Abstract
The purpose of this study was to assess the hypoglycemic and protective activities of the ethanolic extract of Allium saralicum (AS) in streptozotocin (STZ) induced diabetic mice. Diabetes was experimentally induced by intraperitoneal injection of STZ (60 mg/kg bw) in 35 mice. Fasting blood glucose (FBG) levels were measured everyday using glucometer strips. Mice with plasma glucose level > 250 mg/dL were considered diabetic. After 3 days, they were divided randomly into five groups. Groups 1 and 2 served as non-diabetic and untreated diabetic controls, respectively. Group 3 received 0.5 mg/kg glibenclamide orally. Groups 4 and 5 were given 200 and 400 μg/kg, respectively, of AS for 15 days orally. At 16th day, the animals were dissected and their kidney removed. The total volume of cortex, medulla, renal tubules, and glomeruli as well as the length of vessels and renal tubules was estimated using stereological method. Daily treatment of diabetic mice with high dose of AS significantly (p ≤ 0.05) declined FBG levels at days 10–13 of the experiment faster than glibenclamide. High dose of AS could significantly (p ≤ 0.05) improve the volume of cortex, glomeruli, proximal tubules, distal tubules, vessels, and total length of proximal tubules and vessels. Moreover, glomerular loss was significantly (p ≤ 0.05) inhibited with high dose of AS. It concluded that Allium saralicum could improve hyperglycemia and ameliorated renal changes following diabetes in STZ-induced diabetic mice. So, according to these features, it could be used as an antidiabetic supplement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is the most popular endocrine disorder that affects more than 285 million people worldwide. This number is expected to grow to 438 million by 2030, corresponding to 7.8% of the adult population. In addition to the primary complications of diabetes, this disease is associated by increased risk of hypertension, dyslipidemia, decreased fibrinolytic activity, severe atherosclerosis, increased platelet aggregation, and also poor wound healing (Ahmed et al. 2014).
Kidney is one of the organs that is affected in diabetic patients. However, the exact pathogenesis of poor nephropathy in diabetic patients is not clearly understood; the decrease of proximal and distal cell capacity and also the oxidative and inflammatory changes are suggested to be the main causes (Musabayane 2012).
Renal hypertrophy and glomerular hyperfiltration are two known complications that occur in the initial stages of diabetes mellitus (Hostetter et al. 1981). Further, renal disorders following diabetes mellitus may have a specific pattern compared to the other renal diseases (McCrary et al. 1981). Some studies had revealed that in early diabetes, glomerular hyperfiltration and renal hypertrophy could be reversed by insulin treatment (Mogensen and Anderson 1975; Christiansen et al. 1982). Whereas, in chronic diabetes, glomerular hyperfiltration could be improved by severe control of blood glucose level, but renal hypertrophy is irreversible (Wiseman et al. 1985). Although renal hypertrophy and glomerular hyperfiltration play a pivotal role in the progression of diabetic nephropathy, the relationship between them is still unclear (Hostetter 2003).
The enormous costs of modern medicines demonstrate that alternative strategies are needed for better management of diabetes and its related problems (Rahimi et al. 2005). Some plants have the high contents of tannins, saponins, flavonoids, naphthaquinone, triterpenes, and alkaloids. So, they can increase the quality and rate of healing of wounds (Abdel-Barry et al. 1997; Pushparaj et al. 2000). The World Health Organization (WHO) suggested that there should be further studies on antidiabetic properties of medicinal plants (WHO 1981).
In Iranian traditional medicine, herbal medicines have been the basis of treatment and cure for various diseases and physiological conditions. Allium saralicum R.M. Fritsch known as “Valak Soori” in local regions is one of the most important herbal medicines, which is widely consumed in the west of Iran. This plant belongs to the family Amaryllidaceae and Allioideae subfamily. Allium saralicum has a long history of use in Kurdish traditional medicine for treatment of digestive, nervous, and infectious diseases. But there is no evidence of its antidiabetic and/or its nephroprotective effects (Block 2010; Davies 1992; Foroughi et al. 2016).
Therefore, this study was designed to evaluate antihyperglycemic effect of Allium saralicum and its protective effects on renal structural changes in STZ-induced diabetic mice using design-based and unbiased stereological methods.
Materials and methods
Plant collection
Allium saralicum (AS) at maturity was harvested from the Kermanshah suburb during April 2015. The plant was identified for the first time, and a voucher specimen (no. 2738RUH) has been deposited at the Herbarium of Research Center of Faculty of Agriculture, Razi University, Kermanshah, Iran.
Plant extraction
Leaves of the plant were shade dried for 1 week. Dried aerial part of the plants was ground, and about 150 g of the obtained powder was extracted with 450 mL of 100% ethanol for 2 h at 40 °C with continuous shaking. The extract was left for 24 h at room temperature; then, it was filtered through Whatman paper no. 2. The extract was concentrated in rotary evaporator (Panchun Scientific Co., Kaohsiung, Taiwan) then lyophilized.
Animals
Thirty-five healthy male Balb/c mice weighing 36 ± 3 g were provided by the Center of Laboratory Animal Breeding, Kermanshah University of Medical Sciences. The animals were housed under standard environmental (25 ± 3 °C temperature and 12:12-h light and dark) and nutritional (standard pellet diet and water ad libitum) conditions during the experiment.
Institutional Ethics Committee approval was obtained, and all procedures performed in the study were in compliance with the desired ethical rules.
Diabetes was induced by a single intraperitoneal (IP) administration of 60 mg/kg bw of streptozotocin (Sigma, St. Louis, MO, USA). Fasting blood glucose (FBG) level was assessed everyday by glucometer strips. A blood glucose level upper than 250 mg/dL was considered diabetic.
Experimental design
The mice were divided into five following groups (n = 7):
-
(I)
Control group (C) which received 200 μL normal saline orally.
-
(II)
Untreated-diabetic group (UTD).
-
(III)
Treated diabetic mice which received 0.5 mg/kg glibenclamide for 15 days (TDG).
-
(IV)
Treated diabetic mice which received 200 μg/kg of the ethanolic extract of AS for 15 days.
-
(V)
Treated diabetic mice which received 400 μg/kg of the ethanolic extract of AS for 15 days.
Blood sampling
Blood samples were collected daily via tail vein to assess the blood glucose level by glucometer strip. At the end of the experiment, all animals were weighed and euthanized with deep chloroform inhalation. Immediately blood samples were drawn from the animals’ heart. The samples were centrifuged at 10,000 rpm for 15 min and serum separated.
Stereological study
Volume density
After dissection, the left kidneys were removed and then weighed. They were placed in 10% neutral buffered formalin solution for 5 days. Immersion method was then used to determine the primary volume of the kidney. For estimation of final volume of the organs, the amount of tissue shrinkage must be specified (Gundersen et al. 1992; Braendgaard and Gundersen 1986). Isotropic uniform random (IUR) sections must be obtained for estimating tissue shrinkage and tubular length (Gundersen et al. 1992; Nyengaard 1999). Theses sections were obtained using orientator method. Totally, 7–10 slab were obtained from each kidney through orientator method. A circular piece was sampled from a kidney slab, and the area of this piece was calculated. The slabs and circular piece were processed, sectioned (5-μm thicknesses), and stained by Periodic Acid Schiff (PAS) method. The area of the circular piece was calculated again, and tissue shrinkage was estimated as (Mandarim-de-Lacerda 2003)
where AA and AB are the area of the circular piece after and before tissue processing. The total volume of the organ was then estimated using
Tissue sections were examined using a microscope (Olympus CX2, Japan) connected to a video camera (Dinocapture ver.5, dino-lit.com 30.5 mm) and a P4 PC, and the stereological parameters were estimated. The fractional volume of the renal structures was estimated using a point probe (with an area of 100 cm2 and containing 25 points) and following the formula:
“Pstructure” = sum of points dealt with the interested structures.
“Preference” = sum of points dealt with the reference space.
Length density
The length density of the tubules and vessels was estimated using a counting probe (740 × 740 μm) and the following formula:
∑Q = sum of the tubules counted, a (frame) = probe area, 547,600 μm2, and ∑frame = total number of the counted frames.
Numerical density
Physical disector procedure (Sterio 1984) was applied for estimating the numerical density of glomeruli. Two parallel sections with 20-μm distance (first and fifth sections) were prepared. The first section as reference plane and the fifth section as look-up plane. Two counting probes with an area of 547,600 μm2 were attached on the monitor at the final magnification ×135, and the numerical density was estimated using
∑Q− = sum of the counted glomeruli, a (frame) = probe area, ∑P = total number of the examined fields, and h = disector height. The absolute value of each parameter was estimated by multiplying its density by the reference space (Mandarim-de-Lacerda 2003).
Statistical analysis
The data were analyzed by SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA). The one-way ANOVA and Tukey’s post hoc test were used to compare the mean groups, and p < 0.05 was accepted as statistically significant.
Results
Effect of AS on blood glucose level
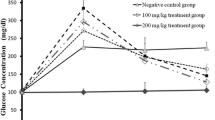
The blood glucose levels of untreated diabetic mice increased to approximately 170% (p ≤ 0.05) of the control mice in a time-dependent manner (Table 1). However, treatment of STZ diabetic mice with the ethanolic extract of As at high dose (400 μg/kg) could significantly (p ≤ 0.05) reduce the blood glucose levels similar to the glibenclamide—treated at the end of the experiment. Also, the difference between low and high dose of AS was significant (p ≤ 0.05) at 10–13 and 14–16 days. The ethanolic extract of AS has most effect on days 10–13 of the experiment (Table 1).
Effect of AS on body weight
The body weight decreased significantly (p ≤ 0.05) in untreated diabetic animals compared to the control ones. High dose of AS could improve the body weight as well as the glibenclamide. The difference between AS 200 group and untreated diabetic mice was not significant (p > 0.05) (Table 2).
Effect of AS on stereological parameters
The data of the kidney weight, mean absolute volume of the kidney, and its subcomponents in treated and untreated diabetic groups are presented in Tables 2 and 3. The results showed that the kidney weight and volume were increased 42 and 56% (p ≤ 0.05), respectively, in the untreated diabetic mice when compared to the control ones. Cortical volume increased 80% (p ≤ 0.05) in this group, but the increase of medullary volume was not significant (p ≥ 0.05) compared to the control one. High dose (400 μg/kg) of AS could significantly (p ≤ 0.05) improve the kidney weight and consequently renal volume compared to the low dose (200 μg/kg).
The volume of PCT (proximal convoluted tubule), DCT (distal convoluted tubule), vessels (VES), and interstitial tissue (IT) was increased significantly (p ≤ 0.05) in untreated diabetic mice in comparison with the control ones (Table 3). Administration of AS at high dose to the diabetic mice could significantly (p ≤ 0.05) decrease the volume of the above structures in comparison with the untreated diabetic group. Low dose of As has no significant (p > 0.05) effect on DCT and collecting duct (CD) volume compared to the untreated diabetic and TDG groups.
The length of the PCT, DCT, LH, and vessels was significantly (p ≤ 0.05) increased in untreated diabetic mice compared to the control ones (Table 4). High dose of AS could significantly (p ≤ 0.05) decrease the length of the PCT and vessels compared to the untreated diabetic group.
The glomerular volume was increased significantly (p ≤ 0.05) following diabetes induction, and treatment with high dose of AS could significantly (p ≤ 0.05) improve glomerular hypertrophy as compared to the untreated group.
The glomerular number in the untreated diabetic mice was reduced significantly (p ≤ 0.05) as compared to the control ones. The glomerular number loss was prevented significantly (p ≤ 0.05) with high dose (400 μg/kg) of AS as compared to the untreated ones (Table 5).
Discussion
This study was the first attempt to evaluate the ethanolic extract of Allium saralicum as antidiabetic and nephroprotective agent using stereological methods. Diabetes mellitus is the most common endocrine disease that affects many populations in the world. In this disease, the purpose of oral treatment is reducing blood glucose to impede later complications (Salvatore and Giugliano 1996).
In the present study, 60 mg/kg of STZ was used for diabetes induction in all mice. This dose can partially damage the beta cells of islets of Langerhans, nephron, and hepatocytes resulting in inexpressive insulin secretion resulting in type 2 diabetes, nephrotoxicity, hepatotoxicity, and hypercholesterolemia (Weir et al. 1981; Heidland et al. 1996; Rabkin et al. 1996).
During the short-term study, the administration of AS extract produced significant antihyperglycemic activity and improved the renal morphological changes at a dose of 400 μg/kg in diabetic mice. In accordance with the obtained results, numerous studies have shown the antidiabetic effects of other plants from genus Allium and family Amaryllidaceae (Eidi et al. 2006; Thomson et al. 2007). These features can be attributed to the sulfur compounds which are found in these plants.
There is some evidence that antidiabetic effects of garlic can be attributed to the antioxidant properties of S-allyl cysteine sulfoxide (Mathew and Augusti 1973; Augusti et al. 1996). Stimulation of pancreatic beta cells for secretion of more insulin or release of bound insulin is another mechanism which can lead to hypoglycemic activity (Jain and Vyas 1975). Accordingly, it is reported that administration of garlic oil or diallyl sulfide to STZ-induced diabetic rats resulted in increased serum insulin levels (Liu et al. 2005).
In the present study, diabetic mice showed some degree of renal hypertrophy which was mainly due to the enlargement of the cortex and its subcomponents. These changes was improved significantly with high dose of AS. It was previously shown that other plants including garlic, ginger (Qattan et al. 2008), and Ginkgo biloba (Welt et al. 2007) can also prevent renal hypertrophy in experimentally induced diabetes. It is well established that renal hypertrophy can be treated at the beginning of the diabetes. However, belated treatment is not successful (Stackhouse et al. 1990; Gondwe et al. 2008). The pathogenesis of the renal hypertrophy can be attributed to the overproduction of oxygen-free radicals following hyperglycemia and inducible nitric oxide synthase (iNOS) which is expressed in response to cytokines (Chaiyasut et al. 2011; Sharma and Ziyadeh 1995; DeRubertis and Craven 1994). Therefore, compounds with antioxidant properties can ameliorate these changes and inhibit the progression of diabetic nephropathy. AS is mainly composed of linolenic acid, gamma tocopherol, and vit E that might be contributed to its antiradical/antioxidant efficacy (Sherkatolabbasieh et al. 2016).
The results of serum glucose levels showed that AS at high dose could restore the blood glucose level toward the normal level. Although there was no significant difference between the experimental doses of AS and classic antidiabetic drug, glibenclamide until day 9 of the experiment, high dose of AS showed its maximum activity between days 10 and 13 with a significant difference as compared to the glibenclamide. But the difference between these was not significant at the end of the experiment (14–16). This indicates that ethanolic extract of AS at high dose only is as effective and potent as glibenclamide and also can acts faster than it.
Conclusion
In conclusion, the present results show that Allium saralicum at high dose may be useful for controlling blood glucose level and alleviation of diabetic complications such as nephropathy generally observed in diabetic patients.
References
Abdel-Barry JA, Abdel-Hassan IA, Al-Hakiem MHH (1997) Hypoglycaemicand antihyperglycaemic effects of Trigonellafoenum-graecum leaf in normal and alloxan-induced diabetic rats. J Ethnopharmacol 58:149–155
Ahmed D, Kumar V, Verma A, Gupta PS, Kumar H, Dhingra V, Mishra V, Sharma M (2014) Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. Complement Altern Med 14:243–260
Augusti KT, Sheela CG (1996) Antiperoxide effect of S-allyl cysteine sulfoxide, a insulin secretagogue, in diabetic rats. Experientia 52:115–120
Block E. Garlic and Other Alliums: The Lore and the Science (2010) Royal Society of Chemistry ISBN978-0-85404-190-9
Braendgaard H, Gundersen HJ (1986) The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods 18(1–2):39–78
Chaiyasut C, Kusirisin W, Lailerd N et al (2011) Effects of phenolic compounds of fermented Thai indigenous plants on oxidative stress in streptozotocin-induced diabetic rats. J Evid Based Complement Altern Med:1–10
Christiansen JS, Gammelgaard J, Tronier B, Svendsen PA, Parving HH (1982) Kidney function and size in diabetes before and during initial insulin treatment. Kidney Int 21:683–688
Davies D (1992) Alliums: the ornamental onions. Timber Press. ISBN0-88192-241-2
DeRubertis FR, Craven PA (1994) Activation of protein kinase C in glomerular cells in diabetes: mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes 43:1–8
Eidi A, Eidi M, Esmaeili E (2006) Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 13:624–629
Foroughi A, Zangeneh MM, Kazemi N, Zangeneh A (2016) An in vitro study on antimicrobial properties of Allium noeanumreut ex regel: an ethnomedicinal plant. Iranian J Publ Health 45(2):32
Gondwe M, Kamadyaapa DR, Tufts M et al (2008) Sclerocaryabirrea[(A. Rich.) Hochst.] [Anacardiaceae] stem-barkethanolic extract (SBE) modulates blood glucose, glomerular filtrationrate (GFR) and mean arterial blood pressure (MAP) of STZ-induceddiabetic rats. Phytomedicine 15:699–709
Gundersen HJ, Bendtsen TF, Korbo L et al (1992) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394
Heidland A, Ling H, Vamvakas S, Paczek L (1996) Impaired proteolytic activity asa potential cause of progressive renal disease. Miner Electrolyte Metab 22:157–161
Hostetter T (2003) Hyperfiltration and glomerulosclerosis. SeminNephrol 23:194–199
Hostetter TH, Troy JL, Brenner BM (1981) Glomerular hemodynamic in experimental diabetes. Kidney Int 19:410–415
Jain RC, Vyas CR (1975) Hypoglycemic action of onion and garlic. Am J Clin Nutr 28:684–685
Liu C-T, Hse H, Lii C-K, Chen P-S, Sheen L-Y (2005) Effects of garlic oil and diallyltrisulfide onglycemic control in diabetic rats. Eur J Pharmacol 516:165–173
Mandarim-de-Lacerda CA (2003) Stereological tools in biomedical research. An Acad Bras Cienc 75(4):469–486
Mathew PT, Augusti KT (1973) Studies on the effect of allicin (diallyldisulphide-oxide) on alloxan diabetes I. Hypoglycaemic action and enhancement of serum insulin effect andglycogensynthesis. Indian J Biochem Biophys 10:209–212
McCrary RF, Pitts TO, Puschett JB (1981) Diabetic nephropathy. Natural course, survivorship, and therapy. Am J Nephrol 1:206–201
Mogensen CE, Anderson MJF (1975) Increased kidney size and glomerular filtration rate in untreated juvenile diabetes: normalization by insulin-treatment. Diabetologia 11:221–224
Musabayane CT (2012) The effects of medicinal plants on renal function and blood pressure in diabetes mellitus. Cardiovasc J Afr 23:462–468
Nyengaard JR (1999) Stereologic methods and their application in kidney research. J Am Soc Nephrol 10(5):1100–1123
Pushparaj P, Tan CH, Tan BKH (2000) Effects of Averrhoa Bilimbileaf extract on blood glucose and lipids in streptozotocin-diabetic rats. J Ethnopharmacol 72:69–76
Qattan KA, Thomson M, Muslim A (2008) Garlic (Allium sativum) and ginger (Zingiberofficinale) attenuate structural nephropathy progression in streptozotocin-induced diabetic rats. Eur J Clin Nutr Metab 3:62–71
Rabkin R, Schechter P, Shi JD, Boner G (1996) Protein turnover in thehypertrophy in kidney. Miner Electrolyte Metab 22:153–156
Rahimi R, Nikfar S, Larijani B, Abdollahi M (2005) A review on the antioxidantsin the management of diabetes and its complications. Biomed Pharmacother 59:365–373
Salvatore T, Giugliano D (1996) Pharmacokinetic-pharmacodynamic relationshipof acarbose. Clin Pharmacokinet 30:94–106
Sharma K, Ziyadeh FN (1995) Hyperglycemia and diabetic kidney disease: the case for transforming growth factor-β as a key mediator. Diabetes 44:1139–1146
Sherkatolabbasieh H, Hagh-Nazari L, Shafiezadeh S, et al (2016) Ameliorative effects of the ethanolic extract of Allium saralicum R.M. Fritsch on CCl4-induced nephrotoxicity in mice: a stereological examination. Archives of Biological Science DOI: https://doi.org/10.2298/ABS160914129S
Stackhouse S, Miller PL, Park SK, Meyer TW (1990) Reversal of glomerular hyperfiltration and renal hypertrophy by blood glucose normalization in diabetic rats. Diabetes 39:989–995
Sterio DC (1984) The unbiased estimation of number and sizes of arbitrary particles using the dissector. J Microsc 134:127–36
Thomson M, Al-Amin ZM, Al-Qattan K et al (2007) Anti-diabetic and hypolipidaemic propertiesof garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes Metab 15:108–115
Weir GC, Clore ET, Zmachiroski CJ, Bonner-Weir S (1981) Islet secretion in a newexperiment model for non-insulin dependent diabetes. Diabetes 30:590–595
Welt K, Weis J, Martin R, et al (2007) Ginkgo biloba extract protects rat kidney from diabetic and hypoxic damage. Phytomedicine 14:(2–3): 196–203
WHO Expert Committee on Diabetes mellitus (1981) Technical Report Series 646, Second Report. World Health Organization Geneva
Wiseman MJ, Saunders AJ, Keen H, Viberti GC (1985) Effect of blood glucose control on increased glomerular filtration rate and kidney size in insulin dependent diabetes. N Engl J Med 312:617–621
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All institutional and national standards for the care and use of laboratory animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zangeneh, M.M., Goodarzi, N., Zangeneh, A. et al. Amelioration of renal structural changes in STZ-induced diabetic mice with ethanolic extract of Allium saralicum R.M. Fritsch. Comp Clin Pathol 27, 861–867 (2018). https://doi.org/10.1007/s00580-018-2674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2674-9