Abstract
Purpose
Lumbar fusion has been shown to be effective in treating a variety of degenerative spinal conditions, though significant differences exist in the magnitude of clinical improvement across different surgical diagnoses. With modern, minimally disruptive approaches for fusion, diagnosis-specific differences in clinical improvement may be reduced. The purpose of this study is to report and compare interim clinical improvements in patients treated with XLIF for various degenerative lumbar conditions.
Methods
160 patients underwent XLIF for either degenerative spondylolisthesis (n = 68), degenerative disc disease (n = 20), adjacent segment disease (n = 26), or post-laminectomy syndrome (n = 46). Average age was 61 years and 66 % were female. Mean BMI was 28.9 kg/m2. 37 % were smokers, 23 % had diabetes mellitus, 22 % had depression. Mean age was highest for ASD patients (66 years) and lowest for DDD patients (48 years) (p < 0.001). There were no other baseline demographic differences between groups. Patient-reported clinical outcomes measures were collected at baseline and prospectively at standard intervals. Interim results at an average of 19 months follow-up are reported here.
Results
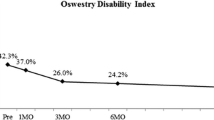
In total, 197 levels were treated with XLIF (mean 1.2 per patient). There were no cases of symptomatic pseudoarthrosis or implant/instrument failure. Overall, 1 patient (0.6 %) had a major complication and 12 % had a minor complication. Approach-related anterolateral thigh/groin sensory changes were present in 14 % and hip flexion weakness in 9 %. At last follow-up, overall ODI decreased 47 % (44.1–23.5), VAS LBP decreased 59 % (6.9–2.8), VAS LP decreased 56 % (7.1–3.1), and SF-36 PCS improved 40 % (30.9–43.2) (all p < 0.001). Baseline ODI was significantly lower for DDD patients (p = 0.052). At last follow-up, mean percent improvements on all outcomes were highest for DSP group, though not all differences were significant. Improvements between diagnostic groups were statistically different for LBP (p = 0.021), but were similar for all other clinical outcomes. Percentage of patients reaching MCID and SCB thresholds ranged from 60 to 95 % in clinical outcomes. Patient satisfaction for the entire group was 93 % when asked whether satisfied with surgical outcome.
Conclusions
XLIF has been demonstrated in the current series to lead to significant improvements in clinical outcomes and high rates of MCID and SCB and reduce the discrepancy in outcomes between well accepted and technically challenging indications compared to traditional open approaches for IBF. Complication rates were low, with only one patient in the series experiencing a major complication. Further investigation with larger cohorts and longer follow-up is warranted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lumbar interbody fusion can be an effective treatment for a variety of degenerative spinal conditions and pathologies [1–8]. However, significant discrepancies in the magnitude of postoperative clinical improvement can exist between different surgical diagnoses [4]. Historically, patients with degenerative spondylolisthesis (DSP) have been reported to have greater improvements in pain and disability after lumbar fusion compared to those with degenerative disc disease (DDD), failed-back or post-laminectomy syndrome (PLS), and degenerative changes adjacent to a prior fusion (adjacent segment disease, ASD) [4]. In addition, for “revision” indications such as ASD and PLS, subsequent surgeries may associate with greater technical challenges and higher risk of postoperative complications, likely due to the presence of scar tissue and muscle denervation associated with traditional, open posterior approaches [9].
Alternatively, modern, less invasive approaches for lumbar interbody fusion have gained in popularity, one such approach being the mini-open lateral transpsoas approach (XLIF®, NuVasive, Inc., San Diego, CA) [10]. Benefits of the lateral approach include the preservation of bony and ligamentous structures and also allows for the placement of a large intervertebral cage [11–16]. These advantages may translate to added clinical improvement for less well established and revision indications, thereby addressing the shortcomings of open, traditional approaches, particularly in treating patients with prior lumbar surgery. Direct comparisons of complications and clinical outcomes stratified by preoperative diagnosis specifically following in lateral fusion, however, are heretofore unreported. Thus, the purpose of this study was to investigate potential discrepancies in clinical improvements in patients treated with XLIF for DSP, DDD, ASD, and PLS. Results at an average of 18.5 months are reported here.
Materials and methods
Data were collected at a single institution as part of an institutional review board (IRB) approved prospective registry. Between 2008 and 2012, 187 patients were treated with XLIF between L1-4 at a single institution. Of those, 160 were included in the current study and were grouped by primary diagnosis into DDD (n = 20), DSP (n = 68), ASD (n = 26), and PLS (n = 46) cohorts. The remaining 27 patients had scoliosis, tumor, vertebral body fracture, disctitis, or pseudoarthrosis and were excluded from this study. Patients treated outside of L1-5 levels were also excluded, and treatment for L5-6 was decided on a case-by-case basis.
Patients with a primary diagnosis of DSP had no history of prior lumbar surgery, included Grade 1 and II slips, and were exclusive of isthmic spondylolisthesis. Patients in the DDD group showed radiographic evidence of intervertebral disc desiccation, greater than 50 % disc space collapse, and/or modic endplate changes, without sagittal plane deformity. Of the 20 patients in this group, 18 (90 %) had preoperative leg pain in addition to back pain, of which 15 (75 %) had leg pain greater than or equal to back pain. ASD patients had symptomatic instability, listhesis, and/or disc degeneration at the level adjacent to a previous fusion. PLS patients had either symptomatic recurrent disc herniation, instability or listhesis, and/or disc degeneration at the index level of a previous laminectomy or microdiscectomy.
Data pertaining to demographic, treatment, complication, and reoperation variables were collected through retrospective chart review. Clinical outcomes, which included the Oswestry Disability Index (ODI), numeric rating scale (NRS) for low back pain (LBP) and leg pain (LP), and short form-36 (SF-36) physical component (PCS) and mental component (MCS) were collected preoperatively and prospectively at intervals of 1, 3, 6, 12, and 24 months postoperative. Thresholds for determining minimal clinically important difference (MCID) and substantial clinical benefit (SCB) were previously defined by Copay et al. [17] and Glassman et al. [18], respectively (Table 1).
Complication data was collected perioperatively, and at regular follow-up intervals. Retrospective chart review was performed to confirm accuracy. Major and minor complications were classified according to previously proposed guidelines by Glassman et al. [19]. Postoperative approach-related thigh/groin sensory changes and/or hip flexion weakness, both known phenomena following XLIF [16, 20] were considered complications in presence of clear neurologic deficit or if hospital readmission or surgical intervention was required. Otherwise, cases of transient nerve-related pain or mechanical hip flexion weakness were classified as “side effects.”
Statistical analyses included frequency tests and Chi squared/Fishers’ Exact tests. One-way ANOVA and post hoc Tukey’s Range test for pairwise comparisons were also performed. Clinical improvements were analyzed as repeated measures using generalized linear mixed models with compound symmetric covariance structures. All statistical analyses were performed using JMP v11 (SAS Institute, Cary, NC) and significance was accepted at the 0.05 level.
Results
Average follow-up for the 160 patients was 18.5 months. Mean patient age was 60.6 years and 66 % were female. Mean body mass index (BMI) was 28.0 kg/m2. Thirty-seven percent (37 %) of patients were smokers, 23 % had diabetes mellitus, and 22 % had depression (self-reported). Preoperative diagnosis was DSP for 68 patients, DDD for 20 patients, ASD for 26 patients, and PLS for the remaining 46. Mean age was highest for patients in the ASD group (66 years) and lowest for those in the DDD group (48 years) (p < 0.001). There were no other baseline demographic differences between groups (Table 2).
In total, 197 levels in 160 patients (mean 1.2, range 1–3) were treated with XLIF. The most common levels treated were L4-5 and L3-4, in 114 (71 %) and 49 patients (31 %), respectively. Direct posterior decompressions were performed in 65 (41 %) cases. Supplemental percutaneous posterior fixation was used in 140 (88 %) cases. There were significantly more patients in the DSP group (100 %) who underwent an additional posterior procedure (decompression, fixation, or both) compared to those in the DDD group (60 %).
Mean operative time (ORT) for all patients was 171 min, mean blood loss (EBL) was 73 mL, and postoperative length of stay (LOS) was 1.3 days. Complete treatment information stratified by diagnosis is presented in Table 3.
There were no cases of symptomatic pseudoarthrosis or hardware failure. For the entire study group, 1 patient (0.6 %) had a major complication and 19 patients (12 %) had a minor complication. Approach-related anterolateral thigh/groin sensory changes were present in 22 patients (14 %) and hip flexion weakness was present in 14 patients (9 %). Both these side effects were transient, resolving between 10 days and 6 months postoperative without additional intervention or sequelae. There were no incidences of femoral neuropathy. Minor complication rate was highest for ASD (19 %) and PLS (17 %) patients. There were no statistically significant differences between incidences in complications or approach-related side effects between diagnosis groups (Table 4). Complications and approach-related side effects are listed in Table 5.
From preoperative to last follow-up, overall ODI decreased 47 % from 44.1 to 23.5. LBP decreased 59 % from 6.9 to 2.8 and LP decreased 56 % from 7.1 to 3.1. SF-36 PCS improved 40 % from 30.9 to 43.2 at last follow-up. All postoperative clinical improvements were statistically significant.
Subgroup comparisons by diagnosis revealed baseline ODI was significantly lower for DDD patients (p = 0.052), with no other differences in clinical scores between diagnoses at baseline. At last follow-up, mean percent improvements on all outcomes were highest for DSP group, though not all differences were significant. Improvements between diagnostic groups were statistically different for LBP (p = 0.021), but were similar for all other clinical outcomes (Table 6).
Using previously published definitions for MCID, percentage of patients reaching the threshold ranged from 60 to 95 % in clinical outcomes. While none of the percentage of patients reaching MCID on each outcome statistically differed between groups, DSP patients had the highest rate on all measures, while ASD patients had the lowest rate on ODI, LBP, and LP. Percentage of patients reaching SCB were statistically different between groups on ODI (p = 0.029) and PCS (p = 0.022). For ODI, DSP patients had the highest rate of reaching SCB (78 %) while 60 % of ASD patients reached the threshold. Similarly for LBP, 93 % of DSP patients reached SCB, while 71 % of ASD patients reached the threshold. Complete MCID and SCB percentages stratified by diagnosis are presented in Table 7.
Patient satisfaction for the entire group was 93 % when asked whether satisfied with surgical outcome. Additionally, 93 % of patients also indicated they would do the surgery again, given their current outcome. Patient satisfaction was not statistically different between groups for either satisfaction with surgical outcome or willingness to undergo the same procedure. Eighty-eight percent (88 %) of ASD, 90 % of DDD patients, 91 % of PLS patients, and 97 % of DSP patients indicated satisfaction with surgical outcome (p = 0.139). Eighty-eight percent (88 %) of ASD patients, 95 % of DDD patients, 93 % of PLS patients, and 94 % of DSP patients indicated they would do the surgery again (p = 0.083).
Discussion
Degenerative spondylolisthesis (DSP) is one of the most responsive diagnoses to lumbar fusion. In addition to high rates of improvements on multiple clinical outcome measures, high rates of patients reaching meaningful clinical improvement thresholds such as MCID have also been reported [4]. This is likely due to the ability of interbody fusion to correct the listhesis through realignment and stabilization. However, indications for fusion other than DSP have been shown to have relatively attenuated clinical improvements [7, 8, 21].
DDD with chronic low back pain, in particular, has been scrutinized due to its diagnostic complexity and outcome variability from within the published literature [21]. Clinical improvement ranging from moderate to high have been reported in several randomized clinical trials for a variety of fusion procedures in the treatment of DDD, with LBP improvement ranging from 22 to 77 % and ODI improvement ranging from 19 to 79 % [21].
Few studies have reported outcomes specifically for DDD patient cohorts following XLIF. Marchi et al. [22] reported in a series of 22 patients treated with XLIF a fusion rate of 93 % with a 70 % improvement in back pain and a 53 % improvement in ODI. Patients in their series also experience short operative times (average 72 min), low EBL (50 mL), and LOS (1 day), all comparable to the results of the current studies. Additionally, Berjano et al. [23] reported on the clinical results of a series of 97 patients, 80 % of which were classified as DDD. In their study, clinical improvement was reported as 61.3 %, leg pain improved 64 %, and ODI improved 55 %.
In the current series of DDD patients treated with XLIF, improvements on clinical outcomes at last follow-up ranged from 46 % on ODI, 65 % on LBP, 55 % on LP, and 45 % on PCS, all of which were statistically similar to those observed in DSP patients. Furthermore, there were no significant differences between the percentage of DDD patients and DSP patients reaching MCID on any clinical outcome measures. The authors hypothesize that this reduced discrepancy in clinical improvement between these two diagnoses may not be solely attributed to a minimally invasive surgical approach, but also to careful patient selection and preoperative evaluation of both radiographic and clinical symptoms. DDD patients selected for XLIF in the current series all exhibited significant disc space collapse, Modic endplate changes, as well as central, lateral recess, and/or foraminal stenosis. Clinically, 90 % of DDD patients in the current series had preoperative radicular pain in additional to back pain and all had failed previous conservative treatment.
Similarly, “revision” indications such as ASD and PLS treated through posterior approaches but can also be associated with significant morbidity due to scar tissue formation and injury to posterior bony structures and musculature [24]. In these cases, the lateral approach allows for the avoidance of scar tissue by allowing access to the spine through a “virgin” anatomic plane and preventing further paraspinal muscle atrophy and fibrosis.
In addition to anatomic and biomechanical advantages of the lateral approach for “revision” indications, clinical outcomes also compare favorably against those reported for open or posterior approaches. Djurasovic et al. [25] previously reported results of clinical improvement in following traditional anterior, posterior, or combined approaches for lumbar fusion in a series of 91 post-decompression patients and 42 ASD patients. For ASD patients at 1year postoperative, LBP improved 26 % (8.0–5.9), LP improved 19 % (7.3–5.9), ODI improved 19 % (55.1–44.4), and PCS improved 7 % (27.2–29.2). For post-decompression patients at 1-year postoperative, LBP improved 31 % (7.4–5.1), LP improved 32 % (7.5–5.1), ODI improved 28 % (54.9–39.5), and PCS improved 21 % (26.1–31.7). In comparison, improvements on these four clinical outcomes in the current series of ASD and PLS patients ranged between 36–43 % and 45–56 %, respectively.
Further comparisons of percentage reaching MCID thresholds were also favorable for patients in the current series treated with XLIF. At 1-year postoperative, Djurasovic et al. [25] reported 42 and 52 % of ASD and post-decompression patients, respectively, reaching MCID on ODI, compared to 60 and 69 % of ASD and PLS patients, respectively, in the current series. Similarly for PCS, 37 % of ASD and 38 % of post-decompression patients reached MCID in Djurasovic’s series compared to 65 % of ASD and 72 % of PLS patients in the current series.
In contextualizing our stratified results against those reported for fusion via traditional posterior approaches, we directly compared our study against Glassman et al.’s [4] stratification study for clinical outcomes following open, traditional approach for posterolateral fusion (PLF) in a series of 327 patients. To our knowledge, this study is among the highest quality literature that reports direct comparisons between diagnoses groups. The study included four diagnostic groups similar to those in the current study: DDD, ASD, PLS, and SP. Descriptions noted in the methodology section of Glassman’s paper described DDD, ASD, and PLS groups to have similar classification guidelines as the current study. However, for Glassman’s SP group, both degenerative and isthmic spondylolisthesis were included, while the current study only examined the degenerative type.
At last follow-up, net score changes were higher for patients in the current series compared to their corresponding groups in Glassman’s series, with the exception of ODI for the DDD groups (Fig. 1). SP patients in both studies experienced the highest improvements on all outcome measures. Particularly of note is the discrepancy in improvement in the “revision” groups, ASD and PLS. Patients in these groups who underwent XLIF had consistently higher improvements on all clinical outcomes compared to those in Glassman’s study who underwent a PLF. In the ASD group, net score improvement on ODI (19.7 vs. 10.0), LBP (2.9 vs. 1.6), and PCS (10.0 vs. 4.0) for patients in the current series were almost double that of net score improvements in Glassman’s group, while LP (3.6 vs. 1.2) improvement for patients in the current series was three times that of Glassman’s group. In addition, the disparity in percentage of patients reaching MCID across diagnostic indications was reduced for those in the current series (Fig. 2). In the current series of patients treated with XLIF, percentage of ASD, DDD, and PLS patients reaching MCID in LP ranged between 70 and 80 %, compared to 82 % of DSP patients. In contrast, Glassman et al. reported between 38 and 58 % of ASD, DDD, and PLS patients reached MCID, compared with 74 % of SP patients.
a–d Comparison of mean change from baseline between patients in Glassman et al.’s [4] series and patients in the current series. ASD adjacent segment disease, DDD degenerative disc disease, PLS post-laminectomy syndrome, DSP degenerative spondylolisthesis, ODI Oswestry Disability Index, LBP low back pain, LP leg pain, PCS physical component score
a–d Comparison of minimum clinically important difference (MCID) differences between Glassman et al.’s [4] series and patients in the current series. ASD adjacent segment disease, DDD degenerative disc disease, PLS post-laminectomy syndrome, DSP degenerative spondylolisthesis, MCID minimum clinically important difference, ODI Oswestry Disability Index, LBP low back pain, LP leg pain, PCS physical component score
Excluding patients with transient, approach-related side effects, percentage of patients with any complication in this series was 12 %, with <1 % classified as a major. In comparison, Glassman reported a 35 % complication rate, including a 45 % rate in the SP group, a 40 % rate in the PLS group, a 40 % rate in the ASD group, and a 9 % rate in the DP group. In addition, the major complication rates in these groups ranged from 3 to 15 %, with the highest rate observed in SP patients. Revision surgery was also required for 21 (10 %) patients in these four diagnostic groups, including surgery for nonunion and implant removals, whereas there were no patients in the current study who required a return to operating room.
To our knowledge, the current study represents one of the first comparisons of SCB and MCID between diagnostic groups. These two measures provide great value for contextualizing clinical improvements by describing both the percentage of patients reaching a minimum improvement and patients who can be considered a clinical success.
Regarding the weaknesses of the current study, while the overall study cohort is fairly large, individual group sizes were still relatively small. In addition, classification of the indication groups were straightforward in cases such as spondylolisthesis or isolated one-level disc degeneration in patients with no prior lumbar surgery, but guidelines were less clear for ASD and PLS groups. In instances where there was ambiguity regarding the most appropriate classification, best judgment was taken to assign according to primary indication for surgery. Finally, as this is an interim report of clinical outcomes, long-term conclusions on efficacy and maintenance of improvement are yet to be determined.
Conclusion
While lumbar IBF is generally well accepted for indications such as spondylolisthesis, consensus regarding its utility for more controversial indications such as DDD, or for more technically challenging indications such as PLS, and ASD are more variable. XLIF has been demonstrated in the current series to lead to significant improvements in clinical outcomes and reduces the discrepancy in outcomes between well accepted, controversial, and technically challenging indications compared to traditional open approaches for IBF. Additionally, high rates of patients from all diagnosis groups reached MCID and SCB thresholds. Complication rates were low, with only one patient in the series experiencing a major complication. Further investigation with larger cohorts and longer follow-up is warranted.
References
Anand N, Hamilton JF, Perri B, Miraliakbar H, Goldstein T (2006) Cantilever TLIF with structural allograft and RhBMP2 for correction and maintenance of segmental sagittal lordosis: long-term clinical, radiographic, and functional outcome. Spine 31:E748–E753
Blumenthal S, McAfee PC, Guyer RD et al (2005) A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine 30:1565–1575
Delamarter R, Zigler JE, Balderston RA, Cammisa FP, Goldstein JA, Spivak JM (2011) Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement compared with circumferential arthrodesis for the treatment of two-level lumbar degenerative disc disease: results at twenty-four months. J Bone Joint Surg Am 93:705–715
Glassman SD, Carreon LY, Djurasovic M et al (2009) Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J 9:13–21
Rampersaud YR, Gray R, Lewis SJ, Massicotte EM, Fehlings MG (2011) Cost-utility analysis of posterior minimally invasive fusion compared with conventional open fusion for lumbar spondylolisthesis. SAS J 5:29–35
Sasso RC, Kitchel SH, Dawson EG (2004) A prospective, randomized controlled clinical trial of anterior lumbar interbody fusion using a titanium cylindrical threaded fusion device. Spine 29:113–122
Tosteson AN, Skinner JS, Tosteson TD et al (2008) The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT). Spine 33:2108–2115
Weinstein JN, Lurie JD, Tosteson TD et al (2009) Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. Four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 91:1295–1304
Huntsman K (2013) XLIF for Adjacent level degeneration. In: Goodrich J, Volcan I (eds) Extreme lumbar interbody fusion (XLIF). Quality Medical Publishing (QMP), St. Louis, pp 317–323
Ozgur BM, Aryan HE, Pimenta L, Taylor WR (2006) Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 6:435–443
Peterson M, Youssef JA (2013) Extreme lateral interbody fusion (XLIF): lumbar surgical technique. In: Goodrich J, Volcan I (eds) Extreme Lateral Interbody Fusion (XLIF). Quality Medical Publishing (QMP), St. Louis, pp 159–178
Berjano P, Lamartina C (2011) Minimally invasive lateral transpsoas approach with advanced neurophysiologic monitoring for lumbar interbody fusion. Eur Spine J 20:1584–1586
Berjano P, Damilano M, Lamartina C (2012) Sagittal alignment correction and reconstruction of lumbar post-traumatic kyphosis via MIS lateral approach. Eur Spine J 21:2718–2720
Berjano P, Lamartina C (2013) Far lateral approaches (XLIF) in adult scoliosis. Eur Spine J 22(Suppl 2):S242–S253
Hu WK, He SS, Zhang SC et al (2011) An MRI study of psoas major and abdominal large vessels with respect to the X/DLIF approach. Eur Spine J 20:557–562
Pumberger M, Hughes AP, Huang RR, Sama AA, Cammisa FP, Girardi FP (2012) Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J 21:1192–1199
Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY (2008) Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J 8:968–974
Glassman SD, Copay AG, Berven SH, Polly DW, Subach BR, Carreon LY (2008) Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am 90:1839–1847
Glassman SD, Hamill CL, Bridwell KH, Schwab FJ, Dimar JR, Lowe TG (2007) The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine 32:2764–2770
Tohmeh AG, Rodgers WB, Peterson MD (2011) Dynamically evoked, discrete-threshold electromyography in the extreme lateral interbody fusion approach. J Neurosurg Spine 14:31–37
Phillips FM, Slosar PJ, Youssef JA, Andersson G, Papatheofanis F (2013) Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine 38:E409–E422
Marchi L, Oliveira L, Amaral R et al (2012) Lateral interbody fusion for treatment of discogenic low back pain: minimally invasive surgical techniques. Adv Orthop 2012:282068
Berjano P, Balsano M, Buric J, Petruzzi M, Lamartina C (2012) Direct lateral access lumbar and thoracolumbar fusion: preliminary results. Eur Spine J 21(Suppl 1):S37–S42
Rodgers WB, Cox CS, Gerber EJ (2009) Minimally invasive treatment (XLIF) of adjacent segment disease after prior lumbar fusions. Internet J Minim Invasive Spinal Technol 3(4):1–7
Djurasovic M, Glassman SD, Howard JM, Copay AG, Carreon LY (2011) Health-related quality of life improvements in patients undergoing lumbar spinal fusion as a revision surgery. Spine 36:269–276
Conflict of interest
Dr. Khajavi is a consultant for and has received research funds from NuVasive, Inc, though none related to the current work. Alessandria Shen, following her position at Georgia Spine and Neurosurgery and INSPIRE became employed directly by NuVasive, Inc. for a short time. She currently has no conflicts to report. No other authors have any conflicts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khajavi, K., Shen, A., Lagina, M. et al. Comparison of clinical outcomes following minimally invasive lateral interbody fusion stratified by preoperative diagnosis. Eur Spine J 24 (Suppl 3), 322–330 (2015). https://doi.org/10.1007/s00586-015-3840-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-3840-2