Abstract
Considering the role of oxidative stress as a molecular mechanism underlying the deleterious side effects of gentamicin, applying antioxidants is likely to have ameliorative effects against GNT-induced tissue damages. This work was aimed to investigate the protective effect of betaine or trimethylglycine upon gentamicin-induced toxicity in mice. Thirty male mice were randomly divided into five groups: group 1 (control; i.p. injection of isotonic saline), group 2 received gentamicin (GNT; 80 mg/kg, i.p.) for 10 days, group 3 received GNT (80 mg/kg, i.p.) for 10 days and betaine (2% in the diet) for 18 days starting 8 days before gentamicin injection, group 4 received GNT (80 mg/kg, i.p.) for 10 days and betaine (2% in the diet) during gentamicin prescription, and group 5 just received betaine (2% in the diet) for 10 days. Gentamicin administration caused a notable increase in creatinine and urea levels compared to controls (p < 0.05). Betaine administration in groups 3 and 4 decreased creatinine values relative to the second group to the amounts that had no significant difference in comparison with the control group. Additionally, a remarkable increase in renal and plasma contents of malondialdehyde (as a lipid peroxidation indicator) and renal glutathione peroxidase (which breakdown hydrogen peroxides and hydroperoxides to harmless molecules) was found upon gentamicin injection which both were attenuated in the betaine-treated groups. Also, the histopathologic studies revealed acute tubular necrosis with hyperemia and hemorrhage triggered by gentamicin. Betaine noticeably attenuated the GNT-induced histopathological lesions of the kidneys. The present study indicates that betaine can attenuate gentamicin-induced nephrotoxicity, which might be related to betaine’s antioxidant potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aminoglycosides are broad-spectrum, bactericidal antibiotics that inhibit protein synthesis and have common chemical, antimicrobial, pharmacological, and toxicological properties (Gonzalez et al. 1998). All antibiotics in this group have a limited therapeutic index and produce similar toxic effects at the therapeutic dose (Wargo and Edwards 2014). Gentamicin (GNT) is one of the aminoglycoside antibiotics used in the treatment of infections, especially against gram-negative bacteria (Tavafi 2012). The clinical use of gentamicin has been restricted due to its side effects (Abdel-Raheem et al. 2009). These antibiotics have a common cause of acute renal failure which accounts for about 10 to 20% of cases (Wargo and Edwards 2014). Apoptosis or programmed cell death and, most significantly, free radical production is one of the mechanisms of gentamicin effect on living body tissues (Martínez-Salgado et al. 2002; Ince et al. 2020). Gentamicin increases the production of superoxide anion, hydrogen peroxide, and hydroxyl radical (Yang et al. 1995; Assadi 2012), which can provoke peroxidation of membrane phospholipids, DNA strand breakage, and denaturation of proteins and ultimately causing tissue damage in several organs like the liver and kidneys (Alarifi et al. 2012). Peroxidation of membrane unsaturated lipids can cause changes in membrane fluidity, and as a result, the membrane becomes permeable to even large enzymes (Oikonomidis et al. 2017). Antioxidant supplementation can support the body against different types of oxidative damage (Abdel-Raheem et al. 2009; Rehman et al. 2012). Antioxidants allow the cell to regain its normal physiological function and block and neutralize free radicals (El-Kashef et al. 2015). Considering the role of oxidative stress as a molecular mechanism underlying the deleterious side effects of gentamicin, applying antioxidants is likely to have ameliorative effects against GNT-induced tissue damages. In this respect, treatment via some antioxidants, e.g., quercetin, vitamin C, allicin, and rosmarinic acid (RA), has suggested beneficial effects versus the majority of GNT-induced biochemical and histopathological alterations in experimental models (Abdel-Raheem et al. 2009; Rehman et al. 2012; El-Kashef et al. 2015; Tavafi and Ahmadvand 2011).
Betaine or trimethylglycine was discovered in the nineteenth century for the first time in the juice of sugar beets (Beta vulgaris) and is dispersed widely in plants, animals, and microorganisms (Zhao et al. 2018). It is quickly absorbed and used as an effective natural agent to maintain the health of the liver, heart, and kidneys (Craig 2004; Zhao et al. 2018). The physiologic function of betaine is either an organic osmolyte to protect cells under stress or as a catabolic source of methyl groups that participate in the methionine cycle, primarily in the liver and kidneys (Craig 2004; Saeed et al. 2017). Betaine metabolism in the methionine cycle via betaine homocysteine methyltransferase (BHMT) can provide methionine and subsequently S-adenosyl methionine which is required for the synthesis of numerous substances such as creatine, proteins, phospholipids, carnitine, and some hormones and neurotransmitters (Alirezaei et al. 2015). Furthermore, the direct antioxidant potential of betaine via ROS scavenging activity and its role in the recovery of antioxidant defense through involvement in the sulfur amino acids metabolomics have been emphasized (Kim et al. 2009; Jung et al. 2013).
Betaine has also been reported as an ameliorative agent against nephrotoxicity (Hagar et al. 2015) and hepatotoxicity (Kim et al. 2009; Jung et al. 2013) induced by various chemicals. This study aimed to determine the ameliorative effects of betaine versus GNT-induced damages in mouse kidneys concerning both antioxidant and methyl donor properties of betaine.
Material and methods
Chemicals
Gentamicin (40 mg/ml) and betaine (≥ 99%) were bought from Darupakhsh Co. (Tehran, Iran) and Sigma Co. (St. Louis, MO, USA), respectively. All the other required chemicals with analytical grade were acquired from Sigma Co. (St. Lewis, MO, USA) or Merck Co. (Darmstadt, Germany).
Experimental protocol and sampling
Thirty male mice weighing approximately 30 ± 3 g were used for the experimental procedures. All animals were maintained in stainless-steel cages in an air-conditioned room at 22 ± 2 °C with a photoperiod of 12 h of light and 12 h of darkness per day. The animals were randomly divided into four equal groups of 6 each as described in Table 1. Mice in group 1 (control group) received a daily i.p. injection of isotonic saline (0.1 ml/kg/day) for 10 days. Group 2 or GNT group received GNT (80 mg/kg, i.p.) for 10 days (16, 17). Mice in group 3 received GNT (80 mg/kg, i.p.) for 10 days and betaine (2% in the diet) for 18 days starting 8 days before gentamicin injection. Animals in group 4 received GNT (80 mg/kg, i.p.) for 10 days and betaine (2% in the diet) during gentamicin prescription. Group 5 animals only received betaine (2% in the diet) for 10 days. At the end of the experiment, the overnight fasted mice were anesthetized with diethyl ether 24 h after the last injection, and blood was taken from their hearts before incision of the abdomen. EDTA-containing blood samples were centrifuged for 10 min at 1000 × g, and plasma samples were separated and frozen at − 20 °C until the time of analysis. The kidneys were removed immediately, cleaned of fat and adhering, rinsed with normal saline, and cut into small pieces. Some parts of tissue samples were kept at − 80 °C for biochemical parameters, and the other pieces were immersed in neutral buffered formalin 10% for histopathological assessment.
Biochemical assessment
Plasma biochemical analyses, including urea, creatinine, and uric acid, were assessed by commercial colorimetric kits (Pars Azmoon, Iran). Just before biochemical analysis, frozen tissue samples were quickly thawed and homogenized with phosphate buffer (0.05 M; pH 7.4), and after centrifugation (4000 × g for 15 min), the supernatant was taken for biochemical assays.
Glutathione peroxidase (GPX) and superoxide dismutase (SOD) activities were evaluated in tissue homogenates by diagnostic kits (RANDOX, Crumlin, UK). Tissue catalase (CAT) activity was assessed following the method of Claiborne (Claiborne and Fridovich 1979), which is based on the absorbance decrease at 240 nm due to the disintegration of H2O2 by CAT. Tissue glutathione (GSH) measurement was determined based on the spectrophotometric analysis of the yellow-color derivative resulting from the reaction of GSH with 5,5-dithiol-bis(2-nitrobenzene acid) at 412 nm (Ellman 1959). The concentration of GSH was displayed as nmol g−1 tissue. The concentration of malondialdehyde (MDA) in plasma and kidney homogenate was assessed according to its reaction with thiobarbituric acid to generate a pink product which can be estimated spectrophotometrically at 539 nm (Todorova et al. 2005). Its concentration was assessed using an extinction coefficient value of 156,000 M−1 cm−1, and the results were expressed as nmol g−1 tissue.
Histopathological assessment
The 5-µm thickness of renal tissue samples was sectioned and stained with hematoxylin and eosin (H&E) to examine some histological structure alterations under a light microscope (Olympus, CH-2). A complete histological examination was accomplished on each sample, and histopathological features were quantified based on the severity of the lesions from 0 to 4 as described in Table 2 (Barangi et al. 2020).
Statistical analysis
The Kolmogorov–Smirnov test was applied to assess the normality in data distribution. For normally distributed data, the differences between the groups were performed using a one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test. For non-normally distributed data, the Kruskal–Wallis H test was used to investigate the differences between the groups using the SPSS/PC software, version 21. A significant level was considered at p < 0.05.
Results
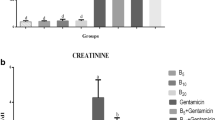
As shown in Fig. 1, GNT significantly enhanced the urea and creatinine concentrations compared with the control mice. Betaine administration in groups 3 and 4 notably reversed the GNT-induced rise of the urea level to the values comparable to the control group. However, the decrease was only significant in group 3 as compared to group 2. Moreover, betaine administration in groups 3 and 4 non-significantly decreased creatinine values relative to the second group to the amounts that had no significant difference in comparison with the control group. Circulating uric acid concentrations were not significantly altered between the experimental groups. GNT and betaine administration showed no considerable alternation in uric acid level (Fig. 2).
As presented in Fig. 3, a significant ascending trend of MDA levels was found in the kidney and plasma of animals in group 2 compared to the control group. Betaine administration in groups 3 and 4 could decrease the MDA values of the plasma and kidney to the amounts comparable with those of group 1. The concentration of plasma MDA in group 3 was also significantly lower compared to those in group 2.
The effects of GNT and betaine on the responses of antioxidant status are presented in Table 3. GNT administration markedly enhanced the GPX activity in group 2 compared with the control group. However, values of other measured antioxidant indices, including SOD, GSH, and catalase, showed no significant alterations following GNT treatment. Betaine administration in group 5 caused a substantial reduction in GPX and catalase enhancement compared to group 2. Moreover, SOD activity showed significant elevation in group 5 as compared to groups 1 and 2.
The histopathological results show that GNT caused degeneration and widespread necrosis of the renal tubular epithelial cells. There was a significant increase in renal tissue degeneration in group 2 compared to groups 1 and 5. Moreover, betaine/gentamicin treatment attenuated the gentamicin-induced renal degeneration significantly as compared to group2. Moreover, gentamicin administration significantly increased renal tissue hyperemia, while the groups that received betaine/gentamicin had a substantial reduction in tissue hyperemia compared with the group that only received gentamicin. There was a significant increase in tissue hemorrhage in groups 1 and 4 compared with those of controls. On the other hand, administration of betaine in group 3, which received betaine 8 days before and during gentamicin administration, caused a notable decrease of tissue hemorrhage to the levels that were not significantly different from the control group (Figs. 4 and 5).
Discussion
It has been shown that GNT exerts its adverse effects on the kidney by the generation of free radicals and affecting redox balance which results in severe renal damage (Ince et al. 2020). Phytochemicals with antioxidant properties could reverse some toxicant-induced histopathological and biochemical alterations. Betaine, concerning its antioxidant, free radical scavenging, and methyl donor abilities, has been documented to display protective effects against organ damage caused by different chemical poisons (Jung et al. 2013; Khodayar et al. 2018). This study mainly focuses on the impact of betaine to reduce GNT-induced renal toxicity in mice.
The injection of gentamicin caused nephrotoxicity, shown by histopathological lesions, including a significant increase in hyperemia, tubular necrosis, and hemorrhage. In agreement with our study, also other studies demonstrated that GNT-induced nephrotoxicity is characterized by tubular necrosis (Ince et al. 2020; Sepehri et al. 2013; Huang et al. 2020). The examination of the renal tissues from gentamicin‐treated mice showed also severe histopathological changes (Maya et al. 2020). Abdel-Raheem et al. (2009) have reported that in renal sections from GNT-treated rats, the glomeruli showed atrophy, and also, hypertrophy was observed in the epithelial cells lining the renal tubules. Moreover, swelling and infiltration of mononuclear inflammatory cells have been recorded following gentamicin administration. On the other hand, betaine showed preventive effects against histopathological injuries caused by gentamicin treatment. Similarly, in a study by Ozturk et al. (2003) betaine’s protective effect against carbon tetrachloride-induced renal histopathological changes was documented.
It has been shown that quercetin as an antioxidant and cytoprotective agent can uptake free radicals and protect the myocardial tissue against global ischemia and reperfusion injury (Abdel-Raheem et al. 2009). Rehman et al. (2012) have shown that vitamin C due to its antioxidant properties can attenuate gentamicin-induced nephrotoxicity. In accord with mentioned works, it has been reported that allicin, via its antioxidant, anti-inflammatory, and immunomodulatory properties, can decrease hydroxyl and peroxyl radicals, superoxide anion, and lipid peroxidation markers (El-Kashef et al. 2015; Hamed et al. 2021).
Betaine, a natural component found in many foods, animals, plants, and microorganisms, is a robust antioxidant agent. Moreover, it has been found that betaine has the capacity to protect the heart, liver, and kidney due to its highly lipotropic properties (Zhao et al. 2018). The current findings show that betaine can attenuate some biochemical and histopathological alterations induced by gentamicin in mouse kidneys. Betaine co-administration in the groups received gentamicin-attenuated observed tissue damages. This result could be due to betaine as an osmotic regulator, which can improve the activity of intracellular enzymes and reduce the rate of degeneration and necrosis (Craig 2004). The present results revealed the nephroprotective nature of betaine against gentamicin-induced damage by improving kidney tissue antioxidant competence. These results agreed with Alirezaie et al. (2015) who reported that the antioxidant and methyl donating properties of betaine are effective against oxidative stress induced by levodopa/benserazide. Moreover, Jung et al. (2013) showed the protective role of betaine against alcoholic liver injury, which may be attributed to the antioxidant defense’s amplification.
The current study results show that gentamicin administration resulted in significant enhancement of plasma creatinine and urea concentrations that are in line with previously published similar studies (El-Kashef et al. 2015; Sepehri et al. 2013). Betaine treatment reduced plasma creatinine and urea levels compared to mice treated with gentamicin alone. In agreement with the present work, previous studies indicated that vitamin C and allicin could decrease serum creatinine and BUN levels in gentamicin-injected experimental animals (Rehman et al. 2012; El-Kashef et al. 2015). Moreover, Hagar et al. (2015) surveyed the effects of betaine on cadmium-induced kidney damage and showed that betaine could attenuate toxicant-induced enhancement of serum BUN and creatinine to the values close to the control group.
In the present study, gentamicin caused a remarkable increase in the renal MDA levels and decreased SOD and GSH values compared to the control group. These results are similar to previously reported data (Abdel-Raheem et al. 2009; Tavafi and Ahmadvand 2011). MDA is generally used for the measurement of lipid peroxidation in biological structures and is considered as a marker for oxidative stress (Tsikas 2017). Gentamicin directly enhances the production of reactive oxygen metabolites from the electron transfer chain, which causes depletion of antioxidant capacity (Abdel-Raheem et al. 2009; Ince et al. 2020). Based on the current findings, betaine administration with gentamicin revealed a decrease in MDA levels of plasma and kidney in comparison with the gentamicin-treated group. Also, betaine administration showed beneficial effects by increasing SOD, GPX, and catalase activities compared to the mice in group 2. This effect may account for scavenging ROS generation and prevent lipid peroxidation (Kim et al. 2009). It has been reported that betaine could ameliorate the restoration of the GSH, which was depleted by acetaminophen (Khodayar et al. 2018). However, the current results revealed that the GSH level was not changed significantly by betaine. Moreover, Ozturk et al. (2003) reported that CCl4-induced enzyme alterations including increases of renal superoxide dismutase and catalase activities and decrease of glutathione peroxidase were not restored by betaine treatment in rats.
The histopathological evaluation of the kidney from gentamicin-treated mice showed degeneration, hemorrhage, and hyperemia of tubular epithelial cells in the renal cortex. These alterations can support by previous authors (Ince et al. 2020; Sepehri et al. 2013). Moreover, betaine administration was protective against degenerative injury caused by gentamicin treatment. The observed differences between the obtained findings in groups 3 and 4 may be mitigated by administering a higher dose of betaine to group 4 or by starting betaine sooner in group 3 prior to gentamicin administration which can be investigated in future studies.
The present study is the first investigation in which protective effects of betaine upon gentamicin-induced nephrotoxicity were evaluated which can promise a new protective agent against gentamicin-induced damage. However, some limitations of the present study including no measurement of additional renal function tests and apoptotic markers as well as the use of only one dose of betaine should be considered when interpreting the results.
In summary, the present findings indicate that betaine administration had been partly successful in restoring GNT-induced alterations in the histopathological features as well as oxidative and biochemical marker indices of kidney damage in mice. The present study indicates that betaine can attenuate gentamicin-induced nephrotoxicity, which might be related to betaine’s antioxidant potential. However, clarification of the molecular basis of its modulating effects versus renal toxicity induced by gentamicin requires further study.
References
Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA (2009) Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull 32(1):61–67
Alarifi S, Al-Doaiss A, Alkahtani S, Al-Farraj SA, Al-Eissa MS, Al-Dahmash B et al (2012) Blood chemical changes and renal histological alterations induced by gentamicin in rats. Saudi J Biol Sci 19(1):103–110
Alirezaei M, Khoshdel Z, Dezfoulian O, Rashidipour M, Taghadosi V (2015) Beneficial antioxidant properties of betaine against oxidative stress mediated by levodopa/benserazide in the brain of rats. J Physiol Sci 65(3):243–252
Assadi F (2012) The epidemic of pediatric chronic kidney disease: the danger of skepticism. J Nephropathol 1(2):61–64
Barangi S, Mehri S, Moosavi Z, Hayesd AW, Reiter RJ, Cardinali DP et al (2020) Melatonin inhibits benzo(a)pyrene-induced apoptosis through activation of the Mir-34a/Sirt1/autophagy pathway in mouse liver. Ecotoxicol Environ Saf 196:110556
Claiborne A, Fridovich I (1979) Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J Biol Chem 254(10):4245–52
Craig SA (2004) Betaine in human nutrition. Am J Clin Nutr 80(3):539–549
El-Kashef DH, El-Kenawi AE, Suddek GM, Salem HA (2015) Protective effect of allicin against gentamicin-induced nephrotoxicity in rats. Int Immunopharmacol 29(2):679–686
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Gonzalez LS 3rd, Spencer JP (1998) Aminoglycosides: a practical review. Am Fam Physician 58(8):1811–1820
Hagar H, Medany AE, Salam R, Medany GE, Nayal OA (2015) Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol 67(2):133–141
Hamed HS, Ismal SM, Faggio C (2021) Effect of allicin on antioxidant defense system, and immune response after carbofuran exposure in Nile tilapia, Oreochromis niloticus. Comp Biochem Physiol C 240:108919
Huang H, Jin WW, Huang M, Ji H, Capen DE, Xia Y et al (2020) Gentamicin-induced acute kidney injury in an animal model involves programmed necrosis of the collecting duct. J Am Soc Nephrol 31(9):2097–2115
Ince S, Kucukkurt I, Demirel HH, Arslan-Acaroz D, Varol N (2020) Boron, a trace mineral, alleviates gentamicin-induced nephrotoxicity in rats. Biol Trace Elem Res 195(2):515–524
Jung YS, Kim SJ, Kwon DY, Ahn CW, Kim YS, Choi DW et al (2013) Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food Chem Toxicol 62:292–298
Khodayar MJ, Kalantari H, Khorsandi L, Rashno M, Zeidooni L (2018) Betaine protects mice against acetaminophen hepatotoxicity possibly via mitochondrial complex II and glutathione availability. Biomed Pharmacother 103:1436–1445
Kim SK, Seo JM, Chae YR, Jung YS, Park JH, Kim YC (2009) Alleviation of dimethylnitrosamine-induced liver injury and fibrosis by betaine supplementation in rats. Chem Biol Interact 177(3):204–211
Martínez-Salgado C, Eleno N, Tavares P, Rodríguez-Barbero A, García-Criado J, Bolaños JP et al (2002) Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int 62(5):1682–1692
Maya NA, Dewan JF, Rashid N, Sharmin K, Uddin MA, Sharmin F (2020) Morphological effect of ethanol extract of Tinospora cordifolia on gentamicin-induced nephrotoxicity in rats. Mymensingh Med J 29(4):871–878
Oikonomidis IL, Kiosis EA, Brozos CN, Kritsepi-Konstantinou MG (2017) Reference intervals for serum reactive oxygen metabolites, biological antioxidant potential, and oxidative stress index in adult rams. Am J Vet Res 78(3):274–278
Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K (2003) Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology 62(2):353–356
Rehman K, Akash MS, Azhar S, Khan SA, Abid R, Waseem A et al (2012) A biochemical and histopathologic study showing protection and treatment of gentamicin-induced nephrotoxicity in rabbits using vitamin C. Afr J Tradit Complement Altern Med 9(3):360–365
Saeed M, Babazadeh D, Naveed M, Arain MA, Hassan FU, Chao S (2017) Reconsidering betaine as a natural anti-heat stress agent in poultry industry: a review. Trop Anim Health Prod 49(7):1329–1338
Sepehri G, Derakhshanfar A, Saburi L (2013) Does propylthiouracil increase the gentamicin-induced nephrotoxicity in rat? Iran J Basic Med Sci 16(11):1190–1195
Tavafi M (2012) Inhibition of gentamicin-induced renal tubular cell necrosis. J Nephropathol 1(2):83–86
Tavafi M, Ahmadvand H (2011) Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell 43(6):392–397
Todorova l SG, Kyuchukova D, Dinev D, Gadjeva V, (2005) Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp Clin Path 13(4):190–194
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524:13–30
Wargo KA, Edwards JD (2014) Aminoglycoside-induced nephrotoxicity. J Pharm Pract 27(6):573–577
Yang CL, Du XH, Han YX (1995) Renal cortical mitochondria are the source of oxygen free radicals enhanced by gentamicin. Ren Fail 17(1):21–26
Zhao G, He F, Wu C, Li P, Li N, Deng J et al (2018) Betaine in inflammation: mechanistic aspects and applications. Front Immunol 9:1070
Funding
This work was supported by a grant from Ferdowsi University of Mashhad, Mashhad, Iran (grant No. 46257).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Research Ethics Committee of Ferdowsi University of Mashhad, Mashhad, Iran, approved all the procedures applied.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalili, N., Ahmadi, A., Ghodrati Azadi, H. et al. Protective effect of betaine against gentamicin-induced renal toxicity in mice: a biochemical and histopathological study. Comp Clin Pathol 30, 905–912 (2021). https://doi.org/10.1007/s00580-021-03285-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-021-03285-2