Abstract
Three adult Holstein-Friesian dairy cows (4 to 6 years old) which had been raised at three different farms were culled with a long history of locomotor dysfunction attributed to dislocation or subluxation of the hip joint (two cows) or foot rot (one cow). They revealed dark brown discoloration of multiple sites of the carcasses at postmortem examination. On histopathological and histochemical investigations, lipofuscin pigments showing autofluorescence, sudanophilia, argyrophilia, periodic acid-Schiff (PAS) positiveness, and acid fastness were stored within otherwise normal cells of various sites in the body, including many thoracic and abdominal viscera, skeletal muscle, diaphragm, and tongue. In the central nervous system, some neurons with massive lipofuscin deposition were prominently shrunken but associated with neither necrotic changes nor glial reaction. Such a generalized pigmentary condition was distinguished from neuronal ceroid lipofuscinosis and thus was diagnosed as generalized lipofuscinosis. Furthermore, distinct proteinaceous eosinophilic inclusions were simultaneously observed to a varying degree within myofibers of the diaphragm, heart, rumen, or small intestine. These inclusions stained weakly positive with PAS and negative with Nile blue, Sudan black B, periodic acid-methenamine silver (PAM), and acid-fast stains. Autofluorescence was not seen. Staining properties demonstrated by phosphotungstic acid-hematoxylin (purple blue) and Masson’s trichrome (bright red) stains were compatible with those of myofibrils, presumably suggesting that the inclusions have chemical composition similar or identical to that of myofibrils. It remains to be elucidated whether the occurrence of such sarcoplasmic inclusions was related to lipofuscinogenesis or a nonspecific phenomenon indicating an incidental finding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While lipofuscin, referred to historically as aging pigment or wear-and-tear pigment, is seen in healthy calves and young cattle (Innes and Saunders 1962), the propensity to store this pigment is rather higher in the skeletal muscles of cattle, particularly old, high-producing dairy cattle (McGavin 1995). Lipofuscinosis in cattle has been an important consideration in the evaluation of their carcasses in meat inspection practice (Innes and Saunders 1962), causing a significant financial problem because the discolored meat is considered, on aesthetic grounds, to be unfit for human consumption (Bradley and Fell 1981; Bradley and Duffell 1982). Even though lipofuscin is sometimes generalized throughout the muscles of the bovine carcass (Bradley and Fell 1981), dark brown discoloration due to deposition of this pigment has been usually recognized in single organ or tissue, principally the heart or skeletal muscles (Innes and Saunders 1962; Bradley and Fell 1981). Review of the English veterinary literature revealed a total of nine cases of generalized lipofuscinosis in cattle, which had been reported more than three decades before (Duffell and Edwardson 1978; Bradley and Duffell 1982). Generalized lipofuscinosis is uncommon in other domestic animal species as well; only a single case of this condition is reported in a cat (Schmidt 1974). As is often the case with cattle, pieces of evidence have shown a significant increase in lipofuscin content in aged compared with young skeletal muscles in human beings (Tohma et al. 2011). Studies have indicated that lipofuscin is not harmless but has multiple negative effects on affected cells (Brunk and Terman 2002a, b; Terman and Brunk 2003, 2004). Although it is believed that lipofuscin is a marker of oxidative stress based mainly on in vitro experiments, this assumption is not generally supported by in vivo studies (Porta 2002).
In terms of the differential diagnosis, generalized lipofuscinosis should be distinguished clinically and pathologically from neuronal ceroid lipofuscinosis, which is an autosomal recessively inherited neurodegenerative disease, taking into account the fact that not only there are some common physicochemical properties between lipofuscin and ceroid (Porta 2002; Tohma et al. 2011), but also the storage of the latter, unlike the former, induces distinctively cerebral dysfunction, including visual and behavioral alterations. Neuronal ceroid lipofuscinosis is classified as one of the lysosomal storage diseases in human beings (Friede 1989; Goebel and Sharp 1998; Wisniewski et al. 2001; Haltia 2003) and a variety of domestic animal species (Jolly et al. 1990; Bildfell et al. 1995; Jolly and Walkley 1997; Url et al. 2001; Cesta et al. 2006), including the bovine species (Harper et al. 1988; Martinus et al. 1991; Hafner et al. 2005).
A variety of inclusions or focally abundant or altered structural components, such as nemaline bodies, lipid droplets, or glycogen deposits, occur in the muscles (Åstrӧm and Adams 1981; Van Vleet and Valentine 2007), whereas reports of accumulation or formation of other substances or structures may be uncommon in domestic animals. The present report describes the histopathological and histochemical features of spontaneously occurring generalized lipofuscinosis with special reference to the simultaneously identified sarcoplasmic eosinophilic inclusions in adult Holstein-Friesian dairy cows.
Materials and methods
Animals
The present study concerns three Holstein-Friesian dairy cows (nos. 1–3) which had been raised at three different farms that were geographically separated. Information on clinical histories of the cows was provided by the referring veterinarians. These cows were reproductively intact but culled on separate days for half a year because of the clinical diagnosis indicative of unfavorable prognosis, as given in Table 1. One cow (no. 1), aged 5 years, showed a sign of lameness in the left hind leg. The cow exhibited difficulty in walking, followed by difficulty to rise, and became recumbent. The referring veterinarian diagnosed the animal as having dislocation of the hip joint. Twenty days after the onset of clinical signs, the cow was culled. Another cow (no. 2), aged 6 years, was affected with foot rot involving the both hind legs. Despite treatments with antibiotics (penicillin) by the referring veterinarian, clinical signs became worsened gradually. The foot lesions were accompanied by suppurative changes with the formation of fistula reaching to the left distal phalange. The animal showed a poor appetite, wide-based hindleg stance, and difficulty in walking, ultimately leading to difficulty to rise. Two months after the onset of clinical signs, the cow was culled. The third cow (no. 3), aged 4 years, presented with a sign of lameness in the left hind leg of unknown cause. Thereafter, the sign remained unchanged, and the animal was progressively emaciated. The referring veterinarian suspected that the cow had been suffered from dislocation of the left hip joint or partial fracture of the pelvis. Despite clinical efforts to improve the condition, clinical manifestations worsened with no sign of improvement. Finally, the decision was made by the owner to cull the cow 5 months after the onset of clinical signs.
On antemortem physical examination just prior to culling of the animals, there were no signs indicative of neurological deficits referable to the central nervous system (CNS) dysfunction, such as blindness, nystagmus, head tremor, loss of conscious proprioception, or altered mental status. Data on the hematology and clinical chemistry were unavailable for evaluation. Information on the pedigree of each cow and on other breeding data was too fragmentary to get a satisfactory breeding history. A detailed and thorough postmortem examination was performed immediately after the death following exsanguination.
Pathological study
Tissues samples available for histopathological examination included those taken, within approximately 50 min of death of the three cows, from the liver, spleen, kidney, heart, lung, adrenal gland, thyroid gland, salivary gland, esophagus, rumen, reticulum, omasum, abomasum, small intestine, large intestine, lymph node (hepatic, tracheobronchial, mediastinal, jejunal, and mesenteric), tongue, masseter muscle, diaphragm, ureter, urinary bladder, longissimus thoracis muscle, lateral vastus muscle, semitendinosus muscle, semimembranosus muscle, gracilis muscle, cerebrum, cerebellum, spinal cord, and trigeminal ganglion. Tissue samples were fixed in 10 % formalin for 72 h before processing, and then, representative slices (3 mm) were processed by routine methods and embedded in paraffin wax. Four-micrometer-thick sections were cut and stained with hematoxylin and eosin (HE). Based on knowledge of the results that were obtained from evaluation of HE-stained histological slides, selected sections taken from various organs or tissues, including the heart, adrenal gland, thyroid gland, small intestine, diaphragm, or cerebrum, were stained with periodic acid-Schiff (PAS), periodic acid-methenamine silver (PAM), luxol fast blue (LFB)-HE, Nile blue, Sudan black B, long (3 h) Ziehl-Neelsen, phosphotungstic acid-hematoxylin (PTAH), or Masson’s trichrome. In some instances, selected unstained sections taken from the liver were examined with fluorescence microscopy under ultraviolet light. For immunohistochemistry, sections from the cerebrum (the medulla oblongata) were cut at a thickness of 4 μm, deparaffinized, and rehydrated in an alcohol series. An EnVision horseradish peroxidase (HRP)-labeled polymer immunoperoxidase staining procedure (Dako, Glostrup, Denmark) was used for immunolabeling according to the manufacturer’s instructions. Briefly, endogenous peroxidase was blocked by immersion in 0.03 % hydrogen peroxidase. Antigen retrieval was achieved by incubating slides in antigen retrieval solution (Dako) in a water bath for 40 min. Next, the slides were washed with Tris-buffered saline (TBS) and incubated at room temperature for 30 min with the primary antibody specific for glial fibrillary acid protein (GFAP; clone 6 F2, Dako, monoclonal mouse, 1:100). The sections were incubated with a peroxidase-labeled dextran polymer conjugated to goat anti-mouse IgG (Dako) for 30 min, followed by washing with TBS. The sections were stained with the chromogen 3′, 3′-diaminobenzidine tetrahydrochloride (Dako) and finally counterstained with Meyer’s hematoxylin.
Results
Gross pathological findings common to the three cows were comprised of dark brown discoloration of multiple sites of the carcass, particularly at the masseter, heart, liver, kidney, small intestinal wall, large intestinal wall, and almost all the skeletal muscles, including the diaphragm and tongue (not shown). Similar discoloration was noted in the adrenal and cerebral cortex. There was no atrophic change in the viscera, such as the heart and liver. The adipose tissue located subcutaneously and viscerally was generally poor, though the entire skeletal musculature did not undergo a reduction in bulk or necrotic changes in all cows. Gross lesions accounting for the antemortem clinical signs characterized by locomotor disturbance were observed in each cow. Dislocation of the left hip joint, which was associated with desmorrhexis and formation of massive granulation tissues accompanied by large encapsulated hematoma (approximately 30 cm in diameter) in the left side of the pelvic cavity, was detected in cow no. 1. Chronic purulent periostitis was seen in the third phalanges of the both hindlegs in cow no. 2. Subluxation of the left hip joint accompanied by hemorrhage in the articular cavity and granulation tissue proliferation in the surrounding regions was noted in cow no. 3.
Histopathologically, the thoracic and abdominal visceral organs of the three cows had cytoplasmic storage of small, round, or irregularly shaped yellowish brown granules (lipofuscin) in a wide range of otherwise normal cell types, including cardiac myofibers; cardiac Purkinje fibers; hepatocytes; renal tubular and pelvic epithelial cells; adrenal cortical cells (particularly cells in the zona glomerulosa); thyroid follicular cells; and epithelial cells of the salivary gland excretory duct, ureter, and urinary bladder (Fig. 1). Lipofuscin revealed juxtanuclear position predisposition in cardiac myofibers and accumulated around the bile canaliculi in hepatocytes. In other cell types, it was randomly distributed within the cytoplasm, showing no specific position predisposition. There was also an accumulation of lipofuscin close to the sarcolemmal nuclear poles in skeletal muscle fibers throughout the range of muscles examined, including the tongue, masseter muscle, esophageal muscle, diaphragmatic muscle, longissimus thoracis muscle, lateral vastus muscle, semitendinosus muscle, semimembranosus muscle, and gracilis muscle (Fig. 2). Smooth muscle fibers of the tunica muscularis in the digestive tracts, such as the rumen, reticulum, omasum, small intestine, and large intestine, contained lipofuscin that was diffusely distributed within the sarcoplasm. In the CNS, lipofuscin was seen in glial cells and neurons of the cerebral cortex, medulla oblongata, cerebellar nuclei, and, to a lesser degree, spinal cord. Some of those neurons in the olivary nuclei of the medulla oblongata, which underwent massive deposition of lipofuscin, exhibited cytoplasmic shrinkage with indistinct nuclei but represented neither significant necrotic changes nor neuronophagia (Fig. 3). Glial cell proliferation was absent in the regions, and immunohistochemistry for GFAP failed to show any appreciable fibrillary gliosis. Degenerative axonal or myelin sheath changes were absent in any sites of the CNS with LFB-HE stain. Neurons in the trigeminal ganglion also represented lipofuscin deposition but were otherwise morphologically unchanged. In addition, lipofuscin was present in macrophages within the medullary sinuses of the lymph nodes examined (Fig. 4). As given in Table 2, lipofuscin displayed sudanophilia, argyrophilia, PAS positiveness, and acid fastness. It stained blue with Nile blue and LFB-HE stains (Fig. 3, inset), deeply violet with PTAH stain, and vividly red with Masson’s trichrome stain. Fluorescence microscopical examination of the liver showed the yellow-orange autofluorescence emission from lipofuscin.
Some neurons in the olivary nucleus of the medulla oblongata show severe lipofuscin deposition and cytoplasmic shrinkage with indistinct nuclei (arrowheads), while other neurons with small to moderate amounts of lipofuscin pigments are otherwise normal (arrows). Sudan black B, ×200. The inset represents lipofuscin pigments (arrow) in a neuron of the cerebellar dentate nucleus. LFB-HE, ×400
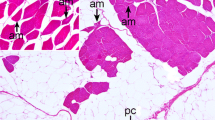
Furthermore, large numbers of proteinaceous, eosinophilic, spherical inclusion bodies in the shape of droplets or granules (<12 μm in diameter) different from lipofuscin pigments occurred in the diaphragmatic muscle (cow no. 2) (Fig. 5). These unique inclusions were recognized in some fascicles. Inclusions involved entire length of myofibers, and inclusions were arranged parallel to myofibrils (Fig. 6). Affected myofibers often displayed internal nuclei and were associated with mild endomysial thickening. To a lesser degree, the same inclusions were observed in cardiac myofibers (cow no. 1), diaphragmatic muscle fibers (cow no. 1), and smooth muscle fibers of the tunica muscularis in the rumen (cow no. 1) and small intestine (cow nos. 1–3). These proteinaceous eosinophilic inclusions stained weakly positive with PAS (Fig. 7) and negative with Nile blue, Sudan black B, PAM, and acid-fast staining methods (Table 2). They stained purple blue with PTAH (Fig. 8) and bright red with Masson’s trichrome stain (Fig. 9). Autofluorescence was not demonstrated.
Myofibers devoid of sarcoplasmic inclusions rarely showed floccular changes or fragmentation, with or without phagocytosis by macrophages, in the heart (cow nos. 1 and 2), masseter muscle (cow nos. 2 and 3), diaphragmatic muscle (cow nos. 1 and 2), and semitendinosus muscle (cow no. 2). Fiber size variation and atrophy suggestive of neurogenic muscular atrophy were absent anywhere. No inflammatory cellular infiltrates were observed in any muscle tissues. A few sarcosporidial cysts, without tissue reaction, occurred in myofibers of the heart, masseter muscle, lateral vastus muscle, and semitendinosus muscle (cow no. 2). Other visceral lesions included acute glossitis, acute bronchiolitis, acute interstitial hepatitis, chronic peritonitis at the abomasum, and splenic hemosiderosis in cow no. 2. In the latter, fibrinoid change was present in arterioles of the heart, small intestine, masseter muscle, diaphragmatic muscle, and semimembranosus muscle. Acute peritonitis at the abomasum and slight coccidial infestation in the epithelia of the small intestinal mucosa were present in cow no. 3.
Discussion
Dark brown discoloration of the visceral organs and skeletal muscles in the present three cows was most likely attributable to widespread and primary deposition of lipofuscin. Importantly, this pigment granule may share with ceroid some physicochemical properties, such as autofluorescence, sudanophilia, argyrophilia, PAS positiveness, and acid fastness (Porta 2002; Tohma et al. 2011). While lipofuscin was hitherto believed to be harmless and thus of no clinical significance (McGavin 1995), ceroid is generated during a variety of pathological conditions (Porta 2002). The current cows showed a history of prolonged clinical signs of locomotor dysfunction ascribed either to traumatic pelvic injury due to hip joint dislocation/subluxation (cow nos. 1 and 3) or to chronic inflammation of the hoofs (cow no. 2), but they exhibited no any neurological clinical signs referable distinctively to the CNS disorder, such as mental or behavioral dysfunctions. Some of the CNS neurons with severe deposition of lipofuscin were shrunken but not associated with necrotic or other significant changes. Ceroid lipofuscinosis, an autosomal recessively inherited neurodegenerative disease occurring in human beings (Friede 1989; Goebel and Sharp 1998; Wisniewski et al. 2001; Haltia 2003), dogs, cats, sheep, goats, mice (Jolly et al. 1990; Bildfell et al. 1995; Jolly and Walkley 1997; Url et al. 2001; Cesta et al. 2006), and cattle (Harper et al. 1988; Martinus et al. 1991; Hafner et al. 2005), is usually associated with destruction of neurons undergoing storage of ceroid granules, incorporation of the granules by macrophages, and massive reactive gliosis. Taken together, the generalized pigmentation identified in the cows under study was distinguished from neuronal ceroid lipofuscinosis, and accordingly, generalized lipofuscinosis was a most likely diagnosis for it.
As alluded to above, lipofuscin deposition itself has been considered to be harmless, but studies have suggested that lipofuscin has negative effects on affected cells (Brunk and Terman 2002a, b; Terman and Brunk 2003, 2004). For instance, lipofuscin may play an important role in the pathogenesis of age-related macular degeneration that gives rise to damage of the retina in human beings (Terman and Brunk 2004). In the current cows, some of those CNS neurons which showed massive deposition of lipofuscin represented no necrotic changes but had shrunken cytoplasm which is an appearance variably designated simple atrophy, cell sclerosis, or chronic cell disease. Although such features of neurons were similar to those of neuronal atrophy that occurs in the process of transynaptic degeneration (Jubb and Huxtable 1993), no significant lesions were observed in the specific anatomical pathways within the CNS. In addition, neither glial cell proliferation nor increased GFAP immunostaining was associated with deposition of lipofuscin. Therefore, it seems likely that even when deposition of lipofuscin was massive to the point of occupying neuronal cell bodies, as in the present cattle, there was probably no association with an adverse effect on neurons. However, it may be necessary for this speculation to await further evaluation, considering that the feature of those lipofuscin-laden neurons, which were morphologically atrophied at least on light microscopical level, appeared to be beyond normal limit. Indeed, some workers stated that, although lipofuscin storage does not induce cerebral dysfunction in young individuals, some deleterious effects cannot be ruled out in aged dogs (Borras et al. 1999).
Lipofuscin pigments are composed mainly of protein (30–70 %), lipid (20–50 %), carbohydrate implied by PAS-positive staining, and metals such as iron (Jolly et al 1995, 2002; Terman and Brunk 2003; Hӧhn and Grune 2013). The significance of lipofuscinosis is known as an indicator either of old age (Terman and Brunk 1998) or of past or present episodes of cachexia or starvation (McGavin 1995). Pieces of evidence indicated that lipofuscin is formed when oxidized lipids, proteins, and carbohydrates become resistant to hydrolysis by lysosomal enzymes (Brunk and Terman 2002a: Hӧhn and Grune 2013). Oxidative stress-related pathological conditions responsible for the occurrence of this pigment include malnutrition, genetic defects, hypoxia, irradiation, trauma, and infections (Porta 2002). Taking into account that lipofuscinosis in the presently reported three cows was generalized one, their organs and tissues were considered likely to have been exposed to systemic oxidative stress, which might have been related to an appreciable stress on the animals caused by the prolonged rigorous damage, such as hip joint injury (cow nos. 1 and 3) and systemic inflammatory process compatible with a disseminated bacteremia possibly attributed to foot rot lesions (cow no. 2). However, other predisposing factors could not be ruled out because it is empirical proof of the author that those cows which have serious traumatic or inflammatory skeletal bone problem over a prolonged period are not necessarily involved with lipofuscinosis, whether generalized or localized, as a common phenomenon.
Studies have suggested the role of inheritance of a simple recessive gene in the development of lipofuscinosis (Duffell and Edwardson 1978; Hayward 1978; Agerholm 2007; Agerholm et al. 2009). A higher prevalence of the condition is confirmed in Ayrshire cattle than other breeds of cattle (Duffell and Edwardson 1978; Hayward 1978). Similarly, renal lipofuscinosis or black kidney, which is a common finding in Danish Red cattle and Danish Holstein cattle, is considered to be inherited autosomal recessively (Agerholm 2007; Agerholm et al. 2009). In the present cases of the Holstein-Friesian breed, no genetic analysis was possible because of inadequate pedigree data. Thus, it is unknown whether genetic factors were implicated in the development of the presently reported generalized lipofuscinosis. Alternatively, the latter definitely involved the tunica muscularis of the intestine as well. In human beings, intestinal lipofuscinosis or brown bowel syndrome is thought to be a consequence of long-standing malabsorption of fat-soluble vitamins, especially vitamin E (Oberhuber et al. 1989; Bialas et al. 2013). Similar intestinal lipofuscinosis has been reported in dogs in which lipofuscin granules, termed leiomyometaplasts, are derived from excess cell membrane lipid peroxidation due to vitamin E deficiency (Barker and van Dreumel Palmer 1993). Vitamin E deficiency accelerates peroxidation of unsaturated membrane lipids, leading to mitochondrial malfunction and degeneration with lipofuscin accumulation in intestinal smooth muscle fibers (Bialas et al. 2013). Therefore, vitamin E deficiency should be taken into consideration among predisposing factors or etiological causes in the present cases, which obviously presented with intestinal lipofuscinosis.
Of special interest was simultaneous identification of varying degrees of sarcoplasmic, proteinaceous, eosinophilic inclusions in the striated, cardiac, or smooth muscles, among which the diaphragmatic muscle of one cow (no. 2) was most prominently involved. These sarcoplasmic inclusions contained material that exhibited a weak PAS positivity and showed no autofluorescence, sudanophilia, argyrophilia, and acid fastness. Such staining properties likely indicated that the components are more or less composed of carbohydrates and contained no lipids, clearly distinguishing them from lipofuscin. It may be highlighted that the staining properties demonstrated by PTAH (purple blue) and Masson’s trichrome (bright red) stains were compatible with those of myofibrils. This evidence presumably suggested that the inclusions have chemical composition similar or identical to that of myofibrils, and thus, they possibly were associated with disintegration of myofibrils. It would appear that the same eosinophilic inclusions have not yet been identified in any degenerative muscle disorders in domestic animals, such as nutritional myopathy due to vitamin E-selenium deficiency and genetically determined muscular dystrophy (Bradley and Fell 1981; Goedegebuure et al. 1983; McGavin 1995; Van Vleet and Valentine 2007), nor in the various constellations of morphological change in muscle conditions of human beings (Åstrӧm and Adams 1981; Carpenter 2001). Moreover, there seem to be no cardiac or smooth muscle conditions associated with the presence of such eosinophilic inclusions in human being and domestic animals. However, an earlier study described that spheroid sarcoplasmic inclusions (<2.5 μm in diameter), which were identical in staining reaction with Giemsa to myofibrils, occurred in myofibers of skeletal muscles of mice (Nagashima 1970). The author of that report considered these sarcoplasmic inclusions to be derived from disorganized myofibrils. In addition, other workers described the presence of spherical inclusions with an average diameter of 4 μm, which are composed of a collection of contracted myofilaments in muscles of human beings (Åstrӧm and Adams 1981). They stated that these inclusions are nonspecific and have been found in several different muscle diseases. Similar cytoplasmic bodies, which possibly contain material derived from Z discs, were previously documented in muscles of human beings (Macdonald and Engel 1969). Further investigations need to be carried out to clarify whether the pathophysiological mechanism responsible for the development of these previously described sarcoplasmic inclusions might have served for the appearance of sarcoplasmic inclusions identified in the current bovine cases.
As this study was limited to using the traditional morphological approach, further studies by ultrastructural investigation, enzyme histochemistry, immunohistochemistry using multiple markers, and measuring the levels of markers of oxidative changes, such as lipid peroxidase, inducible nitric oxide synthase, and cytochrome c oxidase, would be warranted for more accurate understanding of the pathological process involving the development of not only lipofuscin but also sarcoplasmic eosinophilic inclusions, as seen in spontaneous cases of generalized lipofuscinosis reported here.
References
Agerholm JS (2007) Inherited disorders in Danish cattle. Acta Pathol Microbiol Immunol Scand 115(Suppl 122):1–76
Agerholm JS, Christensen K, Nielsen SS, Flagstad P (2009) Bovine renal lipofuscinosis: prevalence, genetics and impact on milk production and weight at slaughter in Danish cattle. Acta Vet Scand 51:7
Åstrӧm KE, Adams RD (1981) Pathological reactions of skeletal muscle fibre in man. In: Walton J (ed) Disorders of voluntary muscle. Churchill Livingstone, Edinburgh, pp 151–208
Barker IK, van Dreumel Palmer N (1993) The intestine. In: Jubb KVF, Kennedy PC, Palmer N (eds) Pathology of domestic animals, 4th edn. Academic Press, San Diego, pp 74–141
Bialas M, Demczuk S, Dyduch G, Drabik G, Chrupek M, Okon K (2013) Brown bowel syndrome (intestinal lipofuscinosis)—a case report and review of the literature. Pol J Pathol 64:228–231
Bildfell R, Matwichuk S, Mitchell S, Ward P (1995) Neuronal ceroid-lipofuscinosis in a cat. Vet Pathol 32:485–488
Borras D, Ferrer I, Pumarola M (1999) Age-related changes in the brain of the dog. Vet Pathol 36:202–211
Bradley R, Duffell SJ (1982) The pathology of the skeletal and cardiac muscles of cattle with xanthosis. J Comp Pathol 92:85–97
Bradley R, Fell BF (1981) Myopathies in animals. In: Walton J (ed) Disorders of voluntary muscle. Churchill Livingstone, Edinburgh, pp 824–872
Brunk UT, Terman A (2002a) The mitochondrial-lysosomal axis theory of aging: accumulation damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem 269:1996–2002
Brunk UT, Terman A (2002b) Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med 33:611–619
Carpenter S (2001) Muscle pathology on semithin resin sections. In: Karpati G, Hilton-Jones D, Griggs RC (eds) Disorders of voluntary muscle, 7th edn. Cambridge University Press, Cambridge, pp 238–295
Cesta MF, Mozzachio K, Little PB, Olby NJ, Sillls RC, Brown TT (2006) Neuronal ceroid lipofuscinosis in a Vietnamese pot-bellied pig (Sus scrofa). Vet Pathol 43:556–560
Duffell SJ, Edwardson R (1978) Xanthosis in cattle. Vet Rec 102:269–270
Friede RL (1989) In: Developmental neuropathology. Springer-Verlag, Berlin, pp 448–460
Goebel HH, Sharp JD (1998) The neuronal ceroid-lipofuscinoses. Recent advances. Brain Pathol 8:151–162
Goedegebuure SA, Hartman W, Hoebe HP (1983) Dystrophy of the diaphragmatic muscles in adult Meuse-Rhine-Yssel cattle: electromyographical and histological findings. Vet Pathol 20:32–48
Hafner S, Flynn TE, Harmon BG, Hill JE (2005) Neuronal ceroid-lipofuscinosis in a Holstein steer. J Diagn Invest 17:194–197
Haltia M (2003) The neuronal ceroid-lipofuscinoses: from past to present. Biochim Biophys Acta 1762:850–856
Harper PA, Walker KH, Healy PJ, Hartley WJ, Gibson AJ, Smith JS (1988) Neurovisceral ceroid-lipofuscinosis in blind Devon cattle. Acta Neuropathol 75:632–636
Hayward AH (1978) Xanthosis, an abnormal pigmentation of cattle. Vet Rec 102:96–97
Hӧhn A, Grune T (2013) Lipofuscin: formation, effects and role of macroautophagy. Redox Biol 1:140–144
Innes JRM, Saunders LZ (1962) Comparative neuropathology. Academic Press, New York and London, pp 147–243
Jolly RD, Douglas BV, Davey PM, Roiri JE (1995) Lipofuscin in bovine muscle and brain: a model for studying age pigment. Gerontol 41(Suppl 2):283–293
Jolly RD, Martinus RD, Shimada A, Fearnley IM, Palmer DN (1990) Ovine ceroid-lipofuscinosis is a proteolipid proteinosis. Can J Vet Res 54:15–21
Jolly RD, Palmer DN, Dalefield RR (2002) The analytical approach to the nature of lipofuscin (age pigment). Archiv Gerontol Geriatr 34:205–217
Jolly RD, Walkley SU (1997) Lysosomal storage diseases of animals: an essay in comparative pathology. Vet Pathol 34:527–548
Jubb KVF, Huxtable CR (1993) Cytopathology of nervous tissue. In: Jubb KVF, Kennedy PC, Palmer N (eds) Pathology of domestic animals, 4th edn. Academic Press, San Diego, pp 292–309
Macdonald RD, Engel AG (1969) The cytoplasmic body: another structural anomaly of the Z disc. Acta Neuropathol 14:99–107
Martinus RD, Harper PA, Jolly RD, Bayliss SL, Midwinter GG, Shaw GJ, Palmer DN (1991) Bovine ceroid-lipofuscinosis (Batten’s disease): the major component stored is the DCCD-reactive proteolipid, subunit C of mitochondrial ATP synthase. Vet Res Commun 15:85–94
McGavin MD (1995) Muscle. In: Carlton WW, McGavin MD (eds) Thomson’s special veterinary pathology. Mosby, St. Louis, pp 393–421
Nagashima N (1970) Inclusion bodies in denervated skeletal muscles of mice. Nagoya J Med Sci 33:189–202
Oberhuber G, Pointner R, Lauer E, Waldenberger P, Radaszkiewicz T (1989) “Brown bowel” syndrome—lipofuscinosis of the intestine as a cause of atonia. Leber Magen Darm 19:270–274
Porta EA (2002) Pigments in aging: an overview. Ann N Y Acad Sci 959:57–65
Schmidt U (1974) Generalized lipofuscinosis in a cat. Berl Munch Tierarztl Wochenscr 87:70–73
Terman A, Brunk UT (1998) Lipofuscin: mechanisms of formation and increase with age. Acta Pathol Microbiol Immunol Scand 106:265–276
Terman A, Brunk UT (2003) Aging and lysosomal degradation of cellular constituents. In: von Zglinicki T (ed) Aging at the molecular level. Kluwer Academic Publications, Netherlands, pp 233–242
Terman A, Brunk UT (2004) Lipofuscin. Int J Biochem Cell Biol 36:1400–1404
Tohma H, Hepworth AR, Shavlakadze T, Grounds MD, Arthur PG (2011) Quantification of ceroid and lipofuscin in skeletal muscle. J Histochem Cytochem 59:769–779
Url A, Bauder B, Thalhammer J, Nowotny N, Kolodziejek J, Herout N, Fürst S, Weissenböck H (2001) Equine neuronal ceroid lipofuscinosis. Acta Neuropathol 101:410–414
Van Vleet JF, Valentine BA (2007) Muscle and tendon. In: Maxie MG (ed) Jubb, Kennedy, and Palmer’s pathology of domestic animals, 5th edn. Elsevier Saunders, Edinburgh, pp 185–280
Wisniewski KE, Kida E, Golabek AA, Kaczmarski W, Connell F, Zhong N (2001) Neuronal ceroid lipofuscinoses: classification and diagnosis. Adv Genet 45:1–3
Conflict of interest
The author has no any financial and personal relationships with other people or organizations that inappropriately influence this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohfuji, S. Bovine generalized lipofuscinosis and sarcoplasmic inclusions: histopathological and histochemical features in three cases. Comp Clin Pathol 24, 1237–1244 (2015). https://doi.org/10.1007/s00580-015-2066-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-015-2066-3