Abstract
Cadmium (Cd), a non-redox metal, has been described as an environmental toxicant that poses a great threat to human health. Therefore, this study aimed to appraise the mitigating potential of the aqueous extract of Bridelia ferruginea (Bf) stem bark against hepato-renal toxicity in Cd-exposed Wistar rats. Thirty (30) adult male Wistar rats (weight 150–200 g) were randomly grouped into five (5) groups of six (6) rats each (n = 6), and experimental rats received a single treatment of cadmium chloride (CdCl2; 30 mg/kg body weight (bw)). Cd-exposed rats were administered 50, 100, and 200 mg/kg bw aqueous extract of Bf stem bark for 14 days. Cd exposure caused hepato-renal toxicity in the untreated control as revealed by a significant (p < 0.05) increase in the serum liver function markers (i.e., AST, ALT, and ALP) as well as a marked (p < 0.05) decrease in hepatic antioxidant enzymes. A similar effect was also noticed in the renal function biomarkers (i.e., creatinine and urea) and its antioxidant enzymes. However, Cd-exposed groups–administered aqueous extract of Bf stem bark revealed a significant (p < 0.05) decrease in serum activities of AST, ALP, and ALT, as well as a significant (p > 0.05) difference in TP level compared to Cd-induced hepato-renal toxicity untreated and normal control. Similarly, hepatic and renal antioxidant parameters (SOD, CAT, and GSH), TP, serum lipid profile, urea, and creatinine were significantly (p < 0.05) improved among the groups-administered aqueous extract of Bf stem bark compared to Cd-induced hepato-renal toxicity untreated and normal control. The renal and hepatic Cd concentrations of groups administered aqueous extract of Bf stem bark significant (p < 0.05) decrease compared to Cd-induced hepato-renal toxicity untreated and normal control. Likewise, histological examinations of kidney and liver tissues of the groups administered aqueous extract of Bf stem bark revealed restoration of normal architecture. Therefore, aqueous extract of Bf stem bark could be suggested to be hepatoprotective and renoprotective in Cd toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals are naturally present in the soils as natural components; however, currently, their presence in the environment has been increased due to several human activities such as mining, fuel processing, electroplating, spent-water treatment, nuclear fuels, and agricultural wastes (Reimann and Caritat 2005). Bioaccumulation of these metallic ions via food chain exposure route is considered injurious to microorganisms, plants, animals, and humans (Vinodhini and Narayanan 2008; Pratush et al. 2018). Nevertheless, there are essential heavy metals needed in certain amounts for biochemical and physiological functions in plants and animals (Jaishankar et al. 2014).
Toxicities of heavy metals like As, Cd, Hg, Pb, Cr, and Se have been reported to perturb several cell components such as cell membrane, mitochondrial, lysosome, endoplasmic reticulum, nuclei, and some enzymes involved in metabolism and detoxification (Wang and Shi 2001; Beyersmann and Hartwig 2008). Several studies have demonstrated that the production of reactive oxygen species (ROS) and ultimately, oxidative stress plays a key role in the toxicity and carcinogenicity of these heavy metal ions (Ercal et al. 2001).
Cd, in particular, is extremely toxic to humans as well as plants (Benavides et al. 2005; Shahid et al. 2016). Cd toxicity has been reported in different ways in the past century (Godt et al. 2006). It specifically affects a broad spectrum of cellular activities such as cell proliferation, differentiation, and apoptosis by interfering with the DNA repair mechanism via the generation of reaction oxygen species (ROS) (Rani et al. 2014; Branca et al. 2018; Erboga and Kanter 2016; Rech et al. 2014). The major sources of human exposure to Cd are the consumption of contaminated food and drinking water (Satarug et al. 2010). Cd toxicity has been associated with serious poisoning effects on tissues like the heart, kidney, liver, and lung (Zhang et al. 2020a). In addition, Cd competes with some essential metals such as Zn, Cu, Se, and Ca, thereby interfering with various cellular processes such as metal membrane transport and energy metabolism (Kar and Patra 2021). At very low concentrations, Cd binds to the mitochondria and causes inhibition of both the cellular respiration and oxidative phosphorylation (Thévenod 2010).
The use of plant parts to treat human or animal ailments is now on the increase with herbal medicine being a major form of medicine in Africa (Agra et al. 2007). The report shows that natural plants are good sources of secondary metabolites that protect the body against the damaging effects of ROS (Afolabi et al. 2018; Oloyede and Babalola 2012). Bridelia ferruginea (Bf), family Euphorbiaceae, is commonly found in the Savannah regions (Ekanem et al. 2008). Its other names are Kizni (Hausa), Marehi (Fulani), Iralodan (Yoruba), Ola (Igbo), and Kensange abia (Boki). Study shows Bf to be rich sources of secondary metabolites (Adesina and Akomolafe, 2014), known to exhibit anti-inflammatory (Olajide et al. 2000), anti-oxidative (Obafemi et al. 2019; Jaiyesimi et al. 2020), anti-microbial activities (Talla et al. 2002), etc. On the other hand, the stem bark component of Bf has also been reported to demonstrate similar activities in vivo and in vitro (Ndukwe et al. 2005; Olajide et al. 1999). Therefore, the current study aimed at appraising the protective effect of the aqueous extract of Bf stem bark on tissues such as kidneys and liver in cadmium-induced toxicity in Wistar rats.
Materials and methods
Chemicals and reagents required
Chemicals used such as cadmium chloride (CdCl2), adrenaline, and Ellman’s reagent (5,5′-dithiobis (2-nitrobenzoic acid), DTNB) were procured from Sigma-Aldrich, Inc., (Saint Louis, MO). All other kits used for biochemical assays were purchased from Randox Laboratory Ltd., Crumlin, Co. Antrin UK. All other chemicals and reagents were of analytical grades and were prepared in all-glass apparatus.
Sample collection
The fresh stem bark of Bf was collected from Ureje farmland, Ado-Ekiti, Ekiti State, Nigeria. A taxonomist at the Plant Science and Biotechnology Department, Faculty of Science, Ekiti State University, Ado-Ekiti, Nigeria, carried out botanical identification and authentication. A voucher specimen (Herbarium number: UHAE2020039) was deposited in the herbarium of the department.
Preparation of extract
The fresh stem bark of Bf was shade-dried for 14 days and the dried materials were ground to a fine powder using an automated blender. Thereafter, a fine powdery sample (5 g) was soaked in 100-ml distilled water for 24 h (w/v %). The mixture was filtered, and the filtrate was stored in the refrigerator before and after daily treatment.
Animal treatment
Thirty (30) adult male Wistar rats (weight 150–200 g) were used in this study. Animals were obtained from one Omisanjana animal house, Ado-Ekiti, Ekiti State, Nigeria. The rats were acclimatized under the humane condition at 25 °C with a light–dark cycle for 7 days, prior to the commencement of the study that lasted for 14 days with free access to a standard pelletized animal feed and water ad libitum.
Induction of cadmium toxicity
A modified protocol by Andjelkovic et al. (2019) was used for the induction of Cd-induced hepato-renal toxicity in the rats. All Cd-induced hepato-renal toxicity groups received a single treatment (CdCl; 30 mg/kg bw). However, 24-h post-treatment observation was considered prior to oral administration of graded doses of Bf extract (i.e., 50, 100, 200 mg/kg).
Animal grouping
Thirty (30) adult male Wistar rats used in this study were randomly divided into five treatment groups of six (6) rats are as follows:
-
(i)
Group 1: Normal control exposed to distilled water for 14 days
-
(ii)
Group 2: Cadmium-induced untreated control
-
(iii)
Group 3: Cadmium-induced + 50 mg/kg bw aqueous extract of Bf stem bark
-
(iv)
Group 4: Cadmium-induced + 100 mg/kg bw aqueous extract of Bf stem bark
-
(v)
Group 5: Cadmium-induced + 200 mg/kg bw aqueous extract of Bf stem bark
Preparation of blood sample
After 14 days of treatment, animals were euthanized and dissected following a mild exposure to diethyl-ether. Tissues (liver and kidneys) were briefly removed and placed on ice. Blood samples were immediately collected by direct heart puncture into both plains. The blood samples were centrifuged at 3000 rpm for 10 min to obtain the sera used. The sera were separated and kept (−20 °C) for various biochemical analyses while the residual tissues were discarded. In addition, organs such as kidneys and liver were carefully isolated and homogenized in preserved for bioassays and histopathological examination.
Preparation of tissue homogenates
Tissues such as the liver and kidney were subsequently dissected and rinsed in 0.1 M tris-buffer (pH 7.4), blotted with filter paper, and placed on ice. Each tissue was weighed and subsequently homogenized in 0.1 M tris buffer (1:5 w/v). Homogenate was centrifuged at 3000 rpm for 10 min to yield a pellet that was discarded, and the supernatant was kept for the various biochemical assays.
Biochemical analyses
Determination of liver enzyme biomarkers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) was carried out using the method described by Reitman and Frankel (1957), while serum TP, urea, creatinine, cholesterol (CHOL), and triglycerides (TG) were determined according to the user manuals (Randox reagent kits).
Determination of antioxidant parameters
Enzymatic antioxidant parameters of the liver and kidney tissues such as superoxide dismutase (SOD) was determined using the method of Misra and Fridovich (1972), catalase by Sinha (1972), while non-enzymatic antioxidant parameter such as reduced glutathione (GSH) was performed using Ellman’s method (1959).
Estimation of Cd concentrations in tissue homogenates
The accumulated concentration of Cd in tissue samples was determined using atomic absorption spectrophotometry (AAS), according to the established method of AOAC (1995).
Histological examination
Histopathological investigations of renal and hepatic tissues were carried out according to the method described by Morakinyo et al. (2008).
Statistical analyses
All data were analyzed using one-way ANOVA with the appropriate Duncan’s multiple range test where necessary. Statistical significance was considered at p < 0.05. Values were expressed as mean ± SD of six trials (n = 6).
Results
Table 1 represents the effect of aqueous extract of Bf stem bark on hepatic enzyme activities and serum TP in Cd-induced toxicity in rat. As shown in the results, there was a significant (p < 0.05) increase in AST, ALP, and ALT activities, as well as a significant (p > 0.05) difference in the TP level of the Cd-induced untreated control compared to normal control. However, treatment with the aqueous extract of Bf stem bark caused a marked significant (p < 0.05) reduction in the activities of these biomarkers among the treated groups compared to the untreated control. Although, little/no significant (p > 0.05) difference was noted in the TP level of the treated groups compared to the normal and untreated controls.
Figure 1I–IV represents the effect of aqueous extract of Bf stem bark on hepatic tissue antioxidant parameters and TP in Cd-induced toxicity in rat. As shown in Fig. 1, there was a noted decrease significantly (p < 0.05) in the SOD and CAT activities, as well as GSH and TP levels of the Cd-induced toxicity control compared to normal control. However, following treatment with 50, 100, and 200 mg/kg bw aqueous extract of Bf stem bark, there was a significant (p < 0.05) increase in SOD and CAT enzymatic activities, as well as GSH level in a dose-dependent manner compared to Cd-induced toxicity control. Similarly, treatment with the extract of Bf stem bark caused a notable significant (p < 0.05) increase in the hepatic tissue TP (especially, 100 mg/kg bw) when compared with Cd-induced untreated control
Figure 2I–IV represents the effect of aqueous extract of Bf stem bark on renal tissue antioxidant parameters and TP in Cd-induced toxicity in rat. As shown in Fig. 2, there was a significant (p < 0.05) decrease in the SOD and CAT activities, as well as GSH and TP levels of the Cd-induced toxicity control compared to normal control. However, treatment with 50, 100, and 200 mg/kg bw aqueous extract of Bf stem bark, caused a significant (p < 0.05) increase in the enzymatic activities of SOD and CAT, as well as GSH level in a dose-dependent manner compared to Cd-induced toxicity control. Similarly, treatment with the extract of Bf stem bark exhibited a notable significant (p < 0.05) increase in the renal tissue TP when compared with Cd-induced untreated control
Table 2 represents the effect of aqueous extract of Bf stem bark on serum lipid profile in Cd-induced toxicity in rat. As shown in the results, there was a significant (p < 0.05) increase in cholesterol and triglyceride levels of the Cd-induced control compared to the normal group. Conversely, treatment with the aqueous extract of Bf stem bark exhibited a significant (p < 0.05) reduction in the lipid profile compared to untreated Cd-induced control, especially in the group treated with 50 mg/kg bw aqueous extract of Bf stem bark.
Table 3 represents the effect of aqueous extract of Bf stem bark on renal function biomarkers in Cd-induced toxicity in rat. As indicated in the results, a significant (p < 0.05) increase was observed in the urea and creatinine levels of Cd-induced control compared to the normal group. Whereas, treatment with 50, 100, and 200 mg/kg bw aqueous extract of Bf stem bark caused a noticeable decrease significantly (p < 0.05) in the levels of these renal clearance markers compared to untreated Cd-induced control.
Table 4 represents the effect of aqueous extract of Bf stem bark on Cd concentrations in liver and kidney tissue homogenates in Cd-induced toxicity in rat. As indicated in the results, a significant (p < 0.05) rise was noted in the residual concentrations of Cd both in the hepatic and renal tissues of the Cd-induced control compared to the normal group. Whereas, following 14 days of administration of 50, 100, and 200 mg/kg bw aqueous extract of Bf stem bark, a reduction was noted significantly (p < 0.05) in the bio-accumulated Cd concentrations both in the liver and kidneys of the treated groups compared to untreated Cd-induced control.
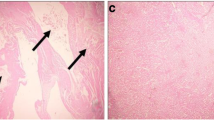
Figure 3A–E represents the histological status of hepatic tissues in Cd-induced toxicity in rat treated with/without aqueous extract of Bf stem bark. As indicated in the hematoxylin–eosin–stained hepatic tissues, Cd-induced control revealed poor oriented and degenerated polyhedral hepatocytes when compared to normal control that revealed a normal histo-architectural polyhedral hepatocytes. However, following treatment with aqueous extract of Bf stem bark, 50 and 100 mg/kg bw aqueous extract of Bf stem bark-treated groups revealed a mild shrunk portal areas and necrosis, while 200 mg/kg bw aqueous extract of Bf stem bark-treated group showed a normal portal area and poorly organized lobular polyhedral hepatocyte.
I–IV Effect of aqueous extract of Bf stem bark on hepatic tissue antioxidant parameters and total protein in cadmium-induced toxicity in rat. Values are expressed as mean ± SD of six trials (n = 6). * indicates statistical (p < 0.05) difference versus normal control, # versus CdCl2-induced control. Keys: Bf, aqueous extract of Bf stem bark
I–IV Effect of aqueous extract of Bf stem bark on renal tissue antioxidant parameters and total protein in cadmium-induced toxicity in rat. Values are expressed as mean ± SD of six trials (n = 6). * indicates statistical (p < 0.05) difference versus normal control, # versus CdCl2-induced control. Keys: Bf, aqueous extract of Bf stem bark
A–E Photomicrographs of hematoxylin–eosin stained hepatic tissues in cadmium-induced toxicity in rat (magnification, × 800; scale bar: 35 µm). A Normal control. B CdCl2-induced untreated control. C Cadmium-induced + 50 mg/kg bw aqueous extract of Bf stem bark. D Cadmium-induced + 100 mg/kg bw aqueous extract of Bf stem bark. E Cadmium-induced + 200 mg/kg bw aqueous extract of Bf stem bark. H; Hepatocytes, white and black circle; portal area/triad (i.e., portal vein (PV), hepatic artery (HA), bile duct (BD))
Figure 4A–E represents the histological status of renal tissues in Cd-induced toxicity in rat treated with/without aqueous extract of Bf stem bark. As indicated in the hematoxylin–eosin–stained renal tissues, Cd-induced control revealed a Bowman’s capsule void of urinary space, disrupted endothelial cells/podocytes, poorly distributed proximal and distal convoluted tubules compared to normal control with a typical architectural renal cortex, Bowman’s capsule, urinary space, and a well-distributed distal and proximal convoluted tubules. However, following the administration of aqueous extract of Bf stem bark, the group treated with 50 mg/kg bw showed a Bowman’s capsule void of urinary space, mild disrupted podocytes with a poor distributed proximal/distal convoluted tubules, whereas, 100 and 200 mg/kg bw treated groups revealed normal Bowman’s capsule, observable urinary space, and a -distributed distal and proximal convoluted tubules compared to untreated control.
A–E Photomicrographs of hematoxylin–eosin stained renal tissues in cadmium-induced toxicity in rat (magnification, × 800; scale bar: 35 µm). A Normal control. B CdCl2-induced untreated control. C Cadmium-induced + 50 mg/kg bw aqueous extract of Bf stem bark. D Cadmium-induced + 100 mg/kg bw aqueous extract of Bf stem bark. E Cadmium-induced + 200 mg/kg bw aqueous extract of Bf stem bark. White arrow; glomerulus, PCT; proximal convoluted tubules, DCT; distal convoluted tubule
Discussion
Cd, a non-redox metal, and its derivatives are considered as most toxic compounds and environmental pollutants of ages (Ognjanovic et al. 2008). Exposure to Cd has increasingly been recognized to pose a serious threat to human health across the globe (Stankovic et al. 2014). Bioaccumulation of Cd in target organs such as the liver and kidney has been shown to underlie pathological diseases associated with these organs via depletion of their enzymatic and non-enzymatic antioxidant system (Arisawa et al. 2007). The ameliorative effect of aqueous extract of Bf stem bark against Cd-induced toxicity in male rats was appraised and reported in this study.
Increased serum hepatic-specific biomarkers ALT, AST, and ALP have been recognized as specific indicators of hepatocellular injury or oxidative damage to functional hepatic membrane architecture (Ozer et al. 2008; Srilaxmi et al. 2010). As indicated in this study, serum activities of these hepatic-specific markers were increased significantly in response to Cd toxicity (Table 1). Cd-induced oxidative damage of the hepatic membrane has been implicated a plausible cause of ALT and AST leakage from the liver cytosol into the bloodstream (Musa and Çelik 2020; Ozer et al. 2008). Loss of these intracellular macromolecules could possibly have contributed to the hepatocellular degeneration/necrosis observed in the histomorphological studies on the hepatic tissue (Fig. 3) (Salama et al. 2019). However, treatment with the extract triggered decreased activities of these biomarkers with a marked improvement in hepatic histological status. This development could possibly suggest the hepatoprotective effect of the extract against Cd-induced hepatotoxicity.
Redox imbalances are known to negatively affect the body system through ROS generation, which destroys proteins, lipids, and DNA by oxidation (Sharma et al. 2014). Antioxidant enzymes primarily provide intracellular defense by catalyzing the decomposition of ROS (Szaleczky et al. 1999). A significant reduction was revealed in SOD, CAT, GSH, and TP in this study, among Cd-exposed hepatic and renal tissues (Figs. 1 and 2). Although cadmium itself does not generate free radicals directly (Hassoun and Stohs 1996), it indirectly generates various radicals such as superoxide radical, reactive nitrogen species (RNS) such as peroxynitrite, nitric oxide, and hydroxyl radical, thus, causing damage consistent with oxidative stress (Stohs et al. 2001). Reports have connected a severe oxidative alteration of enzymatic proteins, and bio-membrane lipids resulting into impaired cellular functions in Cd-fostered overwhelming production of ROS (Cuypers et al. 2010; Liu et al. 2021; Shukla and Kumar 2009). Changes in multiple physiological and biochemical processes, however, have been implicated (Pham-Huy et al. 2008), due to the depletion of low molecular weight antioxidant apparatus via Cd-enzyme interaction mechanisms, that result into perturbation of enzyme structure important for catalytic activities (Kefaloyianni et al. 2005; Zheng et al. 2019; Zhang et al. 2020b). The report of this study corroborates the report of El-Boshy et al. (2017). However, the observed post-treatment sharp rise in hepatic and renal tissue antioxidant systems in this study (Figs. 1 and 2), could possibly be attributed to the ability of the extract to attenuate the deleterious effect of Cd-induced oxidative stress, thereby promoting protection in the organs against Cd-induced hepatotoxicity and nephrotoxicity (Podgórska et al. 2017).
Alteration of lipid profile represents an important and potentially etiological component in the pathophysiogenesis of many disorders (Brewer 2011). In this report, elevated serum CHOL and TRIG were noted in the Cd-induced group. Disruption in the homeostasis of lipids may be responsible for the increase in the levels of CHOL and TRIG (Malhotra et al. 2020). Oxidative injury to cellular membranes, alterations in trans-membrane gradients, and activation or inhibition of enzymes involved in lipid metabolism have been reported to elevate serum TRIG and CHOL levels in Cd toxicity (Ness et al. 2001; Seif et al. 2019; Deng et al. 2017). Similarly, a decrease in the activities of cytochrome P450 enzymes or impaired hepatocytes has also been identified to occasion elevated serum lipid profiles in Cd toxicity (Wang and Shi 2001; Kojima et al. 2004). However, following treatment with the extract, a decline observed in the serum TRIG and CHOL could possibly suggest the ability of the extract to attenuate oxidative stress in the hepatic tissue and reactivation of enzymes involved in lipid metabolism.
Renal diseases that diminish the glomerular filtration rate lead to urea and creatinine retention (Webster et al. 2017). These biochemical parameters are indicators of renal function (Pham 2017). Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals (Walser and Bodenlos 1959; Knepper and Roch–Ramel 1987). Its serum concentration is influenced by the rate of excretion (Levey et al. 1999). While creatinine is a waste product of metabolism primarily excreted by the kidneys (Huang et al. 2002; Donadio et al. 1997). The elevated serum urea and creatinine caused by Cd toxicity (Table 3), could probably be connected to disorder in protein catabolism due to an increase in the synthesis of arginase enzyme involved in urea biosynthesis (Renugadevi and Prabu 2010), and perturbation of glomerular filtration rate implicated in Cd toxicity (Liang et al. 2012; Laskow et al. 1990), which results in poor creatinine clearance (Rani et al. 2014). However, a consequential reduction in serum urea and creatinine levels, as well as improved renal histopathology (Fig. 4), following the administration of the extract at low doses could substantiate the ability of the extract to enhance moderation of arginase induction, increase glomerular filtration rate (Yang and Bankir 2005). This observation suggests the cytoprotective effect of the extract against Cd-induced nephrotoxicity/renal toxicity.
Organs such as liver and kidney tissues have been reported to be the critical targets in Cd toxicity (Kar and Patra 2021; Guirlet and Das 2012). Hepatic and renal lesions abetted by Cd-induced oxidative stress, protein cross-linking, and DNA damage have been implicated in Cd toxicity (Morales et al. 2006). Thus, elevated renal and hepatic Cd concentrations noticed in the Cd-exposed group (Table 4) could consequently have facilitated the reduced antioxidant system and essential biomolecules, as well as responsible for various modifications in the architectural histomorphology of hepatic and renal tissues of the Cd-exposed group (Figs. 3 and 4). It is noteworthy that extract-treated groups reflected a significant reduction in renal and hepatic Cd concentrations. This observation suggests the renoprotective and hepatoprotective effects of the extract against Cd-induced toxicity, probably via mechanisms that facilitate either Cd chelation or its release from these organs. This report corroborates the study of Ige and Akhigbe (2013).
Furthermore, histological examination of the hepatic tissue of Cd-treated rats revealed poor oriented, degenerated polyhedral hepatocytes, and tubular necrosis (Fig. 3A–E). Renal tissue of Cd-induced control as well revealed a Bowman’s capsule void of urinary space, disrupted endothelial cells/podocytes, and poorly distributed proximal and distal convoluted tubules (Fig. 4A–E). Similar studies have also implicated alterations in histological architectures of the kidney (Gabr et al. 2019) and hepatic tissue (Mantur et al. 2014) in Cd toxicity. Accordingly, these observations could be due to several deleterious effects of bio-accumulated Cd-induced ROS in the tissues. On the other hand, treatment with the extract revealed hepatoprotective and renoprotective potentials by demonstrating a reversal to the Cd-induced histological modifications/alterations in these tissues. This effect is in agreement with our observation in Tables 1 and 3 and could be credited to the antioxidative potential of the extract.
Conclusion
The damaging effect of Cd-toxicity in vital tissues namely the kidney and liver were clearly elucidated and established in this report. Conversely, aqueous extract of Bf stem bark increased hepatic antioxidant system, renal clearance of urea and creatinine, as well as mitigated histological alteration in Cd-induced toxicity. Therefore, aqueous extract of Bf stem bark could be suggested to be hepatoprotective and renoprotective in Cd toxicity.
References
Adesina AJ, Akomolafe FS (2014) “Full length research paper nutritional and anti-nutritional composition of Bridelia ferruginea Benth (Euphorbiaceae) stem bark sample. Int J Sci Res Knowl 2(2):92–104
Afolabi OB, Oloyede OI, Agunbiade SO (2018) Inhibitory potentials of phenolic-rich extracts from Bridelia ferruginea on two key carbohydrate-metabolizing enzymes and Fe2+-induced pancreatic oxidative stress. J Integr Med 16(3):192–198
Agra MF, Freitas PF, Barbosa-Filho JM (2007) Synopsis of the plants known as medicinal and poisonous in northeast of Brazil. Rev Bras Farmacogn 17:114–140
Andjelkovic M, Buha DA, Antonijevic E et al (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16(2):274
Arisawa K, Uemura H, Hiyoshi M et al (2007) Cause-specific mortality and cancer incidence rates in relation to urinary β2-microglobulin: 23-year follow-up study in a cadmium-polluted area. Toxicol Lett 173(3):168–174
Association of Official Analytical Chemists (AOAC) (1995) Official methods of analysis. Washington (DC), USA
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17(1):21–34
Beyersmann D, Hartwig A (2008) Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 82(8):493–512
Branca JJ, Morucci G, Pacini A (2018) Cadmium-induced neurotoxicity: still much ado. Neural Regen Res 13(11):1879
Brewer HB (2011) Clinical review: the evolving role of HDL in the treatment of high-risk patients with cardiovascular disease. J Clin Endocrinol Metab 96:1246–1257
Cuypers A, Plusquin M, Remans T et al (2010) Cadmium stress: an oxidative challenge. Biometals 23(5):927–940
Deng Y, Zhang Y, Lemos B, Ren H (2017) Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure’. Sci Rep 7:46687
Donadio C, Lucchesi A, Tramonti G, Bianchi C (1997) Creatinine clearance predicted from body cells mass is a good indicator of renal function. Kidney Int 52:166–168
Ekanem JT, Kolawole OM, Abbah OC (2008) Trypanocidal potential of methanolic extracts of Bridelia ferruginea benth bark in Rattus novergicus. Afr J Biochem Res 2(2):045–050
El-Boshy M, Ashshi A, Gaith M et al (2017) Studies on the protective effect of the artichoke (Cynara scolymus) leaf extract against cadmium toxicity-induced oxidative stress, hepatorenal damage, and immunosuppressive and hematological disorders in rats. Environ Sci Pollut Res 24(13):12372–12383
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Erboga M, Kanter M (2016) Effect of cadmium on trophoblast cell proliferation and apoptosis in different gestation periods of rat placenta. Biol Trace Elem Res 169(2):285–293
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1(6):529–539
Gabr SA, Alghadir AH, Ghoniem GA (2019) Biological activities of ginger against cadmium-induced renal toxicity. Saudi J Biol Sci 26(2):382–389
Godt J, Scheidig F, Grosse-Siestrup C (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1(1):1–6
Guirlet E, Das K (2012) Cadmium toxicokinetics and bioaccumulation in turtles: trophic exposure of Trachemys scripta elegans. Ecotoxicol 21(1):18–26
Hassoun EA, Stohs SJ (1996) Cadmium induced production of superoxide anion and nitric oxide. DNA single strand breaks and lactate dehydrogenase leakage in J774A1 cell cultures. Toxicol 112 (3):219–226
Huang CT, Chen ML, Huang LL, Mao IF (2002) Uric acid and urea in human sweat. Chinese J Physiol 45(3):109–116
Ige SF, Akhigbe RE (2013) Common onion (Allium cepa) extract reverses cadmium-induced organ toxicity and dyslipidemia via redox alteration in rats. Pathophysiology 20(4):269–274
Jaishankar M, Tseten T, Anbalagan N et al (2014) Toxicity, mechanism and health effects of some heavy metals. Inter Toxicol 7(2):60
Jaiyesimi KF, Agunbiade OS, Ajiboye BO, Afolabi OB (2020) Polyphenolic-rich extracts of Andrographis paniculata mitigate hyperglycemia via attenuating β-cell dysfunction, pro-inflammatory cytokines and oxidative stress in alloxan-induced diabetic Wistar albino rat. J Diabetes Metab Disord 19(2):1543–1556
Kar I, Patra AK (2021) Tissue bioaccumulation and toxicopathological effects of cadmium and its dietary amelioration in poultry—a review. Biol Trace Elem Res 6:1–23
Kefaloyianni E, Gourgou E, Ferle V et al (2005) “Acute thermal stress and various heavy metals induce tissue-specific pro- or anti-apoptotic events via the p38-MAPK signal transduction pathway in Mytilus galloprovincialis (Lam.). J Exp Biol 208:4427–4436
Knepper MA, Roch-Ramel F (1987) Pathways of urea transport in the mammalian kidney. Kidney Int 31(2):629–633
Kojima M, Masui T, Nemoto K, Degawa M (2004) Lead nitrate-induced development of hypercholesterolemia in rats: sterol-independent gene regulation of hepatic enzymes responsible for cholesterol homeostasis. Toxicol Lett 154(1–2):35–44
Laskow DA, Curtis JJ, Luke RG et al (1990) Cyclosporine-induced changes in glomerular filtration rate and urea excretion. Am J Med 88(5):497–502
Levey AS, Bosch JP, Lewis JB et al (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130(6):461–470
Liang Y, Lei L, Nilsson J et al (2012) Renal function after reduction in cadmium exposure: an 8-year follow-up of residents in cadmium-polluted areas. Environ Health Perspect 120(2):223–228
Liu L, Liu Y, Cheng X, Qiao X (2021) The alleviative effects of quercetin on cadmium-induced necroptosis via inhibition ROS/iNOS/NF-κB pathway in the chicken brain. Biol Trace Elem Res 199(4):1584–1594
Malhotra P, Gill RK, Saksena S, Alrefai WA (2020) Disturbances in cholesterol homeostasis and non-alcoholic fatty liver diseases. Front Med 7:467
Mantur VS, Somannavarib MS, Yendigeri S et al (2014) Ameliorating effect of black tea extract on cadmium chloride-induced alteration of serum lipid profile and liver histopathology in rats. Indian J Physiol Pharmacol 58(2):2
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Morakinyo AO, Oloyo AK, Raji Y et al (2008) “Effects of aqueous extract of garlic (Allium sativum) on testicular functions in the rat. Nigerian J Health Biomed Sci 7(2):26–30
Morales AI, Vicente-Sanchez C, Sandoval JS et al (2006) Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol 44(12):2092–2100
Musa S, Çelik İ (2020) Determination of hepatoprotective and antioxidant role of thyme (origanum onites l.) infusion against ethyl alcohol induced oxidative stress in rats. Bitlis Eren Uni J Sci Tech 10(2)
Ndukwe KC, Okeke IN, Lamikanra A et al (2005) Antibacterial activity of aqueous extracts of selected chewing sticks. J Contemp Dent Pract 6(3):86–94
Ness GC, Gertz KR, Holland RC (2001) Regulation of hepatic lanosterol 14-demethylase gene expression by dietary cholesterol and cholesterol-lowering agents. Arch Biochem Biophys 395:233–238
Obafemi TO, Onasanya A, Adeoye A et al (2019) Protective effect of methanolic and flavonoid-rich leaf extracts of Synsepalum dulcificum (Danielli) on lead-acetate-induced toxicity in Wistar albino rats. J Appl Pharm Sci 9(5):65–72
Ognjanovic BI, Markovic SD, Pavlovic SZ et al (2008) “Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res 57(3):403–411
Olajide OA, Makinde JM, Awe SO (1999) Effect of aqueous extract of Bridelia ferruginea stem bark corrageenan-induced Oedema and granuloma tissue formation on rats and mice. J Ethnopharmacol 66(1):113–117
Olajide OA, Makinde JM, Okpako DT, Awe SO (2000) Studies on the anti-inflammatory and related pharmacological properties of the aqueous extract of Bridelia ferruginea stem bark. J Ethnopharmacol 71(1–2):153–160
Oloyede OI, Babalola SO (2012) In-vitro Antioxidant activity of ethanolic extract of Bridelia ferruginea. J Acad Res Int 2(3):2223–9944
Ozer J, Ratner M, Shaw M et al (2008) The current state of serum biomarkers of hepatotoxicity. Toxicol 245(3):194–205
Pham TA (2017) Validation of the salivary urea and creatinine tests as screening methods of chronic kidney disease in Vietnamese patients. Acta Odontol Scand 75(8):551–556
Pham-Huy NL, He H, Pham-Huy C (2008) “Green tea and health. An Overview. J Food Agric Environ 6:6–13
Podgórska A, Burian M, Rychter AM et al (2017) Short-term ammonium supply induces cellular defence to prevent oxidative stress in Arabidopsis leaves. Physiol Plant 160(1):65–83
Pratush A, Kumar A, Hu Z (2018) Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: a review. Inter Microbiol 21(3):97–106
Rani A, Kumar A, Lal A, Pant M (2014) Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 24(4):378–399
Rech M, To L, Tovbin A et al (2014) Heavy metal in the intensive care unit: a review of current literature on trace element supplementation in critically ill patients. Nutr Clin Pract 29(1):78–89
Reimann C, de Caritat P (2005) Distinguishing between natural and anthropogenic sources for elements in the environment: regional geochemical surveys versus enrichment factors. Sci Total Environ 337(1):91–107
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Renugadevi J, Prabu SM (2010) Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol 62(5):471–481
Salama SA, Arab HH, Hassan MH, Maghrabi IA (2019) Cadmium-induced hepatocellular injury: modulatory effects of γ-glutamyl cysteine on the biomarkers of inflammation, DNA damage, and apoptotic cell death. J Trace Elem Med Biol 52:74–82
Satarug S, Garrett SH, Sens MA, Sens DA (2010) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118(2):182–190
Seif MM, Madboli AN, Marrez DA, Aboulthana WM (2019) Hepato-renal protective effects of Egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol Rep 6:625–631
Shahid M, Dumat C, Khalid S et al (2016) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241:73–137
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. BioMed Res Inter
Shukla GS, Kumar RL (2009) The present status of biological effects of toxic metals in the environment: lead, cadmium, and manganese. Can J Physiol Pharmacol 62(8):1015–1031
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47(2):389–394
Srilaxmi P, Sareddy GR, Setty OH, Babu PP (2010) Protective efficacy of natansnin, a dibenzoyl glycoside from Salvinia natans against CCl4 induced oxidative stress and cellular degeneration in rat liver. BMC Pharmacol 10(1):1–3
Stankovic S, Kalaba P, Stankovic AR (2014) Biota as toxic metal indicators. Environ Chem Lett 12(1):63–84
Stohs SJ, Bagchi D, Hassoun E, Bagchi M (2001) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 20(2)
Szaleczky E, Prechl J, Feher J, Somogyi A (1999) Alterations in enzymatic antioxidant defence in diabetes mellitus− a rational approach. Postgrad Med J 75(879):13–17
Talla E, Djamen D, Djouldé DR (2002) Antimicrobial activity of Bridelia ferruginea leaves extracts. Fitoterapia 73(4):343–345
Thévenod F (2010) Catch me if you can! Novel aspects of cadmium transport in mammalian cells. Biometals 23(5):857–875
Vinodhini R, Narayanan M (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). Int J Environ Sci Technol 5(2):179–182
Walser M, Bodenlos LJ (1959) Urea metabolism in man. J Clin Invest 38(9):1617–1626
Wang S, Shi X (2001) Molecular mechanisms of metal toxicity and carcinogenesis. Mol Cell Biochem 222(1):3–9
Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic kidney disease. Lancet 389(10075):1238–1252
Yang B, Bankir L (2005) Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol 288(5):F881–F896
Zhang J, Zheng S, Wang S et al (2020a) Cadmium-induced oxidative stress promotes apoptosis and necrosis through the regulation of the miR-216a-PI3K/AKT axis in common carp lymphocytes and antagonized by selenium. Chemosphere 258:127341
Zhang Y, Yin H, Shao B et al (2020b) Antagonistic effect of VDR/CREB1 pathway on cadmium-induced apoptosis in porcine spleen. Ecotoxicol Environ Saf 209:111819
Zheng Y, Shi B, Ma M et al (2019) The novel relationship between Sirt3 and autophagy in myocardial ischemia–reperfusion. J Cell Physiol 234(5):5488–5495
Acknowledgements
The authors of this work hereby appreciate the Provost, College of Sciences, Afe Babalola University for his support and for creating a research-friendly environment for the success of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Supervision of the study by Omotade Ibidun Oloyede. material preparation, data collection, and analysis were performed by Olakunle Bamikole Afolabi, Oluwafemi Emmanuel Babatunde, Adegbolagun Grace Adegboro, and Damilola Oluwaseun Ogunkorode. The first draft of the manuscript was written by Oluwafemi Emmanuel Babatunde and Olakunle Bamikole Afolabi. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animals received humane care with a strict compliance to the ethical guidelines issued according to the principles of laboratory animal care (LAC) of the National Society of Medical Research and in accordance with the principles of laboratory animal care.
Consent to participate
None.
Consent for publication
None.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oloyede, O.I., Afolabi, O.B., Babatunde, O.E. et al. Cytoprotective potential of the aqueous extract from Bridelia ferruginea stem bark against experimental cadmium-induced hepato-renal toxicity in Wistar rat. Comp Clin Pathol 31, 967–978 (2022). https://doi.org/10.1007/s00580-022-03399-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03399-1