Abstract
Brucellosis is a significant zoonotic disease that is emerging or reemerging in many parts of the world. This study was carried out to diagnose and investigate the pathological lesions associated with Brucella abortus in the fetuses and placenta of naturally infected dromedary camels. For diagnosis, the blood samples from infected dams were subjected to Rose Bengal Plate Test (RBPT) and blood PCR. The placental and fetal lung tissue samples were subjected to pathological examination and PCR. Nine cases of abortions and one case of early neonatal mortality was reported in the infected camels. The hematological parameters in infected dams revealed anemia and leukocytosis. The important pathological lesions in the infected fetuses were subcutaneous edema, moderate amount of serohemorrhagic fluid in the body cavities, interstitial pneumonia, degenerative changes in the liver, severe congestion with mononuclear infiltration in the kidney, and congestion in other visceral organs. The placentas were thickened, edematous, and showed necrosis along with mononuclear infiltration in histopathology. The RBPT and PCR for placental and fetal lung tissues detected all ten cases positive for B. abortus infection. However, blood PCR was positive only in two pregnant camels. The findings of the study indicated that B. abortus infection in pregnant dromedary camels causes necrotizing placentitis and fetal pneumonia resulting into abortion during mid to last trimester of pregnancy. The RBPT was found to play an important role in early serological diagnosis, whereas PCR was useful in confirmatory diagnosis of brucellosis from placental and fetal lung tissue samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brucellosis is a major health problem in the developing, tropical, and Mediterranean countries and is the second most important zoonotic disease in the world after rabies (Cutler and Whatmore 2003). Brucellosis can get transmitted to humans through consumption of unpasteurized dairy products or through direct contact with the infected animals, placentas, or aborted fetuses (Dean et al. 2012). Bovine brucellosis is endemic in all the parts of India and appears to be on the increase in recent times, perhaps due to increase trade and rapid movement of livestock (Renukaradhya et al. 2002). Moreover, the lack of confirmed and early diagnostic tests for brucellosis in live animals poses a risk of spread of infection among healthy animals and humans in contact.
The disease in female camels is manifested by abortions, premature birth, still births, infertility, and decreased milk production. The characteristic lesions are primarily placentitis, ovario-bursal adhesions, hydrobursitis, and granulomatous endometritis (Gwida et al. 2012). However, most of the times, the clinical signs of brucellosis in camels largely go unnoticed since many infected camels are silent carriers of brucellosis (Gwida et al. 2012). Being quicker and less expensive, serological methods are used more frequently for the diagnosis of brucellosis (Xavier et al. 2009). Although, there are several studies on Brucella-DNA detection by PCR from camel sera, blood, milk, and pure culture (Gwida et al. 2011; Ghorbani et al. 2013; Shome et al. 2013), no study is available for its detection from placental or fetal tissues. The present study first time describes the clinicopathological features and diagnosis of brucellosis in the naturally infected pregnant dromedary camels and their fetuses.
Material and methods
Animals
The organized camel herd of 350 dromedary camels of the present study was located in the Bikaner district (Rajasthan State, India) which is having arid climatic conditions. These camels were raised under semi-intensive system of management and have never been vaccinated for brucellosis. During the study period, out of total 84 pregnant camels in the herd, sudden abortions were reported in total 13 female camels and neonatal mortality was reported in one female. The affected camels were assessed by clinical examination and by laboratory analysis.
Hematological analysis
Blood samples from ten B. abortus infected camels that have fetal loss were collected from jugular vein in the heparinized vials for estimation of hematological parameters viz., hemoglobin (Hb), total leukocyte count (TLC), differential leukocyte count (DLC), total erythrocyte count (TEC), and packed cell volume (PCV), as per the standard procedure. For comparison, blood samples from ten non-infected pregnant camels from the same herd were used as control. These control groups of camels had normal full-term delivery and detected negative for B. abortus infection by RBPT and blood PCR.
Necropsy and histopathological studies
The fetuses and placentas were examined for any gross lesions during necropsy. Tissue samples from the placenta, lung, liver, kidney, heart, brain, spleen, abomasum, and small intestine were collected in 10 % formal saline for histopathology. The formalin fixed tissue samples were embedded in paraffin, cut into 4–5-μm sections and stained with hematoxylin and eosin (H & E) stain using the standard procedure.
Rose Bengal Plate Test
Serum samples from total 350 dromedary camels including 84 pregnant camels were tested by Rose Bengal Plate Test (RBPT) using Brucella colored antigen, procured from Biological Products Division, Indian Veterinary Research Institute (India). One drop of color antigen was mixed with one drop of serum sample and left for few seconds. Positive samples showed formation of agglutination.
DNA extraction and PCR
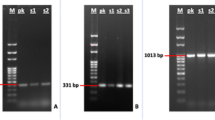
The DNA was extracted from blood and placenta of dam and fetal lung tissues from all 14 cases of fetal loss using DNeasy® blood and tissue kit (Qiagen) by following the procedure of the manufacturer. The DNA was subjected to PCR amplification for Brucella abortus and Brucella melitensis using the specific primer sequence and reaction procedure as described previously (Bricker and Halling 1994).
Statistical analysis
Hematological data were expressed as mean ± standard deviation and analyzed using SPSS 16 statistical software (SAS Institute Inc., Cary, NC, USA).
Results
The details of fetal loss and results of different diagnostic tests for 14 camels are given in Table 1. Out of 14 cases, nine cases of abortion and one case of neonatal mortality were detected positive for B. abortus infection by RBPT and placental and fetal lung tissue PCR, whereas only two aborted infected camels were detected positive in blood PCR. The overall incidence of brucellosis in the farm was 2.85 %.
Clinical and laboratorial findings in dams
The abortions in the brucellosis infected camels were reported from mid (n = 5) to last (n = 4) trimester of pregnancy. One of the infected camels had full-term delivery of a weak calf which died within 24 h of its birth. The majority of the infected camels had history of single (n = 4) or more than one (n = 4) parity, whereas two camels had history of no previous parturition. None of the infected camels had any history of previous abortion or early neonatal mortality. There were no specific clinical signs except mucous vaginal discharge, 5–6 h before abortion in four of the infected females. The results of hematological parameters revealed anemia and leukocytosis in B. abortus infected group as compared to the control group (Table 2).
Gross lesions in placenta and aborted fetuses
The thickening of allantoic membranes with white necrotic spots and edema was the most consistent gross alteration present in the placenta of all the infected aborted camels (Fig. 1). These lesions were absent in the placenta of non-infected camels having abortion or full-term parturition. The important gross pathological alterations in the infected aborted fetuses were subcutaneous edema with foul smell (n = 7), serohemorrhagic fluid inside body cavities (n = 8), severe congestion in lung (n = 9), enlarged and congested liver with pale necrotic patches (n = 8), severely congested and enlarged kidney (n = 8), and moderate to severe congestion of spleen, small intestine, heart, and brain (n = 7) (Figs. 2, 3, and 4). The abomasums of four of the infected aborted fetuses showed a viscous turbid brown-yellow fluid with suspended flecks of fibrin. The infected neonatal calf which died within 24 h of its birth had gross lesions of congestion and foci of consolidation in the lung, enlarged and congested liver with pale necrotic patches, and enlarged kidneys. The four of the non-infected aborted fetuses had no significant gross lesions apart from mild to moderate congestion of vital organs in two fetuses.
Histopathological findings
Microscopically, the placenta of all the infected aborted camels (n = 9) revealed necrosis, edema, and infiltration of mononuclear cells in the allantoic membrane (Fig. 5). Similarly, interstitial pneumonia was the most prominent change in all the infected fetuses examined. Infiltration of mononuclear cells and thickened alveolar and bronchial wall along with necrotic debris inside bronchi and alveoli was observed in the lungs of all the infected fetuses (Fig. 6). The liver showed congestion along with coagulative necrosis and vacuolar degenerative changes of hepatocytes (n = 8) (Fig. 7). In the kidney, glomerular capillary tufts were dilated with hyperemia and severe congestion of capillaries along with small focal interstitial mononuclear leukocyte accumulations were present in the renal cortex and corticomedullar junction of eight infected fetuses (Fig. 8). The majority of the infected fetuses had no splenic lesions, however, slight lymphoid depletion of the white pulp and mild mononuclear infiltration of the red pulp was observed in four of the infected fetuses. In the brain, mild mononuclear infiltration and mild to moderate congestion of capillaries were observed in six of the infected fetuses (Fig. 9). In the heart, no significant microscopical lesions were observed apart from mild mononuclear infiltration in two cases. Histological changes in the abomasum and small intestines were characterized by congestion and infiltration of mild to moderate numbers of mononuclear cells in the lamina propria occasionally extending to the submucosa (n = 7).
Discussion and conclusion
All the infected camels of the present study were adult pregnant females and none of the adult males and calves were detected positive by RBPT. This finding is similar to the previous studies wherein seroprevalence of brucellosis was reported much higher among adult camels than young ones and in females compared to males (Hadush et al. 2013; Adamu et al. 2014; Mohamed et al. 2015). This may be due to the fact that females are comparatively under greater physiological stress during pregnancy and lactation which make them more susceptible to infection (Walker 1999). In addition to that, one of the major factors is erythritol, a polyhydric acid found in higher concentration in the placenta and fetal fluids of females than in seminal vesicles and testis of males which is responsible for sexually mature females being more susceptible than males (Radostits et al. 2007). On the other hand, it is also true that younger animals tend to be more resistant to infection and frequently clear an established infection, although latent infections can occur (Quinn et al. 2013). Higher seropositivity was recorded in she-camels of the present study having single or more than one parity than those with no previous history of parturition. This observation is comparable with the findings of previous studies (Zewolda and Wereta 2012; Hadush et al. 2013). This is mainly because of the repeated exposure of she-camels to parturition and other physiological stress which increases the probability of acquiring Brucella infection (Hadush et al. 2013). Since camels of the present study were grazing in the pasture land common for cattle, sheep, and goats, the source of infection among them may be acquired from contact with the infected material during common grazing.
The hematological alterations such as anemia and leukocytosis observed in the infected camels of the present study are in agreement with a previous study in camels (El-Boshy et al. 2009). However, the infected camels were not exhibiting any external clinical signs except sudden abortion. This observation is similar to a previous study in which all the infected camels were clinically normal at the time of sampling and none had previously shown clinical signs of brucellosis (Gwida et al. 2011). This indicates that many infected camels might be silent carriers for brucellosis. The abortions in infected camels of the present study were reported in mid to last trimester of pregnancy; however, in the previous studies in cattle, the abortions were mostly reported in the last trimester (Sozmen et al. 2004). The possible reasons for this observation may be due to differences in physiology, gestation period, stage of infection, stage of fetal growth, and level of erythritol.
The pathological lesions of necrotizing placentitis, interstitial pneumonia, subcutaneous edema, serohemorrhagic lesions in body cavities, and hyperemic changes in different organs observed in the present study are similar to those reported in experimental and natural infections in the cattle (Sozmen et al. 2004; Xavier et al. 2009; Olsen and Palmer 2014). Necrotic neutrophilic placentitis with perivascular infiltrate was found to be associated with large numbers of B. abortus present intracellularly in the macrophages and trophoblasts, and also extracellularly in the necrotic tissues (Xavier et al. 2009). Trophoblasts are thought to be the primary target cell for invasion and multiplication of B. abortus in the placenta (Anderson et al. 1986). This tropism may be due to the presence of erythritol or to hormone synthesis by trophoblastic cells (Samartino and Enright 1993). These results support the notion that abortion due to B. abortus is dependent on the severity and distribution of placental lesions, which supposedly cause fetal oxygen and nutrient deprivation (Xavier et al. 2009). The fetal infection results from aspiration of amniotic fluid containing B. abortus, a process exacerbated by anoxia secondary to placentitis (Xavier et al. 2009). Following invasion through the respiratory tract, bacteraemia may develop, spreading the organism to other organs of the fetuses (Lopez et al. 1984). The histopathological lesions in lung and other organs of the infected camel fetuses were comparable with previous studies in the cattle (Sozmen et al. 2004; Xavier et al. 2009). These pathological changes and detection of Brucella abortus DNA in infected fetuses and placenta were suggestive of transplacental transmission of the infection.
The results of RBPT in the present study showed that it still plays an important role in serological testing which is carried out routinely and necessary for the early diagnosis and prevention of brucellosis (El-Boshy et al. 2009). The blood PCR of infected dams detected only two out of ten seropositive camels, which is comparable with a previous study wherein only one camel out of 42 seropositive was detected positive by blood PCR (Shome et al. 2013). However, fetal lung and placental tissue PCR detected all the seropositive cases. This might be due to the intracellular nature of Brucella which normally reside in various joints and internal organs or may be due to the absence of bacteria in blood or serum during chronic infection (Morata et al. 1998). Therefore, placental or fetal lung tissue PCR can be used as a complementary tool along with RBPT in the suspected cases with negative bacteriologic culture or where bacteriologic culture is not possible due to the limited containment facilities. In four of the aborted dams, which were detected negative for B. abortus infection by RBPT and PCR, the cause of abortion may be attributed to the factors other than brucellosis. Apart from infectious causes, the physiologic, traumatic, and toxic factors have also been attributed for reproductive loss in camels (Tibary et al. 2006).
In conclusion, brucellosis in camels of the present study was mainly characterized by abortions during mid to last trimester of pregnancy, placentitis, and fetal pneumonia. The infected pregnant camels were mostly asymptomatic hence may pose a permanent risk to other animals and humans in contact. The study therefore emphasizes the need for regular surveillance for the effective prevention and control of brucellosis in camels as well as other livestock species by planning and implementation of joint programs by stakeholders as well as raising public awareness in decreasing the distribution of the disease in the area.
References
Adamu SG, Tijjani AO, Adamu NB, Shettima A (2014) Serological survey for Brucella antibodies in one-humped camel (Camelus dromedarius) herds in North-Eastern Nigeria. Vet World 7:158–161
Anderson TD, Meador VP, Cheville NF (1986) Pathogenesis of placentitis in the goat inoculated with Brucella abortus. I. Gross and histologic lesions. Vet Pathol 23:219–226
Bricker BJ, Halling SM (1994) Differentiation of Brucella abortus bv.1,2, and 4, Brucella melitensis, Brucella ovis and Brucella suis bv.1 by PCR. J Clin Microbiol 32:2660–2666
Cutler S, Whatmore A (2003) Progress in understanding brucellosis. Vet Rec 153(21):641–642
Dean AS, Crump L, Greter H, Schelling E, Zinsstag J (2012) Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis 6. doi:10.1371/journal.pntd.0001865
El-Boshy AH, EL-Khodery S, Osman S (2009) Cytokine response and clinicopathological findings in Brucella infected camels (Camelus dromedarius). Vet Med 54:25–32
Ghorbani A, Rabbani Khorasgani M, Zarkesh-Esfahani H, Sharifi yazdi H, Dehghan Kashani A, Emami H (2013) Comparison of serology, culture, and PCR for detection of brucellosis in slaughtered camels in Iran. Comp Clin Pathol 22:913–917
Gwida MM, El-Gohary AH, Melzer F, Tomaso H, Rösler U, Wernery U et al (2011) Comparison of diagnostic tests for the detection of Brucella spp. in camel sera. BMC Res Notes 4:525–531
Gwida M, El-Gohary A, Melzer F, Khan I, Rösler U, Neubauer H (2012) Brucellosis in camels. Res Vet Sci 92:351–355
Hadush A, Pal M, Kassa T, Zeru F (2013) Sero-epidemiology of camel brucellosis in the Afar region of Northeast Ethiopia. J Vet Med Anim Health 5:269–275
Lopez A, Hitos F, Perez A, Navarro-Fierro RR (1984) Lung lesions in bovine fetuses aborted by Brucella abortus. Can J Comp Med 48:275–277
Mohamed EGS, Elfadi AAM, El Sanousi EM (2015) Epidemiological study of brucellosis in camels (Camelus dromedarius) in Khartoum State, Sudan. Int J Vet Sci 4:39–43
Morata P, Queipo-Ortuno MI, De Dios CJ (1998) Strategy for optimising DNA amplification in a peripheral blood PCR assay used for diagnosis of human brucellosis. J Clin Microbiol 36:2443–2446
Olsen S, Palmer MV (2014) Advancement of knowledge of Brucella over the past 50 years. Vet Pathol 51:1076–1089
Quinn PJ, Carter ME, Markey B, Carter GR (2013) Clinical veterinary microbiology. 2nd edn. Mosby, St Louis.
Radostits OM, Gray CC, Hinchcliff KW, Constable PD (2007). Veterinary medicine: a textbook of the diseases in cattle, horses, sheep, pigs and goats. 10th edn. Saunders Elsevier, London.
Renukaradhya GJ, Isloor S, Rajasekhar M (2002) Epidemiology, zoonotic aspects, vaccination and control/eradication of brucellosis in India. Vet Microbiol 90:183–195
Samartino LE, Enright FM (1993) Pathogenesis of abortion of bovine brucellosis. Comp Immunol Microbiol Infect Dis 16:95–101
Shome R, Gupta VK, Bhardwaj B, Shome BR, Nagalingam M, Rahman H (2013) A report of seroprevalence of camel brucellosis in India. J Camel Pract Res 20:183–186
Sozmen M, Erginsoy SD, Genc O, Beytut E, Ozcan K (2004) Immunohistochemical and microbiological detection of Brucella abortus in aborted bovine fetuses. Acta Vet Brno 73:465–472
Tibary A, Fite C, Anouassi A, Sghiri A (2006) Infectious causes of reproductive loss in camelids. Theriogenology 66:633–647
Walker RL (1999) Brucella. In: Hirsh DC, Zee YC (eds) Veterinary microbiology. Blackwell Science Inc, London, pp. 196–203
Xavier MN, Paixao TA, Poester FP, Lage AP, Santos RL (2009) Pathological, immunohistochemical and bacteriological study of tissues and milk of cows and fetuses experimentally infected with Brucella abortus. J Comp Pathol 140:149–157
Zewolda SW, Wereta MH (2012) Seroprevalence of Brucella infection in camel and its public health significance in selected districts of Afar region, Ethiopia. J Environ Occup Sci 1:91–98
Acknowledgments
The authors are grateful to Indian Council for Agricultural Research (ICAR), New Delhi (India), for providing necessary facilities to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared that they have no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Narnaware, S.D., Dahiya, S.S., Kumar, S. et al. Pathological and diagnostic investigations of abortions and neonatal mortality associated with natural infection of Brucella abortus in dromedary camels. Comp Clin Pathol 26, 79–85 (2017). https://doi.org/10.1007/s00580-016-2348-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-016-2348-4