Abstract
This study investigated the possible neuroprotective properties of two commonly consumed tropical vegetables. The modulatory effects of Vernonia amygdalina (VA) and Struchium sparganophora (SS) on the activities of Na+/K+ ATPase, ecto-5′-nucleotidase, monoamine oxidase (MAO), acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and FeSO4-induced oxidative stress in rat brain homogenate were assessed. The result revealed that both vegetables inhibited AChE, BChE, MAO, and ecto-5’nucleotidase activities, but stimulated Na+/K+ ATPase activity in a concentration-dependent manner. The SS had a significantly higher (P ˂ 0.05) inhibitory effect on AChE (IC50 = 4.83 μg/mL), BChE (IC50 = 5.61 μg/mL), MAO (IC50 = 26.11 μg/mL), ecto-5′ nucleotidase (IC50 = 23.04 μg/mL) than VA [AChE (IC50 = 7.53 μg/mL), BChE (IC50 = 7.32 μg/mL), MAO (IC50 = 26.11 μg/mL), and ecto-5′ nucleotidase (IC50 = 42.35 μg/mL)]. Furthermore, SS (IC50 = 28.30 μg/mL) had a significantly higher (P < 0.05) stimulatory effect on Na+/K+ ATPase activity than VA (IC50 = 34.87 μg/mL). Both extracts exhibited a strong antioxidant properties as typified by their radicals (OH and DPPH) scavenging and Fe2+ chelating abilities, as well as inhibition of Fe2+-induced lipid peroxidation in rat brain homogenate. The HPLC fingerprint of VA and SS extracts revealed the presence of catechin, chlorogenic acid, caffeic acid, p-coumaric acid, rutin, orientin, quercitrin quercetin, and luteolin. The stimulation of Na+/K+ ATPase activity, inhibition of 5′-nucleotidase, AChE, BChE, and MAO activities as well as Fe2+-induced oxidative stress could be a possible mechanism through which VA and SS exerts neuroprotective properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vernonia amygdalina (VA, commonly called bitter leaf) which belongs to the family Asteraceae originated from tropical Africa (Schiffers 2000). The plant is grown as a vegetable in Nigeria, Cameroon, Gabon, Tanzania, and Democratic Republic of Congo. The plant is commonly called bitter leaf due to its bitter taste, but it has a delicious taste when prepared in meals (Oboh et al., 2016a, b). VA has also become popular due to its medicinal uses (Beentje 2000; Atangwho et al. 2013; Oboh et al., 2016a, b). Struchium sparganophora (SS, commonly called water bitter leaf) is an edible herb in most African countries belonging to the family of Asteraceae. The leaves are consumed as a vegetable or added to soup. The leaf has been shown to exhibit antioxidant, antimicrobial, nutritive and anti-malaria activities (Oboh 2006). Phytochemically, sesquiterpene lactone, luteolin, vernodalin, and 3-methyl-2,6-hexacosedienol have been isolated from SS leaf (Jakupovic et al. 1987; Eko et al. 2008; Kasim et al. 2011).
Alzheimer’s disease (AD) is a common neurodegenerative disease of the elderly, currently account for 50–60% cases of dementia. Marked reduction in cholinergic function has been postulated as an activator of AD, and there is currently no known cause. Recently, efforts have been devoted to anti-AD drug discovery, but there are still no efficient therapeutic agents. Therapeutic target enzymes implicated in the management of AD include AChE, BChE, MAO, ecto-5′-nucleotidase, and Na+/K+ ATPase (Thomas 2002; Lane et al. 2006; Oboh et al., 2016a, b; Nwanna et al. 2016). Stimulation of the cholinergic receptor or prolonging of the availability of acetylcholine (ACh) released into the neuronal cleft by inhibiting ACh hydrolysis by AChE is one approach to enhancing cholinergic function in AD, and this is achieved by the use of AChE inhibitors. Synthetic AChE inhibitor drugs such as galantamine, rivastigmine, donepezil, and tacrine have been shown to palliate some symptoms associated with AD and thereby slowing down the progression of AD. The use of MAO and ecto-5′-nucleotidase inhibitors, and Na+/K+ ATPase stimulators has been employed in the therapeutic management of AD (Lane et al. 2006; Ademosun & Oboh 2014; Oboh et al., 2016a, b). A combination of these therapeutic approaches coupled with antioxidative abilities, which is aimed at ameliorating oxidative damage, could be an effective strategy in the management of AD; therefore, this study sought to investigate the antioxidant properties and modulatory effects of VA and SS on the cholinergic, monoaminergic, and purinergic systems. High performance liquid chromatography coupled with diode array detector (HPLC-DAD) was used to quantify the possible phenolic compound. The results of this study can provide the possible mechanism of actions of these vegetables as nootropic and in the management of neurodegenerative diseases.
Experimental procedure
Chemicals and reagents
Chemicals such as acetylthiocholine iodide, butyrylthiocholine iodide, semicarbazide, benzylamine, adenosine triphosphate (ATP), ouabain, ammonium molybdate, thiobarbituric acid (TBA), and 5,5′-dithio-bis(2-nitrobenzoic acid) were purchased from Sigma-Aldrich, ChemieGmH (Steinheim, Germany). The water used was glass distilled.
Sample collection and preparation of extracts
Fresh samples of VA and SS were obtained from the botanical garden, Federal University of Technology, Akure, Nigeria. Authentication of the sample was carried out at the Biology Department, Federal University of Technology, Akure, Nigeria. The leaves of VA and SS were separated from the stem by hand picking, air dried under shade, and pulverized. The extracts were prepared according to the method of Adefegha et al. (2016).
Handling of experimental animals
Animals used for this study were wistar strain male albino rats of the weight ranged between 210 and 215 g. Their treatment was strictly followed in reference to the laid downregulation by the National Academy of Science and National Institutes of Health (NIH 2011) for the care and Use of Laboratory Animals. They had free access to the feed and water ad libitum for a 2-week acclimatization period.
Cholinesterases and monoamine oxidase activities assays
The effects of the VA and SS extracts on cholinesterases [acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)] and Monoamine oxidase (MAO) activities were assessed by colorimetric method described in our earlier report (Oboh et al., 2017a, b). The AChE, BChE, and MAO inhibitory activities of the extracts were expressed as percentage inhibition while the concentration of VA and SS causes 50% of the AChE and BChE inhibition was calculated.
Determination of Na+/K+-ATPase and ecto-5′-nucleotidase activities assays
The effects of the extracts on Na+/K+-ATPase and Ecto-5′-nucleotidase activities in the whole brain homogenate were measured using the spectrophotometer method as earliest described (Oboh et al., 2016a, b). Specific Na+/K+-ATPase activity was calculated by subtracting the ouabain-insensitive activity from the overall activity (in the absence of ouabain) and expressed in nmol of Pi/mg of protein/min while ecto-5′-nucleotidase inhibitory activity was expressed as percentage inhibition.
Antioxidant capacity assays
The antioxidant capacity of VA and SS extracts was determined using different assay methods. Preparation of brain homogenate and lipid peroxidation assay was carried out as mentioned by Oboh et al. (2016a, b). The free radical scavenging ability of VA and SS extracts against DPPH (1,1-diphenyl–2 picrylhydrazyl) radical and hydroxyl (OH) radical as well as the Fe2+ chelating abilities were determined as previously reported (Oboh et al., 2017a, b).
Quantification of compounds by HPLC-DAD
The phenolic constituents of VA and SS were characterized using high performance liquid chromatography coupled with diode array detector (HPLC-DAD); a model of Shimadzu Prominence Auto Sampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A Integrator, SPD-M20A diode array detector, and LC solution 1.22 SP1 software. Differences between groups of HPLC were assessed by an analysis of variance model and Tukey’s test. The level of significance for the analyses was set to P < .05. These analyses were performed by using the free software R version 3.1.1 (Akomolafe et al. 2016).
Data analysis
The results of three replicates were pooled and expressed as mean ± standard deviation (S.D. n = 3). Student’s t test, one-way analysis of variance (ANOVA), and least significance difference (LSD) were carried out. The significance level was taken at P < 0.05. IC50 was determined using nonlinear regression analysis.
Results and discussion
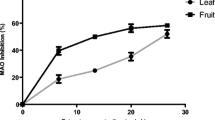
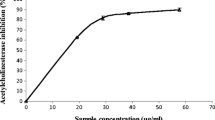
Inhibition of AChE, BChE, and MAO activities are among the therapeutic targets in the management of AD, and the ability of dietary vegetable to inhibit these enzymes emphasized their neuro-protective potentials. AChE inhibition is important in AD management as it increases the concentration of ACh in the brain, thereby enhancing communication between the nerve cells. (Ademosun & Oboh 2012; Nwanna et al. 2016; Oboh et al., 2017a, b). More importantly, BChE inhibition is crucial to the AD management as the activity of BChE rise with the progression of the degenerative condition (Fernandez-Bachiller et al. 2012). Although, the activity of MAO has been reported as one of the risk factors of Parkinson diseases (PD). Its elevation has also been traced to the AD, where it is said to reduce the levels of neuroactive amine, such as norepinephrine, dopamine, and serotonin and increase production of harmful compounds (H2O2 and NH3) that are capable of inducing free radical generation in the AD brain (Nwanna et al. 2016; Oboh et al., 2017a, b). From our investigation, the studied vegetables inhibited these enzymes in a concentration-dependent pattern. As evidence of the result presented in Table 1, SS had significantly (P < 0.05) higher inhibition of AChE (IC50 = 4.83 μg/mL), BChE (IC50 = 5.61 μg/mL), and MAO (IC50 = 8.41 μg/mL) activities than did VA [AChE (IC50 = 7.58 μg/mL), BChE (IC50 = 7.32 μg/mL), and MAO (IC50 = 26.11 μg/mL)]. SS and VA are rich in phenolic compounds, and phenolics have been reported to possess anticholinergic and antimonoaminergic properties (Benamar et al. 2010; Oboh et al., 2017a, b). From the Pearson correlation analysis Table (Table 3), inhibition of AChE correlated (P < 0.05) with catechin (r = 0.934) and chlorogenic acid (r = 0.930) and strongly correlated (P < 0.01) with caffeic acid (r = 0.999), rutin (r = 0.988), orientin (r = 0.962), quercitrin (r = 0.999), and quercetin (r = 0.962), while inhibition of BChE activity correlated (P < 0.05) with p-coumaric acid (r = 0.945) and quercitrin (r = 0.945). The inhibition of MAO, however, strongly correlated (P < 0.01) with cholorogenic and caffeic acids, rutin, orientin, quercitrin, and quercetin with r value of 0.985, 0.965, 0.998, 0.998, 0.965, and 0.998, respectively. This finding of functional properties of the studied vegetables is in line with the report of Nwanna et al. (2016) and Zengina et al. (2016) that consumption of phenolic-rich vegetables capable of inhibiting enzymes (AChE, BChE, and MAO) linked to the degenerative disease could be the best approach to manage this ailment holistically. Moreso, they are relatively cheap, readily available and exhibited little or no side effect.
In addition, the activities of some enzymes of the purinergic signaling system (ecto-5′-nucleotidase and Na+/K+ ATPase) have been implicated in AD. Na+/K+ ATPase plays a crucial role in the central nervous system, and its dysfunction or reduce activity has been shown to contribute to AD development (Ziegelhoffer et al. 2000). Reduced activities of Na+/K+ ATPase trigger cell death, which occurs as a result of Ca2+ bio-accumulation (Choi 1988); therefore, the stimulation of Na+/K+-ATPase could result in an extracellular increase in K+ and also marked intracellular decrease in Ca2+. In the management of AD, inhibition of ecto-5′-nucleotidase is therapeutically important to the central nervous system (Williams 1990). Ecto-5′-nucleotidase catalyzes the breakdown of adenosine monophosphate (AMP), to adenosine and inorganic phosphate; the products that could inhibit the production of neurotransmitters via specific (presynaptic) receptors, thereby impairing the purinergic transmission (Oboh et al., 2016a, b). In this study, SS (IC50 = 28.30 μg/mL) had significantly (P < 0.05) higher Na+/K+-ATPase stimulatory effect than VA (IC50 = 34.84 μg/mL). SS extract (IC50 = 23.04 μg/mL) also inhibited ecto-5′ nucleotidase than VA did (IC50 = 34.44 μg/mL). It is worth mentioning that the stimulation of Na+/K+-ATPase is strongly correlated (P < 0.01) with caffeic and p-coumaric acids, rutin, quacetrin, and quercetin. Catechin, p-coumaric acid, quercitrin (P < 0.05) could be responsible for the inhibition of ecto-5′ nucleotidase (Table 3).
The result further showed that there is an increase (P < 0.05) in the malondialdehyde (MDA) content when Fe2+ solution was incubated with isolated brain homogenate to the tune of 178.23% compared to the control (100%); however, by adding the studied extracts (50 μg/mL), MDA level was decreased, and there was no significant (P > 0.05) difference in the MDA inhibitory abilities [(SS = 114.23%) and VA = 113.93%)]. Table 1 further revealed that SS had significantly (P < 0.05) higher DPPH (IC50 = 16.25 μg/mL) and OH (IC50 = 18.57 μg/mL) radicals scavenging abilities than VA (DPPH radical, IC50 = 42.35 μg/mL; OH radical, IC50 = 1.07 μg/mL). The Fe2+ chelating abilities of the extracts (Table 1) revealed that SS (IC50 = 24.97 μg/mL) chelated Fe2+ better than did VA (IC50 = 44.59 μg/mL). The antioxidative properties of VA and SS were determined in this study using four different methods: lipid peroxidation, DPPH, and OH radical scavenging and Fe2+ chelating abilities, and SS extracts were found to have higher antioxidant activity than VA, which could make it a better agent in the management of oxidative stress-induced neurodegenerative conditions. The antioxidant activities of the studied vegetable could be linked to their phenolic constituents as phenolic compounds are known to posses strong radicals scavenging and metal-chelating abilities (Kwon et al. 2010; Richetti et al. 2011; Javed et al. 2012), abilities that could be related to their structure-function effects (Amic et al. 2003; Adefegha et al. 2015]. We could therefore infer that the higher antioxidant ability of SS could be as a result of its higher total phenolic constituents or synergistic effect of individual phenolics in comparism with the VA (Table 2).
The identification of phenolic constituents in the plant materials, using HPLC, has been proven reliable compared to the colorimetric method of phenolic content. The HPLC fingerprint of VA and SS revealed the presence of four phenolic acids (catechin, chlorogenic, caffeic, and p-coumaric acids) and five flavonoids (rutin, orientin, quercitrin, quercetin, and luteolin) on a dry weight basis (Fig. 1 and Table 3). Although, nine phenolics were identified in each of the sample, however, they were significantly different in amount. Orientin (8.15 mg/g), chlorogenic acid (8.13 mg/g), and rutin (6.08 mg/g) were predominantly present in SS, followed by quercitrin (3.96 mg/g) and luteolin (3.95 mg/g) (P > 0.05), p-coumaric (3.27 mg/g), and quercetin (2.73 mg/g) while caffeic acid (1.54 mg/g) and catechin (1.49 mg/g) were the least (P > 0.05). Contrarily, in VA leaves quercitrin (6.37 mg/g), luteolin (4.15 mg/g), p-coumaric (4.13 mg/g), and quercetin (3.72 mg/g) were the predominant phenolics followed by caffeic acid (2.05 mg/g) and orientin (2.01 mg/g), which were not significantly different (P > 0.05) in amount. It is of interest to know that the phenolic constituents and the amount detected in the tested VA differ from what was reported in the study of Oboh et al. (2016a, b). These differences could be a result of the origin/location, and variation in the genetic makeup as well as the season which the VA leaves were collected (Ademiluyi et al. 2016). The correlation between the phenolics in these samples and the observed functionality is noteworthy. Several studies have reported on the neuroprotective effects of some of these phenolics detected in the studied vegetables, e.g., caffeic and chlorogenic acids, quercetin, and rutin that are known for their anticholonesterases and antioxidant abilities (Oboh et al. 2013; Ademosun et al. 2016). In another study of Oboh et al. (2017a, b), there was a correlation between the quercetin, rutin, and MAO inhibition.
Conclusions
This study was able to show that the aqueous extracts from VA and SS leaves exhibited neuroprotective properties by modulating crucial enzymes relative to neurological impairment as well as antioxidative properties. However, SS was more effective than VA, and could be as a result of the synergistic effect of the individual phenolic constituents that add up to the higher amount of phenolic compound when compared to the VA. The enzyme modulatory and the high antioxidant properties of the SS and VA could be part of the mechanism of actions through which they could be used in the management of neurodegenerative disease such as AD.
References
Adefegha SA, Oboh G, Ejakpovi II, Oyeleye SI (2015) Antioxidant and antidiabetic effects of gallic and protocatechuic acids: a structure–function perspective. Comp Clin Pathol 24:1579–1585
Adefegha SA, Oboh G, Oyeleye SI, Ejakpovi I (2016) Erectogenic, antihypertensive, antidiabetic, anti-oxidative properties and phenolic compositions of almond fruit (Terminalia catappa L.) parts (hull and drupe)–in vitro. J Food Biochem. doi:10.1111/jfbc.12309
Ademiluyi AO, Oyeleye SI, Oboh G (2016) Biological activities, antioxidant properties and phytoconstituents of essential oil from sweet basil (Ocimum basilicum L.) leaves. Comp Clin Pathol 25:169–176
Ademosun AO, Oboh G, Bello F, Ayeni PO (2016) Antioxidative properties and effect of quercetin and its glycosylated form (rutin) on acetylcholinesterase and butyrylcholinesterase activities. J Evid Based Compl Alt Med 21:11–17
Ademosun AO, Oboh G (2012) Inhibition of acetylcholinesterase activity and Fe2+-induced lipid peroxidation in rat brain in vitro by some citrus fruit juices. J Med Food 15:428–434
Ademosun AO, Oboh G (2014) Comparison of the inhibition of monoamine oxidase and butyrylcholinesterase activities by infusions from green tea and some citrus peels. Int J Alzheimers Dis 586407:1–5
Akomolafe SF, Oboh G, Oyeleye SI, Boligon AA (2016) Aqueous extract from Ficus capensis leaves inhibits key enzymes linked to erectile dysfunction and prevent oxidative stress in rats’ penile tissue. NFS J 4:15–21
Amic D, Davidovic-Amic D, Beslo D, Trinajstic N (2003) Structure-radical scavenging activity relationship of flavonoids. Croat Chem Acta 76:55–61
Atangwho IJ, Egbung GE, Ahmad M, Yam MF, Asmawi MZ (2013) Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del. growing in Malaysia. Food Chem 141:3428–3434
Beentje HJ (2000) Compositae (part 1). In: Beentje HJ (ed) Flora of tropical East Africa. A.A. Balkema, Rotterdam, Netherlands, pp 1–313
Benamar H, Rached W, Derdour A, Marouf A (2010) Screening of Algerian medicinal plants for acetylcholinesterase inhibitory activity. J Biol Sci 10:1–9
Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11:465–469
Eko ME, Eteng MU, Eyong EU (2008) Phytochemical composition and effect of aqueous extract of Struchium sparganophora (L) on cockroach crude extract-induced-airway inflammatory responses in wistar rats. Global J Pure Appl Sci 14:417–422
Fernandez-Bachiller MI, P’erez C, Monjas L, Rademann J, Rodr’ıguez-Franco MI (2012) New tacrine-4-oxo-4 Hchromene hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with cholinergic, antioxidant, and β- amyloid properties. J Med Chem 55:1303–1317
Jakupovic J, Zdero C, Boeker R, Warning U, Bohlmann F, Jones SB (1987) Cernocistifolide und andere sesquiterpine lactone aus Vernonia und verwandten Arten. Liebigs Ann Chemie:111–123
Javed H, Khan MM, Ahmad A, Vaibhav K, Ahmad ME, Khan A et al (2012) Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neurosci 210:340–352
Kasim LS, Ferro VA, Odukoya OA, Drumond A, Ukpo GE, Seidel V et al (2011) Antimicrobial agents from the leaf of Struchium sparganophora (Linn) Ktze Asteraceae. J Microbiol Antimicrob 3:13–17
Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH et al (2010) Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. European J Pharmacol 649:210–217
Lane RM, Potkin SG, Enz A (2006) Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol 9:101–124
National Institute of Health (NIH) (2011) Guide for the care and use of laboratory animals. US. Department of Health Education and Welfare. USA: NIH Publication
Nwanna EE, Oyeleye SI, Ogunsuyi OB, Oboh G, Boligon AA, Athayde ML (2016) In vitro neuroprotective properties of some commonly consumed green leafy vegetables in southern Nigeria. NFS J 2:19–24
Oboh G, Ademiluyi AO, Ogunsuyi OB, Oyeleye SI, Dada AF, Boligon AA (2017b) Cabbage and cucumber extracts exhibited anticholinesterase, antimonoamine oxidase and antioxidant properties. J Food Biochem. doi:10.1111/jfbc.12358
Oboh G, Adewuni TM, Ademosun AO, Olasehinde TA (2016b) Sorghum stem extract modulates Na+/K+−ATPase, ecto-5′-nucleotidase, and acetylcholinesterase activities. Comp Clin Pathol 25:749
Oboh G, Agunloye OM, Akinyemi AJ, Ademiluyi AO, Adefegha SA (2013) Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-In vitro. Neurochem Res 38:413–419
Oboh G, Akinyemi AJ, Adeleye B, Oyeleye SI, Ogunsuyi OB, Ademosun AO et al (2016a) Polyphenolic compositions and in vitro angiotensin-I-converting enzyme inhibitory properties of common green leafy vegetables: a comparative study. Food Sci Biotechnol 25:1243–1249
Oboh G, Ogunruku OO, Oyeleye SI, Olasehinde TA, Ademosun AO, Boligon AA (2017a) Phenolic extracts from Clerodendrum volubile leaves inhibit cholinergic and monoaminergic enzymes relevant to the management of some neurodegenerative diseases. J Dietary Suppl 14:358–371
Oboh G (2006) Nutritive value, antioxidant and antimicrobial properties of Struchium sparganophora leaves. J Med Food 9:276–280
Richetti SK, Blank M, Capiotti KM, Piato AL, Bogo MR, Vianna MR et al (2011) Quercetin and rutin prevent scopolamine-induced memory impairment in zebra fish. Behavioural Brain Res 217:10–15
Schiffers RR (2000) African indigenous vegetables: an overview of the cultivated species. University Greenwich Press, England
Thomas T (2002) Monoamine oxidase-B inhibitors in the treatment of Alzheimers disease. Neurobiol Aging 21:343–348
Williams M (1990) Purine nucleosides and nucleotides as central nervous system modulators. Ann New York Acad Sci 603:93–107
Zengin G, Nithiyanantham S, Sarikurkcu C, Uysal S, Ceylan R, Ramya KS et al (2016) Identification of phenolic profiles, fatty acid compositions, antioxidant activities and enzyme inhibition effects of seven wheat cultivars grown in Turkey: a phytochemical approach for their nutritional value. Int J Food Prop. doi:10.1080/10942912.2016.1238391
Ziegelhoffer A, Kjeldsen K, Bundgaard H, Breier A, Vrbjar N, Dzurba A (2000) Na,K-ATPase in the myocardium: molecular principles, functional and clinical aspects. Gen Physiol Biophys 19:9–47
Acknowledgements
The author(s) received no financial support towards this work. Publication of this article and all activities were solely carried out and funded by the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All institutional and national guidelines for the care and use of laboratory animals were followed. Animals were treated in reference to the laid down regulation by the National Academy of Science and National Institutes of Health (NIH 2011) for the care and Use of Laboratory Animals.
Rights and permissions
About this article
Cite this article
Ademosun, A.O., Oboh, G., Oyeleye, S.I. et al. Modulation of cholinergic, monoaminergic, and purinergic enzymes of the brain functions by bitter (Vernonia amygdalina) and water bitter (Struchium sparganophora) leaves extracts: comparison of phenolic constituents versus nootropic potentials. Comp Clin Pathol 26, 1267–1272 (2017). https://doi.org/10.1007/s00580-017-2518-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2518-z