Abstract
Sonchus oleraceus L. is a Mediterranean leafy vegetable. The aerial parts are widely used in Algerian traditional medicine to treat several diseases such as inflammation, hepatitis, and for detoxication. To date, there are no studies corroborating its toxicity profile. This work aimed to investigate the qualitative phytochemical screening (QPS) and to evaluate the toxicity of the hydro-methanolic (HME) and hot aqueous (HAE) extracts of the aerial parts of S. oleraceus L. in female mice. The QPS of both extracts was achieved using simple tests, as well as the acute and subacute toxicities were examined following Lorke’s method and OECD guidelines 407, respectively. The qualitative phytochemical analysis revealed the presence of polyphenols, flavonoids, sterols and triterpenes, anthraquinone glycoside, and reducing compounds in both extracts. The administered doses of the two extracts disclosed no mortality and no visible sign of toxicity. The LD50 was estimated above 5 g/kg body weight. In the subacute test, the extracts did not induce neither mortality nor significant changes in body weight and relative organ weights. The data also revealed that HME leads to a disturbance in LDL, indirect bilirubin, while HAE caused a disorder in cholesterol, ALT, urea, creatinine, and total proteins levels. They caused as well a strong disturbance of hematological parameters. Histopathological examination showed an important hepatic, renal, and lung lesions up to the dose of 2000 mg/kg b.w. Our results showed that the two extracts may elicit toxic effects on the liver, kidney, and lung on prolonged administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbal medicine is considered as a major primary healthcare provider around the globe particularly in rural and remote areas, in spite of the development in synthetic and semi-synthetic drugs for the treatment of different ailments (Sen and Chakraborty 2016; Yuan et al. 2016). This widespread of plant medicine is attributed to several factors such as various claims on the efficacy or effectiveness of plant medicines, high cost and adverse side effects of most modern drugs, and a movement toward self-medication (Bandaranayake 2006). Despite their use over centuries, data about medicinal plants are not sufficient to meet the criteria needed to support worldwide use (WHO 2000). Safety is the major and overriding criterion in the selection of herbal medicines for use in health services, so it is important to validate their safety before use. Experimental data on the potential toxicological profile of traditional medicinal plants and/or their derivatives are imperative for the validation of safety for human use and in the development of pharmaceuticals/functional food (Yuet Ping et al. 2013). Therefore, systematic evaluation of medicinal plants for potential toxicity is a necessary step for the validation of their regular therapeutic use. Several medicinal plants are used in Algerian traditional medicine to treat liver diseases.
Sonchus oleraceus L. (family Asteraceae) is locally known as “Tilfaf” and named as sowthistle, and it is a Mediterranean edible species as leafy vegetables (Quezel and Santa 1963). It is very important in the diets of urban people and provides a relatively inexpensive vegetable to the society (Vilela et al. 2009a). It is used in folk medicine as a therapy for inflammation, hepatitis, gastric spasm, fever, for detoxication, and improvement of blood circulation (Muhammad et al. 2012; Agra et al. 2007). In traditional medicine, the plant extracts are used as an antioxidant, anti-inflammatory, anticancer, antimicrobial, antidepressant, hepatoprotective, antidiabetic, blood purifying, and liver tonic activities (Jain and Singh 2014; Khan et al. 2012a, b, c; Hata et al. 2000). This plant like many other species of Sonchus genus possesses many phytochemical constituents, including terpenes, sterols, flavonoids, coumarins, phenols, saponins, and alkaloids (Jain and Singh 2014). S. oleraceus L. from Brazil showed no toxicity up to 5000 mg/kg body weight of hydroethanolic and dichloromethanic extracts (Vilela et al. 2009a).
However, to the best of our knowledge, no report is available on hydro-methanolic and hot aqueous extracts toxicity of aerial parts of the edible plant, which is required before considering it as medicine in spite of its wide consumption. Based on that, the present study was performed to assess a comparison of the possible toxic effects of the hydro-methanolic and hot aqueous extracts of S. oleraceus L. aerial parts after acute and subacute oral administration in Albino mice, as well as to identify the main components present in these extracts.
Materials and methods
Botanical material and extracts preparations
The aerial parts of the fresh plant were collected in the month of January 2018 from the fields of Boumahra Ahmed commune, City of Guelma (Northeastern of Algeria), and were identified and authenticated as Sonchus oleraceus L. (family Asteraceae) by Prof. Gérard De Belair (Faculty of Sciences, University of Annaba, Algeria), where a voucher specimen was deposited (LBEE.22.01.18). The plant was shade-dried and was ground to fine powder.
The fine powder of Sonchus oleraceus L. was extracted by mixing 200 g of the plant powder with 2 L of 80% methanol (Merck, Germany) for 72 h with occasional agitation at room temperature and filtered. The process was repeated in three times. The filtrates were combined and then concentrated to dryness in a rotary evaporator in reduced pressure (R-215, Büchi Labortechnik AG, Flawil, Switzerland) at 40 °C to obtain hydro-methanolic extract (HME). The concentrated filtrate was dried with a lyophilizer at − 50 °C and vacuum pressure (200 mBar) to remove water. The dried extract was kept in a tight container in the refrigerator until use.
The hot aqueous extract (HAE) was prepared by using the method of Smach et al. (2015). A portion (200 g) of the air-dried aerial parts was macerated in 1 L of disttilled water for 4 h until boiling. The extract was thereafter filtered with Whatman No.1 filter paper, and centrifuged at 2000 × g for 15 min. The supernatant was dried using a lyophilization apparatus and the HAE was kept at 4 °C in a refrigerator for the toxicological study.
Preliminary phytochemical screening
The hydro-methanolic and hot aqueous extracts were subjected to a qualitative phytochemical screening. The phytoconstituents were detected using colorimetric and precipitation reactions, according to the different procedures as described below.
Various solvent extracts of S. oleraceus L. were used to screen for phenolics and flavonoids (Bhandary et al. 2012; Khanam et al. 2015), sterols and triterpenoids (Kumar et al. 2013; Khanam et al. 2015), alkaloids (N’Guessan et al. 2009), saponins (Khanam et al. 2015), cardiac glycosides (Khanam et al. 2015), tannins (John et al. 2015), anthraquinone glycosides (Iqbal et al. 2015), reducing sugars (Singh and Kumari 2015), lipids (Bruneton 2009), coumarins (Rizk 1982), and gums and mucilages (BeMiller and Whistler 2012).
Animals and approval from animal ethical committee
In order to avoid intersex variability, we used only adult female mice of Wistar strain (Mus musculus) weighing between 21 and 36 g. The animals were supplied from the laboratory of the Pasteur Institute of Algiers, and housed at the “Animalerie de Département de Biologie, Université 8 Mai 1945 Guelma, Algérie” under standard experimental conditions. The animals were kept in clean stainless-steel cages and acclimatized in an air-conditioned room at 25 ± 2 °C, 12-h light/12-h dark cycle, and relative humidity of 50–55% for a week. They were provided with standard chow diet and water ad libitum throughout the experiment, except for the short fasting period when no food (except water) was provided 8 h prior to treatment. The principles of the Care and Use of Laboratory Animals (NIH publication 1985) were followed.
Acute toxicity assay
The study of the acute oral toxicity of hydro-methanolic and hot aqueous extracts (HME, HAE) of the plant S. oleraceus L. was conducted according to Lorke’s method (Lork 1983) with some modifications. It consists of determining the 50% lethal dose (LD50). The study was carried out in two phases.
In the first phase, the healthy mice were divided into four groups, consisting of 3 mice per group: groups I, II, III, and IV. Group I was mice treated with a vehicle (1% DMSO for HME and NaCl 9‰ for HAE; normal control) and groups II–IV were mice treated with 10, 100, and 1000 mg/kg body weight (b.w) of the extracts, respectively. All mice were kept under the same conditions and monitored for signs of toxicity or mortality for 24 h. The next day, the signs of mortality are noted and the second phase is carried out.
The second phase involves the use of 9 mice for each extract. They were divided into three groups of 3 mice each and received three higher doses of each extract (1600, 2900, and 5000 mg/kg b.w) to further determine the correct LD50 value. They were also observed periodically during the first 24 h for any toxic sign, then once a day for 14 days, noting weight variations, mortality rate, and any physical and behavioral changes.
Preparation of dose
The two extracts (HME, HAE) of Sonchus oleraceus L. were suspended in vehicles with 1% dimethyl sulfoxide (DMSO) and 0.9% physiological saline (NaCl) respectively prior to single oral administration. After the 12-h fasting period, body weights of the mice were measured and the extract dose was calculated in relation to the body weight, given the extract solution of 10 mL/kg body weight.
Subacute oral toxicity
The subacute toxicity study was performed according to the OECD guideline for testing of chemicals 407 (OECD 2008), and dose selection was made on the basis of acute oral toxicity study (2000 and 5000 mg/kg b.w) according to OECD guidelines 425. The animal experiment was conducted in strict compliance with the ethical standards of the International Guidelines for the Evaluation of the Safety and Efficacy of Medicinal Plants (Organisation for Economic Co-operation and Development) (OECD 2008). Similarly, female mice were randomly distributed into five groups, consisting of six mice per group. Group I was mice treated with 1% DMSO for HME and NaCl 9‰ for HAE (neutral control) and groups II–V were mice treated with 250, 500, 1000, and 2000 mg/kg b.w of each extract. The extracts were suspended in vehicle prior to oral administration. The mice were administered orally once a day for 28 days consecutively. Body weights and signs of toxicity and mortality were observed daily. Weekly measurements of weights were recorded. At the end of the treatment, the mice were fasted for 24 h and sacrificed. Blood samples were collected from mice into plain and EDTA-containing tubes for biochemical and hematological analyses, respectively. After the blood collection procedure, the animals were sacrificed by cervical dislocation and dissected. The organs (spleen, heart, liver, kidneys, and lungs) were excised, rinsed in 0.9% saline, and weighed individually to determine relative organ weights, and the gross lesions were monitored.
The relative organ weight (ROW) for each organ was determined using the following formula:
The kidneys, livers, and lungs were then preserved in 10% formalin for histopathological examination.
Hematological analysis
The blood samples collected in EDTA tubes were immediately used for hematological assays. Hematological parameters were determined using automated Beckman Coulter (Beckman Coulter Inc., Brea, CA, USA). The following hematological indices were assayed: white blood cell count (WBC), neutrophils (NEU), lymphocytes (LYM), eosiophils (EOS), monocytes (MONO), basophils (BASO), red blood cell count (RBC), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLT), and hematocrit (HCT).
Biochemical analysis
The blood samples collected in non-heparinized tubes were allowed to clot under room temperature for 15–30 min and then centrifuged at 3000 rpm, 4 °C for 10 min. The serum obtained was stored at − 20 °C for biochemical analysis. It was conducted using diagnostic kits according to the manufacturers’ manuals.
The clinical biochemistry parameters, including glucose (GLU), urea (UREA), uric acid (URIC), creatinine (CREA), cholesterol (C), triglycerides (TGs), high density lipoprotein (HDL), low density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total protein (TP), and total bilirubin (T-BIL) (direct and indirect bilirubin), were analyzed colorimetrically by using the standard ready-to-use kits.
Histopathological assessment
On the 28th day, histopathological examinations were carried out on the excised and fixed (10% formalin) liver, kidney, and lung tissues of the test mice. They were dehydrated in gradual ethanol concentrations (60–100%), cleared in xylene, and finally embedded in paraffin. Prior to photomicroscope observation, they were prepared and segmented by a microtome into Sects. (5 μm thick) and stained with hematoxylin and eosin (H&E) dye. The stained sections were eventually viewed with Leica Optika B-192 microscope at power magnifications of × 10 and × 40, and photomicrographs were recorded (Martey et al. 2010).
Statistical analysis
The statistical analyses were carried out using GraphPad Prism software, Inc., version 5.00. Experimental results were expressed as mean ± S.D (standard deviation), and the statistical significance between the groups was analyzed by means of one-way analysis of variance (ANOVA) followed by Dunn’s multiple comparison post hoc test. A p-value of p ≤ 0.05 was considered as statistically significant.
Results
Phytochemical investigation
The qualitative phytochemical analysis of the hydro-methanolic (HME) and hot aqueous (HAE) extracts revealed the presence of various secondary metabolites which are listed in Table 1. Strong positive reactions were registered for polyphenols, flavonoids, sterols and triterpenes, anthraquinone glycoside, and reducing compounds in both extracts, whereas saponosids, lipids, gums, and mucilages were not detected. HME contains more compounds than HAE.
Acute toxicity assay
The acute lethal study of SO aerial parts extracts on mice shows that no animal died within 24 h after treatment with the extracts (HME and HAE) in the first phase. Again, no mortality or signs of toxicity in female mice were recorded among all the dose groups throughout the 2-week experimental period. Moreover, the treated groups were physically active, consuming food and water as regular. Any sign of abnormal behavior has not been noticed compared to the control group, and so based on the result of acute toxicity, the LD50 of hydro-methanolic and hot aqueous extracts was estimated to be above 5 g/kg body weight.
At the end of the study, the necropsy of the test animal organs revealed no abnormalities related to the treatment with the two extracts and no significant abnormal changes in color, size, shape, and texture compared with the control. The histopathological examination was not conducted as the gross pathological showed no abnormality. According to OECD (2008), the substance tested is considered of low toxicity and falls in class 5, since the oral lethal dose (LD50) of the two extracts for Wistar female mice is greater than 5000 mg/kg b.w.
Subacute toxicity study
General observations
Daily oral administration of HME and HAE extracts at doses 250, 500, 1000, and 2000 mg/kg b.w for 28 consecutive days to female mice showed no death, changes in behavior, or other physiological changes compared to the control animals, respectively. The doses were selected based on the results of acute toxicity study, wherein the test item was found to be safe up to a dose of 5000 mg/kg body weight. A limit dose of 2000 mg/kg was selected as a high dose in the subacute toxicity study.
Effects of extracts on body weight
The animals and their organs were weighed using the KERN precision balance, ABJ-NM (ABS 220-4 N). The body weights of all animals, both in the control and treated groups, are shown in Figs. 1 and 2.
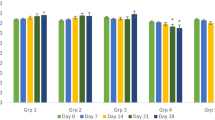
Changes in body weight mean (g) in mice treated orally under subacute toxicity conditions with hot aqueous extract of Sonchus oleraceus L. Values are presented as mean ± SEM of 6 mice. Compared to the control group (one-way ANOVA followed by Dunnett’s post hoc test). *Significantly different, p < 0.05
During the subacute experimental period, all groups of mice showed gradual and normal increase in their body weight (Fig. 1). However, there was no significant change (p > 0.05) in body weight between the treated and control groups at HME doses of 250, 500, 1000, and 2000 mg/kg. On the other hand, compared to the day of administration of the extract (day 14), there was a significant increase (p = 0.05) in the body weight of the animals, after administration of the extract at 1000 mg/kg.
The administration of hot aqueous extract from S. oleraceus L. at a dose of 2000 mg/kg resulted a significant increase (p = 0.01) in body weight of treated mice at D7 compared to the control groups (Fig. 2). There was no statistically significant change in body weight between the control and other treated groups throughout the study period. In addition, no signs of toxicity or animal deaths were observed at any dose up to a maximum of 2000 mg/kg during the 4 weeks of repeated oral treatment. Weekly weighing of the mice implied that the oral application of both extracts up to a dose of 2 g/kg for 28 days did not disturb the growth of the mice at the end of the experimental period.
Based on these results, the hydroalcoholic and aqueous extracts of the plant S. oleraceus L. are considered non-toxic at the treated doses.
Effects of S. oleraceus L. extracts on the absolute and relative weight of the different organs (ROW) under subacute toxicity conditions
On the day of the sacrifice, the weights of the organs (liver, kidneys, lungs, heart, and spleen) were measured. This allowed us to calculate the relative weight of these organs and to evaluate the variation between the different groups. The results obtained, concerning the evolution of the absolute and relative organ weight, are shown in the tables and figures.
Macroscopic examination of the internal organs of the control and treated groups, including liver, kidneys, spleen, heart, and lungs, revealed no abnormalities or morphological changes related to the administration of the extracts. These organs had a normal appearance similar to that of the organs in the control group (Fig. 3).
Effect of the hydro-methanolic and hot aqueous extracts of Sonchus oleraceus L. on the absolute organ mass of female mice treated under the conditions of subacute toxicity. HME, hydro-methanolic extract; HAE, hot aqueous extract. Values are mean ± SEM of 6 mice. Compared to the control group (one-way ANOVA followed by Dunnett’s post hoc test). *Significantly different from the control sets, p < 0.05
Figure 3 shows the effects of S. oleraceus L. extracts on the absolute weight of the liver, kidneys, spleen, lungs, and heart. The absolute weight of each organ recorded at necropsy in the treatment groups did not show any significant difference compared to the control group. In contrast, in mice treated with HME, a significant decrease was noted at 1000 mg/kg compared to control. However, there was no significant change (p > 0.05) in the absolute weight of these organs regardless of the extract administered (Fig. 3). In mice treated with HAE (Fig. 3), there was no significant change (p > 0.05) in absolute organ weights at any dose administered.
According to Fig. 4, there is no significant variation (p > 0.05) in the relative weights of all the organs studied (liver, kidneys, spleen, lungs, and heart). However, there was a non-significant increase in relative liver and kidney weights in HME extract-treated mice compared to controls. When mice were treated with HME extract, we observed a slight weight loss in the spleen, lungs, and heart. In the groups of mice in which hot aqueous extract “HAE” of the plant was administered, there were slight non-significant decreases in relative organ weights compared to the control group.
Effect of the hydro-methanolic and hot aqueous extracts of Sonchus oleraceus L. on the relative organ weights of female mice treated under the conditions of subacute toxicity. HME, hydro-methanolic extract; HAE, hot aqueous extract. Values are mean ± SEM of 6 mice. Compared to the control group (one-way ANOVA followed by Dunnett’s post hoc test). *Significantly different from the control sets, p < 0.05
Effect on biochemical parameters
The biochemical parameters (Table 2) are essential as they provide information on major toxic effects in tissues, specifically the effects on kidney and liver. Some enzymes and proteins can be used to indicate hepatocellular effects (seen in ALT, AST, and bilirubin). Cholesterol levels are an indirect indicator of liver function; creatinine, uric acid, and blood urea nitrogen are biomarkers of nephron functional injury (Brandt et al. 2009).
Tables 2 and 3 summarize the levels or activities of biochemical parameters in female mice treated with both extracts. The biochemical parameters for the assessment of renal function are shown in the figures and tables. The serum creatinine concentration of mice treated with HME extract showed a slight non-significant decrease; however, the creatinine elevation was significant (p ˂ 0.05) compared to that of control animals for mice treated with HAE extract.
It should be noted that the HAE extract caused an increase in urea activity in mice. This increase was very significant (p ˂ 0.01) at 500 mg/kg b.w (0.28 ± 0.06 g/L) compared to the control group (0.14 ± 0.03 g/L) (Table 3). However, fasting glucose and uric acid levels showed no significant difference (p ˃ 0.05) at any dose or extract administred compared to the control groups.
The biochemical markers for the evaluation of liver function are presented in the tables. Liver transaminases (AST: aspartate aminotransferase and ALT: alanine aminotransferase) showed a slight non-significant increase compared to the HME control, whereas HAE caused an increase in ALT. This increase was highly significant (p ˂ 0.001) at 500 mg/kg b.w (128.67 ± 55.38 IU/L) compared to the control (40.70 ± 10.93 IU/L). The enzyme activity of ALT was significantly increased as well in HAE-treated animals compared to the control as shown in Table 3. Furthermore, the action of HME and HAE extracts on liver biochemical markers (AST and ALT) is not dose-dependent.
For the 1000 mg/kg dose, there was a non-significant decrease in “ALP” alkaline phosphatase activity and a slight increase in total protein concentration, but these were not significantly different from those of the vehicle control group (p ˃ 0.05). In contrast to the HAE-treated mice, at 2000 mg/kg b.w, the elevation in total protein concentration was significant (p ˂ 0.05) with values of 65.31 ± 2.21 g/L compared to the control group (42.70 ± 4.94 g/L).
In addition, no significant changes were found between all HME-treated groups in total bilirubin, direct bilirubin, triglyceride, and cholesterol levels, while HAE caused an increase in cholesterol in mice. The increase in concentration was very significant (p ˂ 0.01) at doses of 500–2000 mg/kg b.w (1.20 ± 0.24; 1.03 ± 0.10; 1.04 ± 0.01 g/L) compared to the control group (0.61 ± 0.11 g/L). On the other hand, a significant decrease (p ˂ 0.01) in indirect bilirubin activity was noted in mice treated with 250, 500, and 1000 mg/kg HME extract with concentrations of 0.63 ± 0.1 5; 0.31 ± 0.10; and 0.83 ± 0.79 mg/L compared to the DMSO control group (1.95 ± 0.25 mg/L), while LDL values showed a very significant increase (p ˂ 0.01) at 500 and 2000 mg/kg b.w (0.38 ± 0.15 and 0.39 ± 0.05 g/L) compared to the control group (0.11 ± 0.03 g/L).
This allows us to conclude that repeated oral application of the hydro-methanolic extract of the plant, at the induced dose, leads to a disturbance of LDL as well as hepato-renal function, in particular indirect bilirubin, while the hot aqueous extract caused a lipidic and hepato-renal disorder at the level of cholesterol, ALT, urea, creatinine, and total proteins.
Effect on hematological parameters
The evaluation of hematological parameters (Tables 4 and 5) is important for assessing the physiological and pathological status in humans and animals (Khan et al. 2011).
Effects of HME and HAE on erythrocyte lineage parameters (red blood cells, hemoglobin, hematocrit, MCV, MCH, CCMH)
Red blood cell, hemoglobin, and hematocrit values (Tables 4 and 5) were significantly increased (p ˂ 0.0001) in mice treated with HME. Significant raise in RBCs (at 1000–2000 mg/kg), HGB, and HCT (at 1000 and 2000 mg/kg) was recorded. In contrast, at 500 mg/kg mice showed a highly significant (p < 0.0001) decrease in RBCs, HGB, and HCT compared to the DMSO control group. Mice treated with hot aqueous extract “HAE” showed a slight non-significant increase in HGB compared to the physiological water control group, while RBC and HCT values were constant (Table 5). For MCV level, the increase was very significant (p ˂ 0.001) at 500 and 1000 mg/kg (55.30 ± 0.10 and 54.40 ± 0.30 fl.) compared to controls (49.85 ± 0.98 fl.); in contrast to HAE extract-treated mice which had constant MCV values. There was a very significant variation in the MCH, with lower values (p ˂ 0.001) at the doses (250, 500, and 2000 mg/kg) compared to the control group. Similarly for HAE extract, MCH showed a significant decline (p ˂ 0.001) at all doses except the 1000 mg/kg b.w dose. For MCHC, the decline observed was highly significant (p ˂ 0.0001) at all doses compared to controls (Table 5). For groups treated with HAE, the concentration of MCHC was decreased insignificantly at the different doses tested.
In addition, the hematological analysis of the blood revealed significant changes in the calculated parameters. However, these changes did not occur simultaneously in the groups of animals and were not dose-dependent.
Effects of HME and HAE on leukocyte lineage parameters (white blood cells, neutrophils, lymphocytes, eosinophils, basophils, and monocytes)
Noting that the statistical study by Dunnett’s t-test showed that all differences between the groups for leukocyte lineage parameters (WBC, NEU, LYM, EOS, and MONO) were highly significant for all the tested doses of HME extract. Similarly, mice treated with HAE showed a highly significant (p ˂ 0.0001) increase in MONO and BASO and a large significant (p ˂ 0.0001) decrease in LYM over all doses used. WBCs were significantly decreased (p ˂ 0.001) at doses of 500 and 1000 mg/kg b.w, and NEU were increased at doses of 1000 and 2000 mg/kg b.w. BASO showed a very significant (p < 0.001) increase at 1000 mg/kg (0.40 ± 0.09%) compared to controls (0.02 ± 0.01%) (Tables 4 and 5).
Effects of HME and HAE on blood platelets
HME caused a decrease in blood PLT in mice at all doses used (Table 4). This decrease was more significant at 250 mg/kg (143.00 ± 3.00 103/µL, p ˂ 0.0001) compared to controls (695.70 ± 71.90 103/µL), whereas HAE caused a highly significant (p < 0.0001) decrease at doses of 250, 500, and 1000 mg/kg compared to the control group.
This leads us to conclude that the 28-day repeated-dose oral application of the hydro-methanolic extract and the hot aqueous extract of perennial sow-thistle caused a strong disturbance of hematological parameters.
Histopathological studies
At the end of the treatment, macroscopic examination of the internal organs of the control and treated groups, including liver, kidney, spleen, heart, and lungs, revealed no pathological abnormalities or morphological changes related to the administration of the extracts after preparation and staining with hematoxylin–eosin. These showed a normal appearance similar to that of the organs in the control group.
The general appearance of the organs of HME- and HAE-treated mice, as well as their relative weights, was similar to those of control mice. The organs (liver, kidneys, and lungs) are being studied anatomo-pathologically and histologically, as some of the products could alter any of the functions of these organs.
The results of the histopathological examination are shown in the figures. The observation of the histological sections of the liver, kidneys, and lungs of the treated mice in comparison with the controls allowed to observe the conservation of the cellular architecture (lobular and tubular) of these organs; however, some particularities were considered.
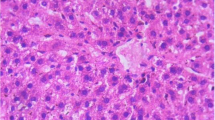
The livers of control female mice are normal with hepatocytes separated by narrow sinusoids. Vascular congestion with tissue infiltrates by inflammatory cells is recorded on some liver tissues in mice treated with hydro-methanolic extract of S. oleraceus L. with the doses of 250 and 2000 mg/kg b.w, respectively (Fig. 5).
Photomicrographs of histological sections from female mice liver tissue in subacute toxicity study of hydro-methanolic extract “HME” of Sonchus oleraceus L. A Control group, B HME 250 mg/kg treated group, and C HME 2000 mg/kg treated group. The slides were stained with hematoxylin/eosin and viewed at 40 × magnification
The kidneys of control mice showed normal glomeruli and tubules; however, examination of the kidney revealed the presence of hemorrhagic nephritis, a few spots of hemorrhagic congestion, and hemorrhagic degeneration in HME-treated mice with the doses of 2000 and 500 mg/kg b.w, respectively. Hemorrhagic degeneration, tissue infiltration by inflammatory cells, hemorrhagic nephritis, intravascular congestion, and presence of blood, i.e., blood infiltration of the distal convoluted tubule, were observed when the effect of the 2000 mg/kg of HME dose was exploited compared with controls (Fig. 6).
Photomicrographs of histological sections from female mice kidney tissue in subacute toxicity study of hydro-methanolic extract of Sonchus oleraceus L. A Control group, B HME 500 mg/kg treated group, and C HME 2000 mg/kg treated group. (C) congestion; (DC) distension of the channels; (N) necrosis; (TD) tissue damage; (D) distal convoluted tube; (P) proximal convoluted tube; (NP) nucleus of a podocyte; (I) infiltration. The slides were stained with hematoxylin/eosin and viewed at 40 × magnification
Whereas histological examination of the lungs revealed hemorrhagic degeneration in the sections from the dose-treated mice (250 mg/kg and 2000 mg/kg b.w, respectively) (Fig. 7), the other batches of organs (liver, kidneys, and lungs) had normal architecture and showed no evidence of damage compared with control sections (Figs. 5, 6, and 7).
Photomicrographs of histological sections from female mice lung tissue in subacute toxicity study of hydro-methanolic extract of Sonchus oleraceus L. A Control group, B HME 250 mg/kg treated group, and C HME 2000 mg/kg treated group. The slides were stained with hematoxylin/eosin and viewed at 40 × magnification
However, the histological sections of the liver, kidneys, and lungs of the mice presented severe histopathological alterations, such as vascular congestion of the liver and intravascular congestion of the kidney, renal nephritis, hemorrhage and degeneration of the kidneys and lungs, blood infiltration of the distal renal tubule, and massive inflammation (mononuclear inflammatory infiltrates). Histological examination also reveals the presence of foci of necrosis and distensions of the renal tubules in the conditions of subacute toxicity.
The results of histopathological examination of organs from mice treated with hot aqueous extract HAE of Sonchus oleraceus L. (250–2000 mg/kg b.w) are shown in Figs. 8, 9, and 10.
Photomicrographs of histological sections from female mice liver tissue in subacute toxicity study of hot aqueous extract “HAE” of Sonchus oleraceus L. A Control group, B HAE 250 mg/kg treated group, C HAE 500 mg/kg treated group, D HAE 1000 mg/kg treated group, and E HAE 2000 mg/kg treated group (C). The slides were stained with hematoxylin/eosin and viewed at 40 × magnification
Photomicrographs of histological sections from female mice kidney tissue in subacute toxicity study of hot aqueous extract “HAE” of Sonchus oleraceus L. A Control group, B HAE 250 mg/kg treated group, C HAE 500 mg/kg treated group, D HAE 1000 mg/kg treated group, and E HAE 2000 mg/kg treated group (C). The slides were stained with hematoxylin/eosin and viewed at 40 × magnification
Photomicrographs of histological sections from female mice lung tissue in subacute toxicity study of hot aqueous extract “HAE” of Sonchus oleraceus L. A Control group, B HAE 250 mg/kg treated group, C HAE 500 mg/kg treated group, D HAE 1000 mg/kg treated group, and E HAE 2000 mg/kg treated group (C). The slides were stained with hematoxylin/eosin and viewed at 40 × magnification
The livers of control female mice revealed normal hepatic architecture, polyhedral hepatocytes separated by narrow sinusoidal spaces, and central veins. However, vascular and intravascular congestion with some spots of hepatic degeneration was observed at the dose of 500 and 2000 mg/kg b.w, respectively (Fig. 8).
The kidneys of mice injected with 250 mg/kg b.w of HAE showed hemorrhagic nephrons with some points of degeneration. The architecture of the nephric tissue of mice treated with 500 mg/kg of extract showed hemorrhagic lesions, with hemorrhagic nephritis and the veins marked by vascular congestions (Fig. 9). Whereas at 1000 mg/kg, the kidneys showed mild nephritis with some hemorrhagic spots and intravascular congestion. In addition, histological incision of the kidney revealed congestion with a few spots of tissue degeneration in the highest dose group (2000 mg/kg b.w) compared with the control group.
Similarly, histological examination of lung appeared to have tissue and hemorrhagic degeneration at the doses of 250 and 500 mg/kg b.w, respectively compared to control. Subacute administration of hot aqueous extract HAE at 1000 mg/kg b.w resulted in accentuated intravascular congestion with some degeneration spots, whereas histology of lung sections from mice given 2000 mg/kg b.w HAE extract revealed hemorrhagic degeneration (Fig. 10).
Discussion
Most of the pharmacological effects can be explained by the phenolic compounds including flavonoids present in all plant parts. The class of flavonoids is important because they are essential components to the humans and animal diet, and they possess a therapeutic potential, including antitumoral activity (Tungmunnithum et al. 2018). Some work has been done on the plant S. oleraceus L., citing; Awaad and his collaborators (2018) report that the total alcoholic extract contains carbohydrates and/or glycosides, flavonoids, sterols and/or triterpenes, phenolic compounds and tannins, and the absence of saponins, anthraquinones, alkaloids, and cardenolides. Teugwa et al. (2013) report that methanolic and ethanolic “EE” extract from the same Cameroonian plant contain a diversity of chemical families including polyphenols, flavonoids, alkaloids, terpenes, sterols, and saponins. However, anthocyanins and lipids were absent, and these results are in agreement with ours except for the absence of saponins in these two extracts. Makkawi et al. (2010) find the presence of flavonoids and tannins only in the EE and not in the HAE, which is not in agreement with our preliminary qualitative study. Thus the absence of these biomolecules in the HAE is due to the heating of the extract and its low solubility in alcohol.
Despite the widespread use of medicinal plants to treat different diseases in developing countries, little scientific data regarding their toxicity are available (Saad et al. 2006). In fact, several studies have reported that some medicinal plant can cause several forms of toxic effects, which can have a negative impact on human health. Thus, to ensure the safety of plant products for human use, systematic studies are needed to predict toxicity risks and provide scientific information to select safe doses in humans (Neergheen-Bhujun 2013), as it is the dose that makes the drug a poison. Although Sonchus oleraceus L. aerial parts have valuable pharmacological effects, the comprehensive awareness about the toxicity potential of hydro-methanolic and hot aqueous extracts has been lacking. Hence, this project was designed to check the toxic effect by performing acute oral toxicity in mice for 14 days and subacute oral toxicity in mice for 28 days.
In the acute study, a single dose of up to 5000 mg/kg b.w of HME extract and HAE showed no signs of toxicity or mortality throughout the observation period (14 days). Therefore, the approximate lethal dose (LD50) of both extracts from the aerial part of S. oleraceus L. is estimated to be greater than 5 g/kg b.w. According to the globally Harmonized Classification system for chemical substances and mixtures (GSH) adopted by the OECD, substances with an oral LD50 greater than 5 g/kg could be assigned as a class 5 drug and then, considered as a non-toxic substance (OECD 2001). These results are comparable to those obtained with hydroethanol or dichloromethane extracts of Sonchus oleraceus from Brazil (Vilela et al. 2009b) in mice. According to these authors, the extracts administered to mice had no effect on their behavioral responses during the 14-day observation period after administration. No mortality was observed up to 14 days of monitoring. The LD50 value of these extracts in mice was therefore estimated to be greater than 5 g/kg b.w.
Changes in general behavior, body, and organ weights are considered as critical indicators for the evaluation of early signs of toxicity caused by drugs and chemicals (Ezeja et al. 2014). They are also markers of pathological and physiological well-being in animals. The weight of the damaged organ would be altered based on the magnitude of the toxicity and the ratio of body weight to organism weight (Yam et al. 2009). In subacute toxicity, no animal was found dead, and no significant changes were observed in the general behavior of the HME- and HAE-treated groups in female mice. The crude extract did not show a significant change in body weight of female mice during the treatment period. However, the increase in body weight was significant at D14 in the 1000 mg/kg b.w group. On the other hand, the other groups showed normal body development similar to that of the control group. A significant increase in body weight at D7 was recorded in mice treated with hot aqueous extract of S. oleraceus L. at a dose of 2000 mg/kg b.w. No significant changes in body weight were observed between the control and other treated groups throughout the study period. Treated mice gained weight with age, and there was no significant change (p > 0.05) in their mean body weight compared to the control groups throughout the study. It can be concluded that oral administration of HME and HAE extracts at a dose of up to 2 g/kg for 28 days did not adversely affect the normal growth of mice.
Assessment of liver and kidney function is a very important part of assessing the toxicity of drugs and plant extracts. The hematopoietic system, which is one of the most vulnerable targets of toxic substances, resides in the bone marrow where the production of red blood cells occurs (Birbrair and Frenette 2016); it is also an important index of the physiological and pathological state in humans and animals (Liju et al. 2013). Toxicological studies have shown that alterations in the hematopoietic system have a higher predictive value for human toxicity when extrapolated from animal studies (Olson et al. 2000). In this study, significant alterations in WBC and platelet counts were observed in female mice treated with the different doses of HME and HAE extracts. However, these changes were not dose-dependent. Blood platelet plays a vital role in hemostasis. Tohti et al. (2006) have reported decrease in WBC due to the presence of some phytochemicals such as saponins and cardiac glycosides. This decrease may also suggest the extract’s inhibitory effect on white blood cell production, possibly by decreasing thrombopoietin secretion. White blood cells function is mainly to fight infection and produce and transport antibodies to various body compartments, which suggest that there is cellular inflammation process. Also, a low platelet count (thrombocytopenia) contributes to morbidity and mortality in several diseases and requires early diagnosis so that appropriate management measures can be taken (Smock and Perkins 2014).
Hematological analysis revealed a disturbance “increase and decrease” in the level of MCV or MGV (mean corpuscular volume or mean globular volume) of mice treated with the crude extract at the doses of 500 and 1000 mg/kg b.w, but this change was not observed in the groups of mice treated with the HAE. Therefore, this difference was not considered a toxic effect because it did not show a clear dose–response relationship, but it suggests that there is some influence of both extracts on the hematopoiesis pathway. In this study, subacute administration of HME and HAE did result a minor significant changes in the hematological profile of mice that received different doses of the extracts compared to the control group, suggesting that HME and HAE are probably toxic to the blood system. This leads us to conclude that the 28-day repeated oral application of the hydro-methanolic and hot aqueous extracts of sow-thistle caused a strong disturbance of the hematological parameters.
The liver is a vital organ that plays a central role in drug biotransformation, and its normal function is assessed by various serum biomarker enzymes (Olorunnisola et al. 2012). Increased serum ALT levels reflect liver tissue hypertrophy and damage (Costa-Silva et al. 2008). AST levels, in addition of being an indicator of liver dysfunction, are also used to assess muscle and heart diseases (Adeyemi and Osubor 2010). Alkaline phosphatase “ALP” is mainly present in the cells lining the bile duct of the liver and is used in the diagnosis of bile duct pathologies (Ramaiah et al. 2011). Assessment of liver function is essential for evaluating the toxicity of drugs and plant extracts (Clarke and Clarke 1977). In fact, AST, ALT, ALP, total serum protein, and total bilirubin concentration in serum are the most common clinical biomarkers of liver status (Al-Mamary et al. 2002; Philip and Zilva-Pannal 1994).
In the present study, HME and HAE extracts of SO produced a non-significant increase in serum levels of ALT, AST, and ALP compared to controls; but the significant increase in ALT level of treated mice could indicate a decline in liver function due to a possible hepatotoxic effect of the HAE extract. This may suggest that there is no apparent hepatotoxicity effect in mice treated with HME. It is known that damage to the structural integrity of the liver results in an increase of specific hepatic enzymes (ALP, ALT, and AST) in the serum, because they are cytoplasmic enzymes and are put into circulation after hepatocellular damage (autolytic degradation or cellular necrosis) (Janbaz and Gilani 1995; Venkateswaran et al. 1997). It is necessary to mention that these transaminases play an important role in the metabolism of amino acids and by providing intermediates necessary for gluconeogenesis (Hanley et al. 1986). It was reported by Khan et al. (2012a) that methanolic extract of the whole plant of S. asper from Pakistan showed strong anti-hepatotoxic activity toward CCl4 (carbon tetrachloride) by reducing the activity of all three enzymes (ALP, ALT, and AST), cholesterol, and triglyceride while raising the levels of HDL. According to the previous studies on other species of the genus Sonchus, it appears that the extracts obtained from this genus are not toxic. Alkreathy et al. (2014) reported that the methanolic extract of S. arvensis is hepatoprotective by reducing cholesterol, HDL and by increasing triglycerides and LDL. Administration of CCl4 significantly (p < 0.01) increased the activity of liver serum marker enzymes such as AST, ALT, and ALP compared to the control groups. The elevations of secretion of these enzymes were significantly (p < 0.01) decreased by 100 mg/kg b.w and 200 mg/kg b.w of extract compared to the CCl4 group. In conclusion, the results of our study are in agreement with the studies already conducted on other species of the genus Sonchus (Khan et al. 2012b; Alkreathy et al. 2014). Nurianti et al. (2014) determined the LD50 of ethyl acetate extract of S. arvensis L. leaves on rats after single and repeated dose administration based on acute (14d) and sub-chronic (90d) oral toxicity studies which is greater than 15 g/kg body weight and was classified as practically non-toxic. No abnormalities in behavioral, hematological, clinical biochemical, and urinary parameters were recorded. The macroscopic and microscopic presentations of the organs were not different between the control and test groups, which is not in agreement with our findings.
Bilirubin is a breakdown product of hemoglobin, and increases in its serum level are associated with diseases such as primary biliary cirrhosis and hepatic cholestasis (Thapa and Walia 2007), whereas serum total protein level is a proximate measure of protein status that may reflect major functional variations in kidney and liver function. Abnormal levels may be associated with liver infections or chronic inflammation (Tatefuji et al. 2014). Proteinuria is a urine marker in the diagnosis of renal dysfunction, and may indicate the development of various renal pathologies (McElreath et al. 2007). Of which, the decrease in total plasma protein concentration has been proposed as an indicator of protein synthesis alteration (Kubena et al. 1993). In the present study, total protein was elevated very slightly not significantly in the HME-treated groups; however, the total protein concentration of HAE-treated mice was significantly increased at 2000 mg/kg b.w due to increased synthesis by the liver, while there was a non-significant decrease in bilirubin levels in the groups treated with both extracts compared to the control groups. This variation may be attributed to hepatic structural damage and inhibition of bile secretion, specifically the interruption of bile flow through the liver (Metushi et al. 2011). These results confirm previous findings regarding hematological parameters and liver marker enzymes, which indicate that the extracts may have an adverse effect on the erythropoietic system and liver. Hayes (2007) also postulated that since the liver is also a major organ of protein synthesis, any decrease in hepatic synthesis can be considered as damage to hepatocytes with impairment of its production capacity. Impaired hepatocellular function may result in reduced total protein and bilirubin concentrations in the insert. The insignificant change in serum total protein and bilirubin concentrations in the treated and control groups further suggests that synthetic liver functions are altered at the test doses of hydro-methanolic and hot aqueous extracts.
The kidney is a vital organ that is highly vulnerable to toxic compounds due to the high volume of blood flowing through it. It filters large types of toxins, which can accumulate in the renal tubules (Akindele et al. 2014). Urea, uric acid, and creatinine are considered sensitive biomarkers of kidney injury. An elevated renal biomarker is an indication that poor excretion may be occurring. This also applies to liver function enzymes, as the liver and kidneys are the primary sites of elimination of substances in the body (Gowda et al. 2010). Glomerular filtration in this study was evaluated through the measurement of creatinine clearance and urea. Serum creatinine of mice treated with HME extract showed a slight insignificant decrease; on the other hand, the elevation of creatinine was significant (p ˂ 0.05) compared to that of control animals for mice treated with HAE extract. Mice treated with HME and HAE with the doses of 250–2000 mg/kg b.w had an increase in urinary protein, which was within normal limits. However, uric acid concentrations recorded a non-significant increase (p ˃ 0.05) regardless of the dose or extract injected compared with the control groups. This increase in urinary protein did not correlate with other biomarkers in renal serum. Moreover, the increase of serum creatinine is considered as the marker leading to nephron damage (Mehta et al. 2007), and increased urea level is usually correlated with renal ischemia or it may be the result of elevated glomerular filtration (Soussi et al. 2018). With that respect, our findings indicate that mice may be suffering from renal ischemia. Another biochemical marker used in the current study, namely uric acid levels in plasma, indicated a dysfunction of kidneys. Uric acid is the end-product of purine catabolism, and it has been shown to reduce oxidative stress by scavenging ROS-like peroxynitrite (Hooper et al. 1998). Furthermore, a body of evidence have shown that the disturbance of urine biochemical markers in animals is a consequence of oxidative stress, and is associated with glomerular and tubule interstitial changes, hyperuricemia, vacuolization, and renal breakdown (Navarro-Moreno et al. 2009). However, the increase in urea, creatinine, and uric acid, even if not significant, could indicate a possible impact of SO on renal function.
The liver is known to be the primary organ for glycogen metabolism. Glycogen depletion in the liver is a common result of liver failure after liver damage caused by toxic compounds. This hepatic failure can cause an increase in blood insulin, and a consequent reduction in blood sugar, which is consistent with the level of glucose and glycogen in the liver (Wu et al. 2005). In our study, the elevation of glucose level suggests that the HME and HAE extracts could directly affect the synthesis, storage, and breakdown of glycogen in the liver. Therefore, we suggest that SO treatment had a significant impact on total protein concentrations.
Hyperlipidemia is well known as one of the main risk factors for atherosclerosis, which leads to coronary heart disease (Fuster and Esko 2005). The elevated cholesterol levels could be explained by the stimulation of lipid anabolism in the hepatocytes by the action of the extract or by an exogenous supply of fatty compounds contained in the extract. In recent years, research in cardiovascular pharmacology has been particularly interested in hypolipidemic drugs derived from medicinal plants. Oral administration of HME extract to mice for 4 weeks produced a significant (p ˂ 0.05) increase in LDL, and a reduction in cholesterol, TGs, and HDL compared with the control groups, whereas oral administration of HAE extract produced a reduction in LDL and a significant (p ˂ 0.05) increase in cholesterol and non-significant increase in triglycerides and HDL. Abnormal lipid metabolism can lead to elevated levels of fatty substances, largely cholesterol and triglycerides in the bloodstream, resulting in hyperlipidemia (Ginsberg et al. 2006). The present study showed marked hyperlipidemia and a marked increase in cholesterol in mice treated with HAE extract, in contrast to the crude extract-induced hypolipidemia by decreasing cholesterol, TG, and HDL levels. These results suggest that the hydro-methanolic extract of Sonchus oleraceus L. may be effective in improving lipid profile, which may protect against atherosclerosis, coronary heart disease, and diabetic complications.
Previous phytochemical studies have shown that the crude extract of SO contains a variety of phenolic compounds and flavonoids (Abhijeet et al. 2018). These bioactive constituents present in the crude extract may explain the observed pharmacological effects. The previous study by Teugwa et al. (2013) reported that polyphenols and flavonoids in the hydroethanol extract of the whole plant of S. oleraceus exert their hypoglycemic effect through different mechanisms, including inhibition of α-amylase and α-glucosidase activities, increased peripheral glucose uptake, and stimulation of insulin secretion by pancreatic ß-cells. This result is in agreement with the previous findings of Schaffer et al. (2005), who investigated the subacute toxicity of 100 mg/kg b.w infusion and 6 mg/kg b.w esculetin of S. oleraceus in Wistar rats. They observed hypolipidemic activity by reducing lipid, cholesterol, triglyceride, LDL, increasing HDL levels, and by stimulating insulin production or by modulating enzymes involved in lipid metabolism. Several plants containing flavonoids with hypoglycemic action also showed hypolipidemic activity (Vinayagam and Xu 2015), which is in agreement with our results. Ginsberg et al. (2006) revealed that covalent binding of the reactive products to hepatocyte components initiates inhibition of lipoprotein secretion and thus increase in total hepatic lipids in the liver of extract-treated mice may be due to blockage of lipoprotein biosynthesis and/or secretion. Hypertriglyceridemia may lead to induction of the hepatic enzyme, which further induces the activity of regulatory enzymes in triglyceride biosynthesis. Multiple hyperlipidemias are often secondary to many factors, e.g., diet, alcohol intake, and therapies, or to diseases such as nephrosis, diabetes, hypothyroidism, or tumors (Havel 1969). The 100 mg/kg and 200 mg/kg methanolic extract of Sonchus asper from Pakistan could have the ability to scavenge free radicals which in turn reduce serum cholesterol and triglycerides which were reported in the investigation of Khan et al. (2012c). These results are comparable to those obtained with the butanolic extract of Sonchus asper from Pakistan (Khan et al. 2012c) in rats for 5 weeks. According to these authors, the plant extract would cause a significant improvement in creatinine, urobilinogen, and decrease in high levels of proteinuria, hematuria, total bilirubin, and direct bilirubin unlikely to our extracts, thereby leading to protection of the kidneys from potassium bromate (KBrO3) induced oxidative damage. This indicates that both extracts may affect normal renal function after subacute exposure compared to the control group.
Liver, kidney, heart, lungs, and spleen are the vital organs of our body which are the major targeted area of any toxic substance metabolically (Auletta 1995). The change in relative organ weights is a reliable indicator that can be used in toxicological investigations to assess toxicity caused by drugs and chemicals (Balogun et al. 2014). Mice did not show signs of toxicity throughout the experimental period. No macroscopic lesions were observed in the organs. Absolute and relative organ weights (liver, kidney, lung, spleen, and heart) indicated no toxic effects in both the control and treated groups, and statistically insignificant differences (p > 0.05) suggested that the plant extracts were not toxic to the animals at the doses tested (250–2000 mg/kg b.w). Furthermore, these changes (non-significant increase or decrease) can be considered toxicologically relevant, as no effects were observed at the highest dose, but histopathological changes were found in the liver, kidney, and lung.
In several organs, cellular damage is followed by the release of a number of cytoplasmic enzymes into the blood, a phenomenon which forms the basis for clinical diagnosis. On the one hand, in our study, the liver function was evaluated by measuring the plasma concentrations of ALT, AST, ALP, serum protein, and bilirubin. Renal function was assessed by measuring plasma creatinine, urea, and uric acid concentrations (Sundberg et al. 1994). On the other hand, the beneficial effects of bioactive molecules of Sonchus oleraceus L. in the protection of liver damage have already been reported and are consistent with the results obtained from biochemical indicators. In addition, herbal drugs are considered to have a lower risk of toxicity. However, they are not completely free from the possibility of inducing toxicity or other adverse effects (Thiesen et al. 2018; Tir et al. 2019). The results of the histopathological investigation showed significant correlations with the results of the biochemical study. The graded observations of the hematoxylin and eosin stained sections are presented in the figures. The histopathological alterations observed are vascular and intravascular congestion of the liver, tissue infiltration of the liver by inflammatory cells, sub-capsular hepatic degeneration, hemorrhagic nephritis, a few spots of congestion and hemorrhage of the kidneys, renal degeneration, and hemorrhagic degeneration of the lungs (Figs. 5–10).
In the present study, treatment with HME extract modulated lipid alterations by maintaining TG, LDL, HDL, and cholesterol levels at near normal values, reduced histopathological alterations, and the degree of steatosis. As mentioned above, treatment with HME extract clearly reduced bilirubin levels to near normal, suggesting that the components of the crude extract may be effective in reducing cholesterol synthesis. Remarkably, the use of HME extract resulted in normalization of all assayed lipid parameters, which may stimulate β-oxidation of fatty acids and which in turn reduce triglyceride biosynthesis in the liver. The mechanism by which HME extract reduced cholesterol levels could be due to the stimulation of 7-α-hydroxylase responsible for the conversion of cholesterol to bile acid (Khan et al. 2016; Lv et al. 2016). It has been reported that the regulation of lipid profile parameter levels by polyphenols could be due to one or more mechanisms, and the mechanisms underlying the lipid-lowering effect of polyphenols may involve inhibition of lipoprotein synthesis and secretion, reduction of LDL receptor expression (Benn et al. 2015). Kassaee et al. (2017) cited that catechins would exert a direct influence on LDL and HDL levels by regulating LDL receptor binding activity of HepG2 cells. Kassaee et al. (2017) showed that Cinnamomum zeylanicum flavonoids can act by making liver cells more efficient at removing LDL-C from the blood by increasing LDL receptor densities in the liver and binding to apolipoprotein. Reduced cellular cholesterol biosynthesis is often associated with increased LDL receptor activity, which in turn leads to the removal of LDL cholesterol from plasma, resulting in lower plasma cholesterol concentrations (Kassaee et al. 2017). In support of our present results, several studies have demonstrated the protective effect of some polyphenols on liver toxicity and improvement of hepatocyte antioxidant defense (Liu et al. 2014; Bahmani et al. 2015). Jimoh et al. (2011) and Obeid et al. (2018) confirmed the richness of methanolic and ethyl acetate extract in flavonoids, flavonols, proanthocyanidins, and total polyphenols respectively and that these extracts are endowed with considerable antioxidant power. These results are consistent with those observed in the study of the hepatoprotective activity of Sonchus arvensis against (CCL4)-induced liver injury in rats (Alkreathy et al. 2014). Another study showed the hepatoprotective effect of Sonchus asper against CCL4 toxicity, and it is closely related to the presence of the antioxidant molecules (Khan et al. 2012a), which is not in accordance with our study.
The results suggest that HAE-treated mice were more sensitive than HME-treated mice. The observed differences in the pattern of acute and subacute toxicity between the different types of treatment may be explained by differences in phytochemical composition. Several factors may be involved as well, such as drug metabolizing enzymes (cytochrome P450), transport proteins (P-glycoprotein), organ size, higher percentage of body fat, and lower glomerular filtration rate in females than in males (Marzolini et al. 2004).
In addition, histological examination showed little difference between control mice and those given 250 and 2000 mg/kg/day of HME extract, which is further evidence of the nonsafety of the crude extract in mice. Our results from acute and subacute toxicity studies indicate that oral administration of HME extract (250–2000 mg/kg/day) did not affect normal growth in mice. To our knowledge, this is the first publication reporting a toxicological safety evaluation of an orally administered hydro-methanolic extract. Although HME extract is widely used to treat many diseases by replenishing vital essence, no comprehensive safety evaluation has been performed regarding its toxicological profile in animals. Thus, the results of this study provide an experimental basis for the clinical practice of the crude extract formula.
As observed in this study, non-significant elevations in serum AST, ALT, and ALP levels may be due to liver necrosis in HAE extract-treated mice. However, the remarkable ability of the liver to regenerate on its own allows it to tolerate moderate zonal or diffuse necrosis. Over a period of several days, necrotic cells are eliminated and replaced by new cells; and normal liver architecture and function are restored (Roberts et al. 2000). However, animals in the control group had intact hepatocytes, portal vein, glomeruli, and tubules. The occurrence of lymphocytic infiltration in the organs (liver and kidney) was attributed to the presence of glycosides as reported by Adedapo et al. (2003), and the result of this study is consistent with the findings of Builders et al. (2012), who studied the toxicity of Parkia big lobos a stem bark extracts in rats. It has been reported that the toxicity of some herbal drugs could be the result of phytochemical constituents.
Among the most abundant bioactive compounds that exist in HMA (Aissani et al. 2021) are quininic acid, aesculin, 3-(acetyl-oxy)-1-methoxy-1-(3,4,5-trimethoxyphenyl) propane, corchoionoside C, sonchuside H, loliolid, melampolide,15-O-β-D-glucopyranosyl-11β,13-dihydro urospermal A, tanacetin, and macrocliniside A. The different roles that these agents may play in the biological system suggest that they should be considered in future studies. Muhammad et al. (2012) also reported that high consumption of tannins can cause kidney and liver damage. High doses of N. campestris caused elevation of some serum biochemical parameters and histological changes in the target organs of toxicity (liver and kidney). The plant is however a promising agent in pharmacy, but may cause slight organ damage at high doses.
In summary, since the relative weights of the major organs affected by the extracts showed no significant treatment-related alterations, it can be said that the crude HME and hot aqueous HAE extracts induced an obvious adverse effect on the vital organs (liver, kidney, and lung) of the treated groups in the subacute treatment. In addition, histopathological examination showed several histological lesions that were observed in the organs after treatment with HME and HAE extracts. Therefore, these results may indicate that the extracts of Sonchus oleraceus L. have a toxic effect on the vital organs of mice. Overall, we suggest paying attention to this study to show how hydro-methanolic, hot aqueous extracts, and/or their bioactive components may interact at the molecular level, especially with specific organs in the body. This study provides insight into possible interactions with plants.
In conclusion, oral doses of extracts of the aerial parts of S. oleraceus L. can be considered unsafe as they showed adverse effects in liver, kidney, and lung tissues of subacute toxicity in female mice. The present study provides additional data on the use of S. oleraceus L. in traditional medicine and may lend credence to its medical use. Chronic toxicity, mutagenicity, and carcinogenicity assessments should be conducted to better understand the safety profile of the plant.
Conclusion
S. oleraceus L. is a good source of phytoconstituents (polyphenols, flavonoids, sterols and triterpenes, anthraquinone glycoside, and reducing compounds) in both hydro-methanolic and hot aqueous extracts. Acute and subacute toxicity studies were conducted to establish the safety of both extracts of S. oleraceus L. aerial parts in mice. The LD50 of the acute toxic study of the extracts HME and HAE were superior to 5 g/kg b.w; thus not toxic and safe by oral dose according to the protocol of Lorke, whereas the 28-day repeated-dose subacute toxicity test of the two extracts revealed an important hepatic, renal, and lung lesions up to the maximum dose of 2000 mg/kg b.w but did not cause any lethality. The samples exhibited no signifiant changes in behavior and organ-body weight ratios on tested models. Some changes in biochemical, hematological, and histological parameters suggest that precautions is advised when it is given over longer periods. Further studies should be carried out to establish safe and effective doses for the extracts and also to assess other types of toxicity such as chronic toxicity, reproductive toxicity, genotoxicity, and carcinogenicity using other models to establish a complete toxicity profile of this herb.
Availability of data and material
All data supporting the findings in this work are adequately contained within the manuscript.
Code availability
None.
References
Abhijeet VP, Prakash DK, Yunus NA (2018) A review on ethnomedicinal, pharmacological and a review on ethnomedicinal, pharmacological and phytochemical aspects of Sonchus oleraceus LINN (Asteraceae). IJPBS 8(3):01–09
Adedapo AA, Abatan MO, Akinloye AK, Idowu SO, Olorunsogo OO (2003) Morphometric and histopathological studies on the effects of some chromatographic fractions of Phyllanthus amarus and Euphorbia hirta on the male reproductive organs of rats. J Vet Sci 4(2):181–185
Adeyemi O, Osubor CC (2010) Toxicological evaluation of the effect of Clarias gariepinus (African catfish) cultivated in water contaminated with phthalate, benzene and cyclohexane on liver of albino rats. AJFS 4(1):026–031
Agra MDF, Baracho GS, Nurit K, Basílio IJLD, Coelho VPM (2007) Medicinal and poisonous diversity of the flora of Cariri Paraibano Brazil. J Ethnopharmacol 111(2):383–395
Aissani F, Grara N, Bensouici C, Bousbia A, Ayed H, Idris MHM, Teh LK (2021) Algerian Sonchus oleraceus L.: a comparison of different extraction solvent on phytochemical composition, antioxidant properties and anti-cholinesterase activity. Adv Trad Med 1–12
Akindele AJ, Adeneye AA, Salau OS, Sofidiya MO, Benebo AS (2014) Dose and time-dependent sub-chronic toxicity study of hydroethanolic leaf extract of Flabellaria paniculata Cav. (Malpighiaceae) in rodents. Front Pharmacol 5:78
Alkreathy HM, Khan RA, Khan MR et al (2014) CCl4 induced genotoxicity and DNA oxidative damages in rats: hepatoprotective effect of Sonchus arvensis. BMC Complement Altern Med 14(1):452
Al-Mamary M, Al-habori M, Al-Aghbari AM, Baker MM (2002) Investigation into the toxicological effects of Catha edulis leaves: a short term study in animals. Phytother Res 16(2):127–132
Auletta CS (1995) Acute, subchronic and chronic toxicology. CRC Press, London
Awaad AS, Almoqren SS, Safhi AA, Zain YM, El-Meligy RM, Al-Asamary FA (2018) Gastroprotective extracts of Sonchus oleraceus L. U.S. Patent No. 10, 137, 162. Washington, DC:U.S. Patent and Trademark Office
Bahmani M, Shirzad H, Rafieian S, Rafieian-Kopaei M (2015) Silybum marianum: beyond hepatoprotection. J Evid Based Complementary Altern Med 20(4):292–301
Balogun SO, da Silva Jr IF, Colodel EM et al (2014) Toxicological evaluation of hydroethanolic extract of Helicteres sacarolha A. J Ethnopharmacol 157:285–291
Bandaranayake WM (2006) Quality control, screening, toxicity, and regulation of herbal drugs. In: Ahmad I, Aqil F, Owais M (eds) Modern phytomedicine. Turning Medicinal Plants into Drugs, p 25–57
BeMiller JN, Whistler RL (2012) Industrial gums: polysaccharides and their derivatives. Academic Press
Benn T, Kim B, Park YK et al (2015) Polyphenol-rich blackcurrant extract exerts hypocholesterolaemic and hypoglycaemic effects in mice fed a diet containing high fat and cholesterol. Br J Nutr 113(11):1697–1703
Bhandary SK, Kumari S, Bhat VS, Sharmila KP, Bekal MP (2012) Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. J Health Sci 2(4):35–38
Birbrair A, Frenette PS (2016) Niche heterogeneity in the bone marrow. Ann N Y Acad Sci 1370(1):82–96
Brandt AP, Oliveira LFSD, Fernandes FB, Alba J (2009) Evaluation of prospective hypocholesterolemic effect and preliminary toxicology of crude extract and decoction from Vitex megapotamica (Spreng) Moldenke (V. montevidensis Cham.) in vivo. Rev Bras De Farm 19(2A):388–393
Bruneton J (2009) Pharmacognosie, phytochimie, plantes médicinales (4e éd.). Paris: Tec & Doc/Lavoisier, pp 279–281
Builders MI, Isichie CO, Aguiyi JC (2012) Toxicity studies of the extracts of Parkia biglobosa stem bark in rats. J Pharm Res Int 2(1):1–16
Clarke EGC, Clarke CML (1977) Veterinary toxicology. Cassel and Collier Macmillan Publishers, London, pp 268–277
Costa-Silva JH, Lima CR, Silva EJR et al (2008) Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J Ethnopharmacol 116(3):495–500
Ezeja MI, Anaga AO, Asuzu IU (2014) Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. J Ethnopharmacol 151:1155–1164
Fuster MM, Esko JD (2005) The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 5(7):526–542
Ginsberg HN, Zhang YL, Hernandez-Ono A (2006) Metabolic syndrome: focus on dyslipidemia. Obesity 14(2S):41S-49S
Gowda S, Desai PB, Kulkarni SS et al (2010) Markers of renal function tests. N Am J Med Sci 2(4):170–173
Hanley KS, Schmidt E, Schmidt FM (1986) Enzymes in serum, their volume in diagnosis. Illinois: Charles Thomas Springfield, pp 79–81
Hata K, Hori K, Konishi T (2000) Differentiation-inducing activity of lupeol, a lupane-type triterpene from Chinese dandelion root (Hokouei-kon), on a mouse melanoma cell line. Biol Pharm Bull 23:962–967
Havel RJ (1969) Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med 15:117
Hayes AW (2007) Principles and methods of toxicology. Informa Healthcare, New York
Hooper DC, Spitsin S, Kean RB et al (1998) Uric acid, anatural scavenger of peroxynitrite, in experimentalallergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA 95(2):675–680
Iqbal E, Salim KA, Lim LBL (2015) Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Univ Sci 27(3):224–232. https://doi.org/10.1016/j.jksus.2015.02.003
Jain SK, Singh GK (2014) Prelminary phytochemical screening and in vitro antioxidant activity of extracts of whole plant of Sonchus oleraceus Asteraceae. J Pharm Sci 3:1–12
Janbaz KH, Gilani AUH (1995) Evaluation of the protective potential of Artemisia maritima extract on acetaminophen-and CCl4-induced liver damage. J Ethnopharmacol 47(1):43–47
Jimoh FO, Adedapo AA, Afolayan AJ (2011) Comparison of the nutritive value, antioxidant and antibacterial activities of Sonchus asper and Sonchus oleraceus. Rec Nat Prod 5(1):29–42
John KMM, Ayyanar M, Arumugam T, Enkhtaivan G, Jin K, Kim DH (2015) Phytochemical screening and antioxidant activity of different solvent extracts from Strychnos minor Dennst leaves. Asian Pac J Trop Dis 5(3):204–209
Kassaee SM, Goodarzi MT, Hayati Roodbari N, Yaghmaei P (2017) The effects of Cinnamomum zeylanicum on lipid profiles and histology via up-regulation of LDL receptor gene expression in hamsters fed a high cholesterol diet. Jundishapur J Nat Pharm Prod 12(3):e37340
Khan RA, Khan MR, Sahreen S et al (2012c) Amelioration of kidney function markers by Sonchus asper butanolic extract against KBr O3-induced toxicity in rat. J Med Plant Res 6(7):1224–1228
Khan RA, Khan MR, Sahreen S, Ahmed M (2012a) Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem Cent J 6(1):1–7
Khan RA, Khan MR, Sahreen S, Shah NA (2012b) Hepatoprotective activity of Sonchus asper against carbon tetrachloride-induced injuries in male rats: a randomized controlled trial. BMC Complement Altern Med 12(1):1–8
Khan SA, Epstein JH, Olival KJ et al (2011) Hematology and serum chemistry reference values of stray dogs in Bangladesh. Open Vet J 1(1):13–20
Khan SR, Morgan AG, Michail K et al (2016) Metabolism of isoniazid by neutrophil myeloperoxidase leads to isoniazid-NAD+ adduct formation: a comparison of the reactivity of isoniazid with its known human metabolites. Biochem Pharmacol 106:46–55
Khanam Z, Wen CS, Bhat IUH (2015) Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali). J King Saud Univ Sci 27(1):23–30
Kubena LF, Harvey RB, Huff WE et al (1993) Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poult Sci 72(1):51–59
Kumar D, Ghosh R, Pal BC (2013) α-Glucosidase inhibitory terpenoids from Potentilla fulgens and their quantitative estimation by validated HPLC method. J Funct Foods 5(3):1135–1141
Liju VB, Jeena K, Kuttan R (2013) Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L). Food Chem Toxicol 53:52–61
Liu T, Yu XH, Gao EZ et al (2014) Hepatoprotective effect of active constituents isolated from mung beans (P haseolus radiatus L.) in an alcohol‐induced liver injury mouse model. J Food Biochem 38(5):453–459
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54:275–287
Lv O, Wang L, Li J, Ma Q, Zhao W (2016) Effects of pomegranate peel polyphenols on lipid accumulation and cholesterol metabolic transformation in L-02 human hepatic cells via the PPARγ-ABCA1/CYP7A1 pathway. Food Funct 7(12):4976–4983
Makkawi TA, Yaseen NY, Zgheir ZR (2010) Study of the effect of crude extracts of Sonchus oleraceus on cancer cell growth (In vivo). IJVM 34(1):218–227
Martey OK, Armah G, Okine LKNA (2010) Absence of organ specific toxicity in rats treated with Tonica, an aqueous herbal haematinic preparation. Afr J Tradit Complement Altern Med 7(3):231–240. https://doi.org/10.4314/ajtcam.v7i3.54781
Marzolini C, Paus E, Buclin T, Kim RB (2004) Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther 75(1):13–33
McElreath R, Luttbeg B, Fogarty SP, Brodin T, Sih A (2007) Evolution of animal personalities. Nature 450(7167):E5–E5
Mehta RL, Kellum JA, Shah SV et al (2007) Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11(2):1–8
Metushi IG, Cai P, Zhu X, Nakagawa T, Uetrecht JP (2011) A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin Pharmacol Ther 89(6):911–914
Muhammad Z, Ahmad SHABIR, Ullah R, Ullah F, Jan SALEEM (2012) Isolation and characterization of two new compounds from genus Sonchus. Biomed Pharmacol J 5(1):65–70
N’Guessan K, Kadja B, Zirihi G, Traoré D, Aké-Assi L (2009) Screening phytochimique de quelques plantes médicinales ivoiriennes utilisées en pays Krobou (Agboville, Côte-d’Ivoire). Sciences & Nature 6(1)
Navarro-Moreno LG, Quintanar-Escorza MA, González S et al (2009) Effects of lead intoxication on inter-cellular junctions and biochemical alterations of the renal proximal tubule cells. Toxicol in Vitro 23(7):1298–1304
Neergheen-Bhujun VS (2013) Underestimating the toxicological challenges associated with the use of herbal medicinal products in developing countries. Biomed Res Int 2013:1–9
NIH Publication #85–23 (1985) Respect for life. National Institute of environment. Health Sci NIEHS. http://www.niehs.gov/oc/factsheets/wri/studybgn.htm. Accessed 3 Mar 2019
Nurianti Y, Hendriani R, Sukandar E, Anggadiredja K (2014) Acute and subchronic oral toxicity studies of ethyl acetate extract of Sonchus arvensis L. leaves. Int J Pharm Pharm Sci 6(5):343–7
Obeid HA, Saeed AE, Khalid HS (2018) Isolation and structure elucidation of a flavone and tannic acid from Sudanese S.Oleraceus plant. Int J Curr Res 10(02):65435–38
OECD (2001) Harmonised integrated classification system for human health and environmental hazards of chemical substances and mixtures. OECD, Paris, adopted 14th August 2001, (Chapter 2.1)
OECD (2008) Repeated dose 90-day oral toxicity study in rodents. OECD Guideline for testing of chemicals No. TG 408, OECD, Paris
Olorunnisola OS, Bradley G, Afolayan AJ (2012) Acute and sub-chronic toxicity studies of methanolic extract of Tulbaghia violacea rhizomes in Wistar rats. Afr J Biotech 11(83):14934–14940
Olson H, Betton G, Robinson D, Thomas K et al (2000) Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 32(1):56–67
Philip DM, Zilva-Pannal M (1994) Clinical chemistry in diagnosis and treatment. 6th edn. London, UK: Taylor & Francis Ltd, pp 284–85
Quezel P, Santa S (1963) Nouvelle flore de l’Algérie et des régions desertiques méridionales. Centre National de la Recherche Scientifique, Paris
Ramaiah M, Jain A, Baldwin JC, Karthikeyan AS, Raghothama KG (2011) Characterization of the phosphate starvation-induced glycerol-3-phosphate permease gene family in Arabidopsis. Plant Physiol 157(1):279–291
Rizk AM (1982) Constituents of plants growing in Qatar. Fitoterrapia 52(2):35–42
Roberts SM, James RC, Franklin MR (2000) Hepatotoxicity: toxic effects on the liver. Principles of toxicology: environmental and industrial applications 111–128
Saad B, Azaizeh H, Abdu-Hijleh G, Said O (2006) Safety of traditional Arab herbal medicine. Evidence-Based Complement Altern Med 3(4):433–439
Schaffer S, Schmitt-Schillig S, Muller WE, Eckert GP (2005) Antioxidant properties of Mediterranean food plant extracts: geographical differences. J Physiol Pharmacol Supplement 56(1):115–124
Sen S, Chakraborty R (2016) Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: importance, challenges and future. J Tradit Complement Med 7(2):234–244
Singh R, Kumari N (2015) Comparative determination of phytochemicals and antioxidant activity from leaf and fruit of Sapindus mukorrossi Gaertn. A valuable medicinal tree. Ind Crops Prod 73:1–8
Smach MA, Hafsa J, Charfeddine B, Dridi H, Limem K (2015) Effects of sage extract on memory performance in mice and acetylcholinesterase activity. Ann Pharm Fr 73(4):281–288
Smock KJ, Perkins SL (2014) Thrombocytopenia: an update. Int J Lab Hematol 36:269–278
Soussi A, Gargouri M, El-Feki A (2018) Potential immu-nomodulatory and antioxidant effects of walnut Juglans regia vegetable oil against lead-mediated hepatic damage and their interaction with lipase activity in rats. Environ Toxicol 33(12):1261–1271
Sundberg A, Appelkvist EL, Dallner G, Nilsson R (1994) Glutathione transferases in the urine: sensitive methods for detection of kidney damage induced by nephrotoxic agents in humans. Environ Health Perspect 102(suppl 3):293–296
Tatefuji T, Yanagihara M, Fukushima S, Hashimoto K (2014) Safety assessment of melinjo (Gnetum gnemon L.) seed extract: acute and subchronic toxicity studies. Food Chem Toxicol 67:230–235
Teugwa CM, Mejiato PC, Zofou D et al (2013) Antioxidant and antidiabetic profiles of two African medicinal plants: Picralima nitida (Apocynaceae) and Sonchus oleraceus (Asteraceae). BMC Complement Altern Med 13(1):175
Thapa BR, Walia A (2007) Liver function tests and their interpretation. Indian J Pediatr 74(7):663–671
Thiesen LC, de Oliveira Nunes ML, Meyre-Silva C et al (2018) The hydroethanolic Litchi chinensis leaf extract alleviate hepatic injury induced by carbon tetrachloride (CCl4) through inhibition of hepatic inflammation. Biomed Pharmacother 107:929–936
Tir M, Feriani A, Labidi A, Mufti A et al (2019) Protective effects of phytochemicals of Capparis spinosa seeds with cisplatin and CCl4 toxicity in mice. Food Biosci 28:42–48
Tohti I, Tursun M, Umar A, Turdi S, Imin H, Moore N (2006) Aqueous extract of Ocimum basilicum L. (sweet basil) decrease platelet aggregation induced by ADP and thrombin in vitro and rats arterio-veneous shunt thrombosis in vivo. Thromb Res 118:733–739
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A (2018) Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 5(3):93
Venkateswaran S, Pari L, Viswanathan P, Menon VP (1997) Protective effect of Livex, a herbal formulation against erythromycin estolate induced hepatotoxicity in rats. J Ethnopharmacol 57(3):161–167
Vilela FC, De Mesquita Padilha M, Dos Santos-E-Silva L, Alves-da-Silva G, Giusti-Paiva A (2009a) Evaluation of the antinociceptive activity of extracts of Sonchus oleraceus L. in mice. J Ethnopharmacol 124(2):306–310
Vilela FC, Soncini R, Ginsti AL (2009b) Anxiolytic like effect of Sonchus oleraceus L. in mice. J Ethnopharmacol 2:325–327
Vinayagam R, Xu B (2015) Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab 12(1):60
WHO (2000) General guidelines for methodologies on research and evaluation of traditional medicine. World Health Organization, Geneva
Wu H, Zhang X, Li X, Wu Y, Pei F (2005) Acute biochemical effects of La (NO3)3 on liver and kidney tissues by magic-angle spinning 1H nuclear magnetic resonance spectroscopy and pattern recognition. Anal Biochem 339(2):242–248
Yam MF, Sadikun A, Ahmad M, Akowuah GA, Asmawi MZ (2009) Toxicology evaluation of standardized methanol extract of Gynura procumbens. J Ethnopharmacol 123(2):244–249
Yuan H, Ma Q, Ye L, Piao G (2016) The traditional medicine and modern medicine from natural products. Molecules 21(5):559
Yuet Ping K, Darah I, Chen Y, Sreeramanan S, Sasidharan S (2013) Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. Biomed Res Int 2013:1–14
Author information
Authors and Affiliations
Contributions
FA conducted the experimental work, collected the data, and wrote the draft of the manuscript. NG conceived and designed the experiment, and furnished the designated work. RG analyzed the data and critically reviewed the manuscript. All authors had read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The current work involved animal use. The principles of the Care and Use of Laboratory Animals (NIH publication 1985) were followed. The authors also would like to declare that the present manuscript and data have not been published and are not currently under review for publication elsewhere.
Consent to participate
None.
Consent for publication
None.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aissani, F., Grara, N. & Guelmamene, R. Phytochemical screening and toxicity investigation of hydro-methanolic and aqueous extracts from aerial parts of Sonchus oleraceus L. in Swiss albino mice. Comp Clin Pathol 31, 509–528 (2022). https://doi.org/10.1007/s00580-022-03349-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03349-x