. NRF-2013R1A1A2009495).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
H.-J. Park, T. Sim and S.-W. Suh contributed equally.
Rights and permissions
About this article
Cite this article
Park, HJ., Sim, T., Suh, SW. et al. Analysis of coordination between thoracic and pelvic kinematic movements during gait in adolescents with idiopathic scoliosis. Eur Spine J 25, 385–393 (2016). https://doi.org/10.1007/s00586-015-3931-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-3931-0