Abstract
Cystic echinococcosis due to Echinococcus granulosus is a serious public health and economic concern in India. The disease is endemic in most of the food producing animals such as cattle, buffalo, sheep, goat and pigs in the country. In this study, tissues comprising of pieces of liver and lungs were collected in 10 % formal buffered saline. The formalin fixed tissues (liver and lungs) from 10 cattle, buffalo, sheep, goat and pigs each were selected and further processed by acetone benzene method for histopathological examinations. The cysts were surrounded by outer fibrous layer over the inner germinal layer and filled with clear hydatid fluid. Echinococcal protoscolices were also noticed in some of the sections. Histologically, slight hemorrhage, leucocyte infiltration and mild hepatocellular degeneration in the liver were noticed. The adjacent hepatic paraenchyma showed atrophy, variable degeneration and infiltration. The parenchyma adjacent to cysts was markedly congested and showed multiple small haemorrhagic areas. In lungs, there was proliferation of fibrous connective tissue and infiltration of mononuclear cells.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The disease echinococcosis occurs due to cestodes belonging to the genus Echinococcus (family Taeniidae). The important parasite species include Echinococcus granulosus, E. multilocularis, E. oligarthrus and E. vogeli (Rausch 1975; Thompson 1995). Carnivorous animals act as definitive hosts and shed eggs in the faeces. Ingestion of food or water contaminated with eggs leads to infection in the intermediate hosts (Thompson 1995). A number of herbivorous and omnivorous animals act as intermediate hosts. Numerous animal species such as animals belonging to families Bovidae, Cervidae, Suidae, Equidae, Camelidae, Giraffidae, Elephantidae, Hippopotamidae, Leporidae, primates and marsupials could act as intermediate and aberrant hosts (WHO 1984). Humans act as accidental intermediate hosts. Cystic echinococcosis (CE) is endemic in many countries across the world (Sakamoto et al. 1987; Ghorui and Sahai 1989; Uysaler et al. 1998; Yildiz and Tuncer 2005; Lahmar et al. 2009).
In intermediate hosts, the disease mostly remains asymptomatic and is usually detected at post mortem inspection. The hydatid cysts grow slowly and take several years to grow big enough and cause symptoms. The cysts are primarily located in liver and lungs but could be found in many other organs such as spleen, heart, kidneys and omentum etc. (WHO and OIE 2001).
However, the disease causes huge production and economic losses. CE due to E. granulosis is a serious economic concern in India (Singh et al. 2014) and the disease is endemic in most of the food producing animals such as cattle, buffalo, sheep, goat and pigs in the country (Singh et al. 2012). The present study was contemplated to understand histopathological changes associated with E. granulosus echinococcosis in food producing animals in north India.
Materials and methods
The present work was carried out in the Department of Veterinary Public Health, in collaboration with Department of Epidemiology and Preventive Veterinary Medicine, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab (INDIA). Samples for investigating hydatidosis of food animals were collected from a Municipal Corporation slaughter house Ludhiana (Punjab), private (individual) butchers’ places at Ludhiana (Punjab) and Saharanpur (Uttar Pradesh), or where livestock were slaughtered and at Post Mortem Halls at Ludhiana and Ladowal (Punjab). Due to ban on cow slaughter on religious beliefs and lack of organized buffalo slaughter houses, the animals dying of naturally/any other disease were also included in the study. The visceral organs of every animal included in the survey were examined visually, palpated and incised for the detection of hydatid cysts on post-mortem inspection. The organs infected with hydatid cyst were collected in plastic bags and transported to laboratory for further examination. Tissues comprising of pieces of liver and lungs were collected in 10 per cent formal buffered saline for histopathological examination. We examined carcasses of 4,130 food producing animals and collected 66 hydatid cyst containing livers and lungs. The formalin fixed tissues (liver and lungs) from 50 animals (10 cattle, buffalo, sheep, goat and pigs each) were selected and further processed by acetone benzene method (Luna 1968). The paraffin blocks were prepared and sections of 4–5 µm thickness were obtained on glass slides with rotatory microtome. These paraffin sections were stained with haematoxylin and eosin stain for routine histopathology (Luna 1968).
Results
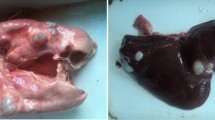
We encountered numerous cysts embedded at different depths usually in liver and lungs of all the mentioned animal species (Fig. 1). The cysts were also found embedded in the spleen and heart of two pigs and one sheep, respectively. There was gross enlargement of infected organs. The cysts were surrounded by outer fibrous layer over the inner germinal layer and filled with clear hydatid fluid. The results re-confirmed that germinal layer is the primary site of parasite development. Echinococcal protoscolices were also noticed in some of the sections.
There was slight hemorrhage, leucocyte infiltration and mild hepatocellular degeneration in the liver (Figs. 2, 3). Due to pressure effects from developing cysts, adjacent hepatic paraenchyma showed atrophy, variable degeneration and lympho-mononuclear infiltration (Fig. 4). The parenchyma adjacent to cysts was markedly congested and showed multiple small haemorrhagic areas. In lungs, there was proliferation of fibrous connective tissue and infiltration of mononuclear cells (Fig. 5).
Discussion
The disease mostly remains asymptomatic in intermediate animal hosts (Eckert et al. 2001). Hydatid cysts have been commonly recorded in liver followed by lungs and majority of cysts are slow growing (Gemmell 1966). The results in the present study are in parity with many previous studies (Hussain et al. 1992; Ahmedullah et al. 2007). Barnes et al. (2011) reported that adventitial layer in the multilocular cysts was thick in sheep which could be restrictive to growth while simultaneously protecting the hydatid from the host immune response. Barnes et al. (2011) further reported that the acellular zone had an outer rim of viable granulation tissue in which there was a mild lymphoplasmacytic and histiocytic infiltrate, including scattered multinucleated giant cells, admixed with rare eosinophils. Occasional lymphofollicular proliferations or remnants of the peribronchiolar lymphoid tissue were seen in the outer part of the adventitia or adjacent lung parenchyma (Barnes et al. 2011). Rashed et al. (2004) also reported a case of severe hydatidosis in a liver of a Najdi sheep. Histopathological changes included the formation of fibrotic capsules around biliary tracts and portal vein and also leaky liver. Pre-malignant changes were also seen in the different foci particularly around the biliary tracts and portal veins. Joshi and Yamasaki (2010) also reported histopathological changes associated with CE in pigs in Nepal. Kul and Yildiz (2010) characterised multivesicular and unilocular hydatid cysts in cattle. They reported that severity of calcification; fibrous capsule formation and giant cell layer were similar for multivesicular and unilocular cysts. However, the severity of subcapsular inflammation, inflammatory cell infiltration into adjacent organ parenchyma and eosinophil leucocyte infiltration into the cyst lumen was higher in multivesicular cysts.
References
Ahmedullah FM, Akbor MG, Haider MM, Hossain MA, Khan HNA, Hossain MI, Shanta IS (2007) Pathological investigation of liver of the slaughtered buffaloes in barisal district. Bangladesh J Vet Med 5(1–2):81–85
Barnes TS, Hinds LA, Jenkins DJ, Bielefeldt-Ohmann H, Lightowlers MW, Coleman GT (2011) Comparative pathology of pulmonary hydatid cysts in macropods and sheep. J Comp Path 144:113–122
Eckert J, Deplazes P, Craig PS, Gemmell MA, Heath D et al (2001) Echinococcosis in animals: clinical aspects, diagnosis and treatment. In: Eckert J, Gemmell MA, Meslin FX, Pawlowski Z (eds.) WHO/OIE manual on Echinococcosis in humans and animals: a public health problem of global concern, Paris, pp. 72–99
Gemmell MA (1966) Immunological responses of mammalian host against tapeworm infections. IV. Species specificity of hexacanth embryos in protecting sheep against Echinococcus granulosus. Immunology 11:325–335
Ghorui SK, Sahai BN (1989) Studies on the incidence of hydatid disease in ruminants. Indian J Anim Health 28:39–41
Hussain A, Maqbool A, Hussain S, Athar M, Shakoor A, Amin MK (1992) Studies on prevalence and organ specificity of hydatidosis in ruminant slaughtered at Karachi and Faisabad abattoir, Pakistan. Indian J Vet Sci 45(9):454–456
Joshi DD, Yamasaki H (2010) Histopathological and molecular confirmation of Porcine Cystic Echinococcosis (CE)/Hydatidosis in Nepal. J Inst Med 32(3):54–58
Kul O, Yildiz K (2010) Multivesicular cysts in cattle: characterisation of unusual hydatid cyst morphology caused by Echinococcus granulosus. Vet Parasitol 170(1–2):162–166
Lahmar S, Rebaï W, Boufana BS, Craig PS, Ksantini R, Daghfous A, Chebbi F, Fteriche F, Bedioui H, Jouini M, Dhibi M, Makni A, Ayadi MS, Ammous A, Kacem MJ, Ben Safta Z (2009) Cystic echinococcosis in Tunisia: analysis of hydatid cysts that have been surgically removed from patients. Ann Trop Med Parasitol 103:593–604
Luna LG (1968) Manual of histological staining methods.Armed Forces Institute of Pathology. McGraw Hill Book Company, New York
Rashed AA, Omer HM, Fouad MA, Al Shareef AM (2004) The effect of severe cystic hydatidosis on the liver of a Najdi sheep with special reference to the cyst histology and histochemistry. J Egypt Soc Parasitol 34(1):297–304
Rausch RL (1975) Taeniidae. In: Hubbert WF, McCulloch WF, Schurrenberger PR (eds) Diseases transmitted from animals to man. Thomas, Springfield, Illinois, pp 678–707
Sakamoto T, Shigekazu T, Hutchinson GW, Copeman DB, Thompson RCA, Sakamoto H (1987) Studies on echinococcosis in Australia. I. histopathological observations on echinococcosis of cattle in Australia. J Fac Agric Iwate Univ 18:323–337
Singh BB, Sharma JK, Ghatak S, Sharma R, Bal MS, Tuli A, Gill JPS (2012) Molecular epidemiology of echinococcosis from food producing animals in North India. Vet Parasitol 186(3–4):503–506
Singh BB, Dhand NK, Ghatak S, Gill JPS (2014) Economic losses due to cystic echinococcosis in India: need for urgent action to control the disease. Prev Vet Med 113(1):1–12
Thompson RCA (1995) Biology and systematics of Echinococcus. In: Thompson RCA, Lymbery AJ (eds) Echinococcus and hydatid disease. CAB International, Wallingford, pp 1–50
Uysaler A, Yazicioglu L, Aral A, Akalin H (1998) A multivesiculer cardiac hydatid cyst with hepatic involvement. Eur J Cardiothorac Surg 14:335–337
WHO, OIE (2001) WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern. Paris
World Health Organization (WHO) (1984) Guidelines for surveillance, prevention and control of echinococcosis/hydatidosis. In Eckert J, Gemmell M, Matyas Z, Soulsby EJL (eds). World Health Organization, Geneva, pp. 1–147
Yildiz K, Tuncer C (2005) Kirikkale’de sigirlarda kist hidatik’in yayilisi. Turkiye Parazitol Derg 29:247–250
Conflict of interest
No financial or personal relationships between the authors and other people or organizations have inappropriately influenced (bias) this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, B.B., Sharma, R., Sharma, J.K. et al. Histopathological changes associated with E. granulosus echinococcosis in food producing animals in Punjab (India) . J Parasit Dis 40, 997–1000 (2016). https://doi.org/10.1007/s12639-014-0622-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-014-0622-4