Abstract
Purpose
Leakage is the most common complication of percutaneous cement augmentation of the spine. The viscosity of the polymethylmethacrylate (PMMA) cement is strongly correlated with the likelihood of cement leakage. We hypothesized that cement leakage can be reduced by sequential cement injection in a vertebroplasty model.
Methods
A standardized vertebral body substitute model, consisting of aluminum oxide foams coated by acrylic cement with a preformed leakage path, simulating a ventral vein, was developed. Three injection techniques of 6 ml PMMA were assessed: injection in one single step (all-in-one), injection of 1 ml at the first and 5 ml at the second step with 1 min latency in-between (two-step), and sequential injection of 0.5 ml with 1-min latency between the sequences (sequential). Standard PMMA vertebroplasty cement was used; each injection type was tested on ten vertebral body substitute models with two possible leakage paths per model. Leakage was assessed by radiographs using a zonal graduation: intraspongious = no leakage and extracortical = leakage.
Results
The leakage rate was significantly lower in the “sequential” technique (2/20 leakages) followed by “two-step” (15/20) and “all-in-one” (20/20) techniques (p < 0.001). The RR for a cement leakage was 10.0 times higher in the “all-in-one” compared to the “sequential” group (95 % confidence intervals 2.7–37.2; p < 0.001).
Conclusions
The sequential cement injection is a simple approach to minimize the risk for leakage. Taking advantage of the temperature gradient between body and room temperature, it is possible to increase the cement viscosity inside the vertebra while keeping it low in the syringe. Using sequential injection of small cement volumes, further leakage paths are blocked before further injection of the low-viscosity cement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous vertebroplasty (VP) and kyphoplasty (KP) have become important techniques for the treatment of weakened or collapsed vertebrae in osteoporotic and traumatic spinal fractures. However, injection of polymethylmethacrylate (PMMA) cement in these augmentation techniques must be done with caution. There are potentially life-threatening complications that may occur after cement injection into fractured or porous vertebra. The risk of extraosseous cement leakage in various studies ranges between 3.0 and 74.0 % [1–4]. Neurological deficits such as radiculopathy and spinal cord compression have been reported to occur in 0–3.7 and 0–0.5 % of cases, respectively [1–4], whereas the incidence of pulmonary embolism has been reported to lie between 3.5 and 23.0 % [5–7]. The leakage rate in KP is significantly lower compared to VP [1, 8], due to the cavity created by the balloon allowing low-pressure and high-viscosity cement injection. Once the cavity is filled out, the leakage behavior is similar to that of VP [9]. Another adverse effect of early leakage is the subsequent injections of low volumes of cement with inadequate fracture stabilization and less pain reduction [10].
Besides the bone structure of the spine, fracture pattern and the severity of the fracture [11, 12], the low-viscous cement is the major risk factor for cement leakages [13]. The use of high-viscous PMMA cement may reduce the rate of cement leakages significantly [14].
The viscosity of PMMA cements increases over time during the polymerization process, and the rate of polymerization is exponential and strongly temperature dependent (Fig. 1) [15]. To reduce the risk of leakage the cement should be injected at the latest possible time point, which of course reduces the total amount of working time. The use of low-viscous cement allows for a longer cement handling time and makes operation more flexible, but increases the risk for leakage. An injection of very high-viscous cement requires high physical load from surgeon and is limited by human strength [13]. This, in turn, requires high-pressure injection devices, which are more expensive and lack from a tactile feedback. Another undesirable effect of a high-viscous cement is its limited penetration into trabecular bone which may compromise the mechanical strength of the augmented vertebral body [1].
Viscosity versus time curves at different temperatures (from Boger et al. [15])
Cement cures much faster inside the body at 37 °C than extracorporeal at about 20 °C (Fig. 1). Thus, the intracorporeal cement will become highly viscous within a short time while the cement outside the body remains less viscous and injectable even with simple syringe systems. We hypothesized that a sequential injection of small amounts should result in highly viscous cement within the vertebral body, which is less likely to leak out, while the cement in the syringe remains at a lower viscosity and, hence, remains injectable. The aim of this study was to prove experimentally the leakage safety of a sequential cement injection in a standardized vertebroplasty model.
Materials and methods
For this study, a vertebral body substitute leakage model, similar to the one presented by Baroud et al. [14], was used. This is an established experimental vertebroplasty model to investigate the leakage phenomenon. The model consists of aluminum oxide foam coated by acrylic cement. A predefined path, simulating a blood vessel, facilitates cement leakage. The following important adaptations were made to the experimental protocol of Baroud et al. Before the experiment, the vertebral body substitutes were stored at 37° for 24 h, and during the experiment, the cylinders were placed in a water bath at 37 °C to simulate human body conditions. Moreover, the aluminum cylinders were filled with a form-stable bone marrow simulant that is described below.
In the study, regular, commercially available vertebroplasty cement with a medium initial viscosity (Vertecem V+ Cement Kit, Synthes GmbH, Oberdorf, Switzerland) was used. This bone cement, syringes of 2 ml and Jamshidi needles of eight gages (4.2 mm outer diameter, 150 mm long; MD Tech, Gainesville, FL) are the standardly used materials for vertebroplasty.
Study groups
Three injection techniques for 6 ml of bone cement were evaluated: (1) all-in-one—injection in one single step; (2) two-step—injection of 1 ml in 30 s as the first step, and injection of 5 ml as the second step with 1 min of latency in-between; (3) sequential—sequential injection of 0.5 ml with 1 min of latency between each sequence (Fig. 2).
Preparation of the leakage model and the bone marrow substitute
The leakage model consisted of a trabecular bone and a bone marrow substitute. The trabecular bone substitute was made of aluminum oxide foams cut to round shape (Fig. 3). We used a porosity of 20 ppi, which is approximately the porosity of osteoporotic spongious bone. One predefined central drill hole was applied as described in the study of Baroud et al. [14] by the Jamshidi needle.
To act as a bone marrow substitute, a starch mixture, which is stable at 37 °C, was prepared by mixing cornstarch powder (MAIZENA, Knorr-Nährmittel AG, CH-8240 Thayngen, Switzerland) and cold water at a ratio of 1:10. By stirring at room temperature a uniform and homogeneous appearance was achieved. The aluminum bodies got soaked into the fluid while the mixture was heated under constant stirring until it began to get thick and boil. Then, stirring was stopped and the mixture was left on heat for another 1–2 min before heating was stopped and the foam removed. After the mixture cooled down, the foam samples were placed in a refrigerator for 1 h.
To simulate the cortical shell of the vertebral body a thin layer of 3 mm acrylic cement (SCS-Beracryl D-28, Suter-Kunstoffe AG, Fraubrunnen, Switzerland) was attached to the aluminum foam cuts afterwards. Before that, a Jamshidi needle was placed in the central part of the foam at 26 mm depth. After hardening of the Becracyl shell, a predefined 3-mm-wide leakage path was drilled in the front and another one perpendicular to the tip of the Jamshidi needle (Fig. 4).
Cement preparation
The cement was prepared according to the manufacturer’s instructions using a closed mixing device at a room temperature of 20 °C. At the moment when the mixing process was initialized, a stopwatch was started to measure the exact time points during the whole experiment.
Cement injection procedure
30 min prior to and during the cement injection, the models were placed in a 37 °C water bath to definitely reach thermal equilibrium at the simulated body temperature. Injections started 4 min after the initialization of the mixing process for all injection techniques.
For each model, the total amount of 6 ml PMMA cement was injected. First, two 2-ml syringes were used followed by two 1-ml syringes. The injection rate was manually kept constant by the investigator with 2 ml/min for all the three injection techniques (Fig. 5). Each injection technique was tested on ten vertebral body substitutes.
Schematic drawing of the experimental setup. An eight-gage Jamshidi needle is attached to a 2-ml standard syringe. The distal end of the cannula is placed in the bone substitute so that the distance to the 3-mm drill hole that simulates a vein is 5 mm. The tip of the needle is placed 100 mm from the water surface. The water bath temperature is 37 °C and the room temperature is 18 °C. The injection speed is held constant with 2 ml/min
Analysis of leakage behavior
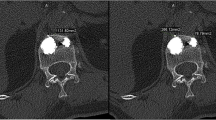
To evaluate the leakage in the models, an X-ray of each model using a C-Arm (SIREMOBIL Iso-C 3D, Siemens AG Medical Solutions, Erlangen, Germany) was made. The leakage was assessed using a zonal graduation: no leakage was defined if cement did not cross the inner border of the cortical shell; the cement crossing the inner border of the cortical shell was defined as leakage (Fig. 6). The two leakage paths per model and ten models per injection technique result in the maximum of 20 possible leakages per injection technique.
The proportions of cement leakages were compared between the injection techniques using the Fisher’s exact test. Relative risk (RR) for a cement leakage was calculated in the groups 1 and 2 in comparison to group 3. α was set to 0.05 throughout the study. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
All-in-one injection technique showed a leakage in both available paths in all models (20 out of 20 possible paths; 100.0 %). Two-step injection techniques resulted in a leakage in 15 out of 20 possible paths (75.0 %). The sequential cement injection showed a leakage in two out of 20 possible paths (10.0 %). These proportions were significantly different (p < 0.001).
The RR for a cement leakage was 10.0 times higher in the group 1 (“all-in-one”) compared to group 3 (“sequential”) [95 % confidence intervals (CI) 2.7–37.2; p < 0.001]. The RR of a cement leakage in group 2 (“two-step”) was still 7.5 times higher compared to group 3 (“sequential”) (95 % CI 2.0–28.6; p < 0.001).
Discussion
The objective of this study was to examine the occurrence of cement leakage of a sequential injection technique of commercially available vertebroplasty cement compared to two standard techniques. To estimate the cement leakage behavior we used a standardized experimental model, similar to the one by Baroud et al. [14].
Several attempts have been made to reduce PMMA leakage in augmentation techniques. Aspiration and lavage have been shown to be feasible in reducing cement leakage in vertebroplasty model [16] as well as in cadaveric and animal studies [17, 18].
It has been shown previously that cement viscosity is one major risk factor for the occurrence of cement leakages [13]. The use of high-viscous PMMA cements was shown to reduce the rate of cement leakages significantly in experimental [14] as well as in clinical [19] studies. To overcome the disadvantages of high-viscous cement injection devices, namely the price and the lack of tactile feedback, we investigated whether it is possible to influence the leakage rate by simply adapting the cement injection technique. In our model, sequential injection of small cement amounts had a significantly lower RR for a cement leakage than “two-step” or “all-in-one” injection techniques.
Knowing that the polymerization of PMMA cement is a radical reaction, which is accelerated at higher temperatures, one can make use of the temperature gradient between body (37 °C) and ambient temperature in the OR (20 °C). A high volume of PMMA cement injected over a short period of time is less likely to harden inside the vertebral body before the subsequently injected cement pushes it further down the path of least resistance. This may explain why the “All in one” injection procedure resulted in 100 % leakage in our model. In contrast, smaller cement amounts can adapt to the body temperature and polymerize between injection sequences. With this technique, it seems to be possible to block potential leakage channels during the injection of low-viscous cement.
Even if the time for the augmentation of one vertebra is prolonged with the sequential technique, the working time of the PMMA cement is long enough to inject the necessary cement volume. In a clinical setting, most often more than one vertebra has to be treated in one session; so waiting may be used for the augmentation for adjacent vertebrae.
Some limitations of the study deserve mention. As pointed out by Baroud et al. [14], the used vertebra model and particularly the “all-in-one” injection technique favor leakage, representing the worst-case scenario. Furthermore, the drill holes of 3 mm simulating the leakage paths are relatively large compared to the diameter a vertebral vein, which is between 0.5 and 2 mm. This has to be regarded as experimental setting. Smaller diameters of the side holes have been shown to prevent cement leakages, whereas greater diameters facilitate them [13]. We tested 2-mm drill holes in a pre-study and observed low leakage rates even using the “all-in-one” cement injection scenario. Therefore, the use of 3-mm drill holes as the “worst-case” scenario was preferred. The bone marrow substitute used in this study, namely cornstarch powder and water in the ratio 1:10, may have been more difficult to displace than normal bone marrow, thereby increasing the risk of cement leakage [13]. Different bone marrow substitutes such as butter, gelatin and cornstarch are used in vertebroplasty models [13, 14]. The main challenge is to find a substitute that is form-stable at 37 °C, which our substitute was. Nevertheless, as we used a standardized model, we suggest that the physical properties of the bone marrow substitute influenced all three injection techniques to a similar extend.
Moreover, vertebral fractures in vivo may have cortical defects that may alter cement flow dynamics, which we have not accounted for.
Furthermore, as we used PMMA cement with certain polymerization properties, other cements may have different properties that surgeons should be aware of.
Finally, we did not assess the potential influence of the starting point of the sequential cement injection that may exist, as the cement polymerization is a continuous process. As the sequential cement injection took 12 min, further cement polymerization took place and may have further reduced the cement leakage rate. However, this does not falsify the comparative results of the studied injection techniques.
Conclusions
The sequential cement augmentation is a safe method to reduce the risk for PMMA leakage in the vertebral body model. Sequential injection of small amounts of cement allows making use of the temperature-dependent and non-linear properties of PMMA polymerization without increasing the waiting time or decreasing the working time. Using the temperature gradient between body and room temperature accelerates the polymerization progress of the PMMA cement within vertebral body. Using sequential injection of small cement amounts possible leakage paths can be blocked before reinjection of the low-viscous cement.
References
Hulme PA, Krebs J, Ferguson SJ, Berlemann U (2006) Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine 31(17):1983–2001
Ryu KS, Park CK, Kim MC, Kang JK (2002) Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg 96(1 Suppl):56–61
Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE (1997) Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 18(10):1897–1904
Klazen CA, Lohle PN, de Vries J et al (2010) Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (vertos II): an open-label randomised trial. Lancet 376(9746):1085–1092
Choe DH, Marom EM, Ahrar K, Truong MT, Madewell JE (2004) Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. AJR Am J Roentgenol 183(4):1097–1102
Seo JS, Kim YJ, Choi BW, Kim TH, Choe KO (2005) MDCT of pulmonary embolism after percutaneous vertebroplasty. AJR Am J Roentgenol 184(4):1364–1365
Duran C, Sirvanci M, Aydogan M, Ozturk E, Ozturk C, Akman C (2007) Pulmonary cement embolism: a complication of percutaneous vertebroplasty. Acta Radiol 48(8):854–859
Papanastassiou ID, Phillips FM, Van Meirhaeghe J et al (2012) Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 21(9):1826–1843
Berlemann U, Franz T, Orler R, Heini PF (2004) Kyphoplasty for treatment of osteoporotic vertebral fractures: a prospective non-randomized study. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 13(6):496–501
Roder C, Boszczyk B, Perler G, Aghayev E, Kulling F, Maestretti G (2013) Cement volume is the most important modifiable predictor for pain relief in BKP: results from SWISSspine, a nationwide registry. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 22(10):2241–2248
Tome-Bermejo F, Pinera AR, Duran-Alvarez C, Lopez-San Roman B, Mahillo I, Alvarez L (2014) Identification of risk factors for the occurrence of cement leakage during percutaneous vertebroplasty for painful osteoporotic or malignant vertebral fracture. Spine 39(11):E693–E700
Nieuwenhuijse MJ, Van Erkel AR, Dijkstra PD (2011) Cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: identification of risk factors. Spine J Off J N Am Spine Soc 11(9):839–848
Bohner M, Gasser B, Baroud G, Heini P (2003) Theoretical and experimental model to describe the injection of a polymethylmethacrylate cement into a porous structure. Biomaterials 24(16):2721–2730
Baroud G, Crookshank M, Bohner M (2006) High-viscosity cement significantly enhances uniformity of cement filling in vertebroplasty: an experimental model and study on cement leakage. Spine 31(22):2562–2568
Boger A, Wheeler KD, Schenk B, Heini PF (2009) Clinical investigations of polymethylmethacrylate cement viscosity during vertebroplasty and related in vitro measurements. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 18(9):1272–1278
Mohamed R, Silbermann C, Ahmari A, Bohner M, Becker S, Baroud G (2010) Cement filling control and bone marrow removal in vertebral body augmentation by unipedicular aspiration technique: an experimental study using leakage model. Spine 35(3):353–360
Benneker LM, Heini PF, Suhm N, Gisep A (2008) The effect of pulsed jet lavage in vertebroplasty on injection forces of polymethylmethacrylate bone cement, material distribution, and potential fat embolism: a cadaver study. Spine 33(23):E906–E910
Benneker LM, Krebs J, Boner V et al (2010) Cardiovascular changes after PMMA vertebroplasty in sheep: the effect of bone marrow removal using pulsed jet-lavage. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 19(11):1913–1920
Georgy BA (2010) Clinical experience with high-viscosity cements for percutaneous vertebral body augmentation: occurrence, degree, and location of cement leakage compared with kyphoplasty. AJNR Am J Neuroradiol 31(3):504–508
Acknowledgments
We thank Synthes GmbH, Oberdorf, Switzerland for providing PMMA cement for the study.
Conflict of interest
The authors have no commercial associations or sources of support that might pose a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sven Hoppe and Sebastian Wangler have contributed equally to the manuscript and share first authorship.
Rights and permissions
About this article
Cite this article
Hoppe, S., Wangler, S., Aghayev, E. et al. Reduction of cement leakage by sequential PMMA application in a vertebroplasty model. Eur Spine J 25, 3450–3455 (2016). https://doi.org/10.1007/s00586-015-3920-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-3920-3