Abstract
Brucellosis, caused by Brucella bacteria, is a common zoonotic infectious disease with various clinical manifestations in humans and animals. The disease is endemic in human and ruminant populations in Iran, with a particular prevalence in areas where humans have close interactions with livestock. Since domestic animals serve as the primary reservoir for brucellosis, this study aimed to identify the presence of Brucella spp. among aborted small ruminants in southeast Iran. Between 2021 and 2022, aborted fetuses of small ruminants (46 sheep and 4 goats) were collected from Zarand County in the Kerman province. Swab samples from the abomasum contents of these fetuses were obtained and subjected to DNA extraction. The samples were then tested for Brucella spp. detection using the polymerase chain reaction (PCR) method. Out of the 50 aborted fetuses examined, Brucella spp. was detected in 15 (30%) specimens, comprising 13 (28%) sheep and 2 (50%) goats. Species typing revealed the presence of Brucella ovis (6 sheep and 1 goat), Brucella melitensis (6 sheep), and Brucella abortus (1 sheep) among the positive specimens. This cross-sectional study highlights the high prevalence of various Brucella species in samples from small ruminant abortions in southeast Iran. Additionally, the identified Brucella species were not limited to their primary host livestock. These indicated potential cross-species transmission among small ruminants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brucellosis is a highly prevalent zoonotic disease that impacts domestic animals, humans, and wildlife. It is caused by various species within the Brucella genus. These slow-growth bacteria possess the ability to survive and multiply in different types of cells, including epithelial cells, trophoblasts of the placenta, dendritic cells, and macrophages [1, 2]. In humans, the most significant clinical manifestations of brucellosis include acute fever, chronic hepatomegaly, splenomegaly, and arthritis. In livestock, brucellosis primarily affects the organs of the reproductive system, leading to abortion, reduced fertility, and decreased milk production [3]. The conventional approach to diagnosing Brucella involves several steps, including clinical examination, cultivation of bacterial isolates from various biological samples, microscopy, biochemical tests (such as fermentation tests, catalase, oxidase, and urease), and serological tests (such as the Rose Bengal test, serum/latex agglutination test, complement fixation test, and enzyme-linked immunosorbent assay). These methods are usually time-consuming, often taking days to observe visible growth. Some methods are not very sensitive or specific, and they require trained personnel and a biosafety level 3 facility. In contrast, molecular techniques, such as the PCR method, have improved the fast and safe identification of Brucella [4].
Brucellosis is a public health concern worldwide, especially in endemic areas in the Mediterranean, North and East Africa, the Middle East, and parts of Latin America and Asia [5]. The high prevalence of brucellosis in livestock flocks with a history of abortion increases the risk of transmission to humans [6]. Brucella species, including Brucella abortus, Brucella melitensis, Brucella suis, Brucella canis, and Brucella ovis, mainly infect cattle, goats and sheep, swine, and dogs, respectively [7]. Among Brucella species, B. abortus, B. suis, and B. melitensis are the most pathogenic and invasive species for humans [7].
Brucellosis is a prevalent and endemic disease in both small ruminants (sheep and goat flocks) and humans in many regions of Iran. This disease not only affects the ruminant population but also poses a significant health concern for humans, as evident by the annual reports of clinical human cases nationwide. According to a study by Zeinali et al. [8] in 2022, the incidence rate of human brucellosis in Iran is estimated to be 29.83 cases per 100,000 population, and most of the cases were via contact with infected livestock (91%). From 2002 to 2006, in Iran’s South Khorasan province, the occurrence rate of brucellosis reached 340 cases per 10,000 individuals in sheep and goats and 37 cases per 100,000 individuals in humans [9].
In Iran, brucellosis in small ruminants, primarily caused by biovar 1 of B. melitensis, imposes significant economic losses [10].
Due to the health and economic challenges arising from this disease, it is crucial to prioritize comprehensive research and implement effective control programs to address these infections in both animals and humans.
This study aimed to identify Brucella species in abortion samples collected from sheep and goat flocks in the southeast region of Iran between 2020 and 2021. The findings of this research can play a significant role in the control and prevention of this widespread disease.
Materials and methods
Area study and sample size
In this study, a completely random sampling method was used, and sampling was conducted based on the abortion reports among sheep and goat flocks from March 20, 2020, to March 20, 2021, in Zarand County, located in Kerman Province, southeast Iran. The specific study area is depicted in Fig. 1.
Based on official information provided by the Veterinary Department of Zarand County in Kerman province, the total livestock population (sheep and goats) in the city consisted of 290 sheep flocks and 46 goat flocks from March 20, 2020, to March 20, 2021. These flocks encompassed a total of 140,000 sheep and 30,000 goats.
During the sampling period, 50 flocks of sheep and goats (46 sheep flocks and 4 goat flocks) with abortion reports were identified. A total of 50 aborted fetus samples (46 sheep and 4 goats) were randomly collected, with one sample collected from each of these flocks.
Sampling method
In this study, samples were selected blindly without considering any previous tests, including serological examinations. To clarify, no tests were conducted prior to our sample selection process. The comprehensive data was collected for fifty aborted fetuses, comprising crucial details such as age, birth order, history of abortion, and vaccination records. Subsequently, these samples were transported under appropriate cold chain conditions to the Faculty of Veterinary Medicine at Shahid Bahonar University in Kerman. Upon opening the fetus carcass, the contents of the abomasum were carefully collected using a sterile swab and transferred into microtubes containing 200 µl of sterile physiological solution. To enable future research, the samples were then stored at a temperature of − 20 °C [11].
DNA extraction
In this study, DNA extraction was performed from abomasum swab samples obtained from the aborted sheep and goat fetuses. A commercial Tissue Genomic DNA Extraction Kit (Pars Tous Co., Iran) was utilized, following the manufacturer’s instructions. The quantity and quality of the extracted DNA were assessed using a microplate spectrophotometer (Epoch bioTek, USA) at wavelengths of 260 nm and 280 nm. Subsequently, the DNA samples were stored at a temperature of − 20 °C for subsequent analysis.
The conventional polymerase chain reaction (PCR) and quantitative real-time polymerase chain reaction (qPCR) for detection of Brucella spp
To detect the presence of Brucella spp., a specific region (317 bp) of the IS711 insertion sequence belonging to the Brucella genus was amplified. The specific primers (Pishgam Biotechnology Co., Iran) used for amplification were as follows: forward primer (IS 711-F): 5′-GAGAATAAAGCCAACACCCG-3′ and reverse primer (IS 711-R): 5′-GATGGACGAAACCCACGAAT-3′ [12].
For the PCR reaction, a mixture was prepared to contain 2.5 µl of DNA sample (1 µg), 12.5 µl of X Taq PCR Master Mix2 (Pars Tous Co., Iran), and 1 µl (1 uM) of each forward and reverse primer. The final volume of the reaction was adjusted to 25 µl with deionized sterile distilled water. Brucella melitensis strain Rev1 was used as the positive control, while sterile distilled water served as the negative control.
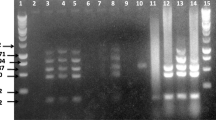
The PCR amplification was performed using the following temperature program in the thermal cycler machine: pre-denaturation: 95 °C for 3 min, denaturation: 95 °C for 30 s, annealing: 58 °C for 30 s, extension: 72 °C for 60 s, and final extension: 72 °C for 10 min [12]. After amplification, the DNA products were analyzed by agarose gel electrophoresis, and the bands were visualized using a UV transilluminator.
To confirm and identify the species of Brucella, the positive samples were sent to the National Reference Laboratory for Plague, Tularemia, and Q fever at the Pasteur Institute of Iran.
For the confirmation of positive Brucella spp., the qPCR method targeting the IS711 of the Brucella genus was employed. The qPCR used specific probe and primers with the following sequences: probe: FAM-AAGCCAACACCCGGCCATTATGGT-TAMRA (IS 711-probe), forward primers: 5′- GCTTGAAGCTTGCGGACAGT-3′ (IS 711-F), and reverse primer: 5′-GGCCTACCGCTGCGAAT-3′ (IS 711-R) [12]. The qPCR mixture consisted of the following components: 4 μl of the extracted DNA sample, 10 μl of RaelQ plus Master Mix2x (Ampliqon Co., Denmark), 200 nM of the probe, and 900 nM of the forward and reverse primers. To reach a total volume of 20 μl, deionized sterile distilled water was added to the mixture. The Rotor-Gene system 6000 Corbett (Corbett, Victoria, Australia) was utilized for the qPCR. The following thermal cycling conditions were programmed: initial denaturation at 95 °C for 10 min, 45 cycles of denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 60 s [12]. Ten consecutive dilutions (10-folds) were used for the standard positive control stock standard curve. Quantification of the approximate bacterial load and the quantification cycle (Cq) values were achieved using this standard. Finally, all samples were tested against the standard curve, and Cq values for all samples were determined. The Real Time-TaqMan PCR results and Cq were analyzed using the Rotor-Gene® Q 2.3.5 software from QIAGEN.
Conventional PCR for Brucella species identification
The confirmed positive samples underwent analysis to identify the specific Brucella species present. The following Brucella species were targeted for identification: B. melitensis, B. abortus, B. suis, B. ovis, B. canis, and B. neotomae.
In a previous study, seven PCR tests (PCR2-7) were specifically designed to differentiate between these Brucella species (B. melitensis, B. abortus, B. suis, B. ovis, B. canis, and B. neotomae) [12]. PCR2 yields positive results for melitensis, B. ovis, and B. neotomae. PCR3, PCR5, and PCR7 yield positive results for B. abortus, B. ovis, and B. neotomae, respectively. PCR4 detects positivity in B. suis, B. canis, and B. neotomae, while PCR6 detects positivity in B. suis and B. canis [12]. In the current study, we employed this specific protocol for differentiating between Brucella species. In this study, the PCR2 test was initially conducted. If PCR2 produced positive results (indicating B. melitensis, B. ovis, and B. neotomae), subsequent PCR5 and PCR7 tests were performed to determine the species of B. ovis and B. neotomae, respectively. If PCR2 was positive but PCR5 or PCR7 was negative, the species was identified as B. melitensis. In cases where PCR2 yielded negative results, PCR3 (specific for B. abortus) was carried out.
Since all samples in this study were successfully classified into a specific species using PCRs 2, 3, 5, and 7, additional PCR tests (PCR4 and PCR6) were not performed.
The PCR reaction mixture used in this study comprised 4 µl of DNA sample (1 µg), 10 µl of Taq DNA Polymerase 2 × Master Mix RED (Ampliqon Co., Denmark), and 0.7 µl (1 uM) of forward and reverse primers as indicated in Table 1. The final volume of the reaction mixture was adjusted to 20 µl using deionized sterile distilled water. The PCR reaction was carried out in a thermal cycler machine using the following temperature program: an initial denaturation step at 95 °C for 3 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 56–61 °C for 30 s, extension at 72 °C for 30 s, and a final extension step at 72 °C for 10 min [12].
Statistical analysis
For the statistical analysis of this study’s data, SPSS software version 18 (SPSS Inc., Chicago, IL, USA) was used. A p-value below 0.05 was regarded as statistically significant in determining associations between the variables.
Results
Sample characteristics
The average age of the 50 included cases of aborted small ruminants (46 sheep and 4 goats) in this study was 2.92 years (minimum 1 year and maximum 6 years). In total, 8 (16%) of all animals (8 of 46 sheep and none of 4 goats) had at least one previous abortion history. The median of parturitions was 2 times (minimum 1 time and maximum 4 times). All livestock included in this study were vaccinated (FD Rev1) against brucellosis as part of a national control program.
Brucella spp. detection in the samples
In the present study, the Brucella spp. was detected in 15 samples (30%) using conventional PCR. The results of conventional PCR were confirmed by the qPCR method. Among these positive samples, 13 (26%) were from sheep and 2 (4%) were from goats. There were no significant differences (p > 0.05) in Brucella infection rates based on animal type (goats vs. sheep), age, history of abortion, or number of parturitions (Table 2).
Brucella species identification in the samples
Out of the 13 positive Brucella samples obtained from sheep abortions, 6 samples (46%) were identified as B. ovis, 6 samples (46%) were classified as B. melitensis, and 1 sample (8%) was classified as B. abortus. Among the two positive goat samples, one sample (50%) was identified as B. ovis and the other sample (50%) was identified as B. abortus. In total, B. ovis was detected in 7 (14%) samples, B. melitensis in 6 (12%) samples, and B. abortus in 1 (4%) sample in the present study (Table 3).
Discussion
Various factors, such as infectious and noninfectious factors, can contribute to abortion in small ruminants [13,14,15]. According to a comprehensive review study conducted in 2019, it was recognized that the most prevalent bacteria responsible for sheep and goat abortion in Iran were Brucella, Toxoplasma, Chlamydophila, Campylobacter, and Salmonella species [15]. Therefore, our study specifically highlighted the molecular detection of Brucella spp. as one of the significant infections contributing to abortions in Iranian small ruminants in samples from aborted goats and sheep. The findings of this research confirmed that Brucella infection is one of the potential causes of abortion within the studied population in a specific region of Southeast Iran. However, it would be advantageous to offer a more comprehensive outlook on the causes of other infectious abortions in sheep and goats across Iran.
In the present study, we observed that 46.7% of brucellosis-induced abortions were attributed to B. ovis, while B. melitensis and B. abortus accounted for 40% and 13.3%, respectively. In a study conducted in the Sistan and Baluchistan province of eastern Iran in 2016, B. melitensis was identified in approximately 2.19% of the abomasum and spleen samples collected from aborted sheep fetuses [16]. Additionally, a study conducted from 2016 to 2019 in Iran revealed that all Brucella isolates obtained from abortion samples of sheep and goats were B. melitensis (8.99%) and B. abortus (0.52%) [17]. In the present study, we observed distinct Brucella species in the positive samples obtained from sheep abortions. Specifically, the identified species were B. ovis, B. melitensis, and B. abortus. This distribution of Brucella species in the sheep abortion cases provides important insights into the prevalence and diversity of Brucella infections in our study population. Additionally, out of two positive bacteria in the goat samples, one Brucella spp. was identified as B. ovis and the other as B. abortus. In our study, we found B. abortus in sheep and goats despite it being considered the principal host in cattle [18]. Several studies have explored cross-species transmission between small ruminants, providing valuable insights for comparison. For instance, a similar investigation in a neighboring region found a considerable number of sheep infected with B. abortus due to mixed farming, where small and large ruminants are raised together and share the same pasture, along with the presence of reservoir hosts on a farm [19]. Another study conducted in Egypt in 2015 [17] showed that B. abortus (principal host: cattle) could be transmitted from infected cattle to other livestock species raised in close contact with them. Furthermore, although B. ovis typically infects sheep [20], the present study identified this species in both sheep and goat aborted fetuses. Lastly, while goats are the natural host of B. melitensis [21], it was only identified in sheep in the present study. Our results confirm infections with non-specific species in sheep and goats and highlight the importance of considering cross-species transmission and infection dynamics in brucellosis epidemiology. Moreover, the presence of B. ovis and B. abortus in aborted goat fetuses indicated that these species are not limited to their principal livestock host. This understanding the mechanisms and factors involved in cross-species transmission is crucial for designing effective control strategies [22, 23]. Further investigation is needed to assess the specific pathways through which species spread between different hosts. Comparative genomics studies analyzing strains from cattle, sheep, and goats could provide insights into genetic adaptations that allow species to infect multiple hosts [24, 25]. In addition, the results of the present study demonstrate that Brucella infection in non-principal hosts not only disrupts disease control and prevention systems in the livestock population but also could facilitate the transmission of more significant species, such as B. melitensis and B. abortus, to humans. In a study conducted in various regions of Iran from 2016 to 2019, it was found that a B. melitensis vaccine strain Rev1 was detected in a small number of sheep and goat abortion cases [17]. However, it should be noted that the present study did not include any additional tests to distinguish the B. melitensis Rev.1 vaccine strain from other B. melitensis strains. This represents a limitation of our study. In Iran, vaccination practices involve the use of RB51 strains of B. abortus in cattle and Rev1 B. melitensis strain in smaller livestock populations such as sheep and goats. Therefore, these strains should also be taken into consideration when assessing the potential causes of abortion. Additionally, since B. abortus species was observed in sheep and goats, further research is crucial to explore the usage of recombinant vaccines that can effectively target all Brucella species across different livestock species.
In conclusion, this study highlighted the significant occurrence of diverse Brucella species in abortion samples from sheep and goat flocks in southeast Iran. While our study focused on Brucella species detection, considering the multifactorial nature of abortion, we acknowledge that it is essential to conduct further research to establish any causal relationship and explore the involvement of other potential causes of abortion in small ruminants in this region, in conjunction with Brucella species. Moreover, the study revealed infections in livestock with non-principal species of Brucella, such as B. abortus in sheep and goats, B. ovis in goats, and B. melitensis in sheep. This suggests that keeping and raising livestock together, even nearby, could contribute to the spread of non-principal species infections among different livestock species. This poses challenges for epidemiological studies and reduces the effectiveness of preventive measures like vaccination. To address these challenges, future studies should focus on identifying Brucella species in livestock to understand the mechanisms and factors that contribute to cross-species transmission. Additionally, it is crucial to provide training to livestock farmers, emphasizing adherence to livestock and health management principles. Such efforts, when combined with accurate species identification and the utilization of suitable vaccine strains, can immensely contribute to the control and prevention of brucellosis.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Tekle M, Legesse M, Edao BM, Ameni G, Mamo G (2019) Isolation and identification of Brucella melitensis using bacteriological and molecular tools from aborted goats in the Afar region of north-eastern Ethiopia. BMC Microbiol 19(1):1–6
Golshani M, Buozari S (2017) A review of brucellosis in Iran: epidemiology, risk factors, diagnosis, control, and prevention. Iran Biomed J 21(6):349
de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG (2015) Pathogenesis and immunobiology of brucellosis: review of Brucella-Host interactions. Am J Pathol 185(6):1505–1517
Kurmanov B, Zincke D, Su W, Hadfield TL, Aikimbayev A, Karibayev T, Berdikulov M, Orynbayev M, Nikolich MP, Blackburn JK (2022) Assays for identification and differentiation of Brucella species: a review. Microorganisms 10(8):1584
Ezama A, Gonzalez J-P, Majalija S, Bajunirwe F (2018) Assessing short evolution brucellosis in a highly Brucella endemic cattle keeping population of Western Uganda: a complementary use of Rose Bengal test and IgM rapid diagnostic test. BMC Public Health 18(1):1–5
Abnaroodheleh F, Emadi A, Dadar M (2021) Seroprevalence of brucellosis and chlamydiosis in sheep and goats with history of abortion in Iran. Small Rumin Res 202:106459
Kolo FB, Fasina FO, Ledwaba B, Glover B, Dogonyaro BB, van Heerden H, Adesiyun AA, Katsande TC, Matle I, Gelaw AK (2018) Isolation of Brucella melitensis from cattle in South Africa. Vet Rec 182(23):668
Zeinali M, Doosti S, Amiri B, Gouya MM, Godwin GN (2022) Trends in the epidemiology of brucellosis cases in Iran during the last decade. Iran J Public Health 51(12):2791–2798
Bokaie S, Sharifi L, Alizadeh H, Advances V (2008) Epidemiological survey of brucellosis in human and animals in Birjand, east of Iran. J Anim Vet Adv 7(4):460–463
Dadar M, Alamian S, Behrozikhah AM, Yazdani F, Kalantari A, Etemadi A, Whatmore AM (2019) Molecular identification of Brucella species and biovars associated with animal and human infection in Iran. In: Vet Res Forum: 2019: Faculty of Veterinary Medicine, Urmia University, Urmia, Iran 315
Nazish A (2021) Fozia, Khattak B, Ali Khan T, Ahmad I, Ullah R, Bari A, Asmari MM, Mahmood HM, Sohaib M: Antinematode activity of Abomasum bacterial culture filtrates against Haemonchus contortus in small ruminants. Animals (Basel) 11(6):1843
Hinić V, Brodard I, Thomann A, Cvetnić Ž, Makaya P, Frey J (2008) Abril C 2008 Novel identification and differentiation of Brucella melitensis, B. abortus, B. suis, B. ovis, B. canis, and B. neotomae suitable for both conventional and real-time PCR systems. J Microbiol Methods 75(2):375–378
Mellado M, Valdez R, Lara L, Garcıa J (2004) Risk factors involved in conception, abortion, and kidding rates of goats under extensive conditions. Small Rumin Res 55(1–3):191–198
Aytekin I, Aypak SU (2011) production: Levels of selected minerals, nitric oxide, and vitamins in aborted Sakis sheep raised under semitropical conditions. Trop Anim Health Prod 43:511–514
Behzadi Shahrbabak MJ (2019) A review on infectious agents of sheep and goats abortion in Iran. NFVM 1(2):102–113
Roshan HM, Saadati D, Najimi M (2018) Molecular detection of Brucella melitensis, Coxiella burnetii and Salmonella abortusovis in aborted fetuses of Baluchi sheep in Sistan region, south-eastern Iran. Iran J Vet Res 19(2):128
Dadar M, Alamian S (2021) Identification of main Brucella species implicated in ovine and caprine abortion cases by molecular and classical methods. Arch Razi Inst 76(1):51
Fiebig A, Vrentas CE, Le T, Huebner M, Boggiatto PM, Olsen SC, Crosson S (2021) Quantification of Brucella abortus population structure in a natural host. Proc Natl Acad Sci U S A 118(11):e2023500118
Saleem MZ, Akhtar R, Aslam A, Rashid MI, Chaudhry ZI, Manzoor MA, Shah BA, Ahmed R (2019) Yasin MJPJZ: Evidence of Brucella abortus in non-preferred caprine and ovine hosts by real-time PCR assay. Pak J Zool 51:1187–1189
Ridler AL, West DM (2011) Control of Brucella ovis infection in sheep. Vet Clin North Am Food Anim Pract 27(1):61–66
Rossetti CA, Maurizio E, Rossi UA (2022) Comparative review of brucellosis in small domestic ruminants. Front Vet Sci 9:887671
Viana M, Shirima GM, John KS, Fitzpatrick J, Kazwala RR, Buza JJ, Cleaveland S, Haydon DT, Halliday JE (2016) Integrating serological and genetic data to quantify cross-species transmission: brucellosis as a case study. Parasitology 143(7):821–834
Hegazy YM, Abdel-Hamid NH, Eldehiey M, Oreiby AF, Algabbary MH, Hamdy ME, Beleta EI, Martínez I, Shahein MA, García N (2022) Trans-species transmission of Brucellae among ruminants hampering brucellosis control efforts in Egypt. J Appl Microbiol 132(1):90–100
Wang Y, Wang Z, Chen X, Zhang H, Guo F, Zhang K, Feng H, Gu W, Wu C, Ma L (2016) The complete genome of Brucella suis 019 provides insights on cross-species infection. Genes (Basel) 7(2):7
Wareth G, Melzer F, Tomaso H, Roesler U, Neubauer H (2015) Detection of Brucella abortus DNA in aborted goats and sheep in Egypt by real-time PCR. BMC Res Notes 8(1):1–5
Acknowledgements
We express our sincere appreciation and gratitude to the following organizations and departments for their invaluable contributions and support in conducting this study: the Department of Bacteriology at the Faculty of Veterinary Medicine of Shahid Bahoner Kerman University, the Department of Epidemiology and Biostatistics at the Pasteur Institute of Iran, the Reference and Bacteriology Laboratories of the Faculty of Veterinary Medicine of the Shahid Bahoner University of Kerman, the General Veterinary Department of the province, the veterinary network of Zarand city, and the veterinary treatment centers of the private sector of Zarand city.
Their collaboration and assistance were instrumental in carrying out our research successfully, and we are truly grateful for their support.
Funding
This research received funding from Shahid Bahonar University of Kerman (Kerman, Iran) as part of an MSc thesis grant.
Author information
Authors and Affiliations
Contributions
AA: conceptualization, data curation, investigation, methodology, software, validation, visualization, writing – original draft. MKh: conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision, validation, visualization. NB: investigation, methodology, writing – original draft, writing – review and editing. SE: conceptualization, investigation, resources, validation, writing – review and editing. EM.d: methodology. SK: methodology. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Research Ethics Committee of the Shahid Bahonar University of Kerman and Pasteur Institute of Iran (No. IR.PII.REC.1398.05). All methods and instructions were performed under institutional guidelines and regulations and were reported in accordance with ARRIVE guidelines (https://arriveguidelines.org) and the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Farmers permitted (with informed consent) their animal samples to be included in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Maria Aparecida Scatamburlo Moreira
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alirezaei, A., Khalili, M., Baseri, N. et al. Molecular detection of Brucella species among aborted small ruminants in southeast Iran. Braz J Microbiol 55, 911–917 (2024). https://doi.org/10.1007/s42770-023-01191-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01191-z