Abstract
Many ectomycorrhizal (ECM) fungi produce commercially valuable edible sporocarps. However, the effects of nitrogen (N) application on ECM fungal sporocarp formation remain poorly understood. In this study, we investigated the effect of application of various N concentrations (0, 5, 25, 50, 100, and 200 mg/L) on the growth of Laccaria japonica mycelia in vitro for 1 month. The results showed that L. japonica mycelial biomass was highest in the 50 mg/L treatment and was significantly inhibited at N concentrations higher than 200 mg/L. Next, we investigated the effects of N application on mycorrhizal colonization and sporocarp formation in L. japonica colonizing Pinus densiflora seedlings in pots. The seedlings were watered with nutrient solutions containing 0, 5, 25, 50, or 100 mg N/L. The biomass, photosynthetic rate, and mycorrhizal colonization rates of the seedlings were measured at 45 days (first appearance of primordia), 65 days (sporocarp appearance on the substrate surface), and 4 months after seedlings were transplanted. The numbers of primordia and sporocarps were recorded during the experimental period. Total carbon (C) and N content were determined in seedlings at 4 months after transplantation, and in L. japonica sporocarps. Both mycelial growth and sporocarp production reached their maximum at an N application concentration of 50 mg/L, suggesting that the most suitable N concentration for ECM fungal sporocarp formation can easily be estimated in vitro during mycelial growth. This finding may help determine the most suitable N conditions for increasing edible ECM fungus sporocarp production in natural forests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ectomycorrhizal (ECM) fungi commonly form symbiotic relationships with various higher plant species (Smith and Read 2008). ECM fungi gain carbon (C) and other essential organic substances from plants; in return, they help their host plants to take up minerals, water, and metabolites (Genre et al. 2020). Many ECM fungi produce edible sporocarps, including the ascomycete Tuber spp. (true truffles), basidiomycete Amanita caesarea (Scop.) Pers., Boletus edulis Bull., and Tricholoma matsutake (Ito & Imai) Singer. These sporocarps are considered culinary delicacies with high medicinal and nutritional value (Wang et al. 2012). However, a number of high-demand, commercially valuable ECM sporocarps, such as T. matsutake, cannot be produced artificially, and instead are collected only in the wild (Sakamoto 2018; Guerin-Laguette 2021).
Culinary demand for edible sporocarps has stimulated research to understand the mechanism of sporocarp formation, improve the yield of edible ECM fungi in the wild, and derive techniques for the cultivation of edible ECM fungi (Guerin-Laguette 2021). Environmental factors including temperature, light, and soil humidity, as well as soil nutrient availability and interactions with other microbes, are of fundamental importance for the growth and sporocarp formation of ECM fungi (Sharma 2017; Zhang et al. 2019). For example, T. matsutake primordia usually begin to form after mid-August when soil temperatures reach 16–19 °C in Japan. However, primordia are aborted at soil temperatures of > 19 °C or < 15 °C (Wang et al. 1997). Ambra et al. (2004) reported that blue light inhibits Tuber borchii ascocarp growth. Soil microorganisms also have an important influence on the growth and development of ECM fungi. For example, ECM fungi deliver plant photosynthates to mycorrhizosphere bacteria (Gorka et al. 2019), whereas bacteria promote ECM fungal mycelial growth by producing growth stimulators, such as vitamins (Aslani et al. 2020), and facilitate the production of ECM fungal sporocarps (Obase 2020). ECM fungi tend to co-occur on the same host plant, resulting in competition; various effects of such competition on sporocarp formation in Laccaria japonica Popa &Nara have been reported (Zhang et al. 2019). Cenococcum geophilum and Suillus luteus were reported to exert a negative impact on sporocarp formation in L. japonica but Pisolithus sp. had no effect (Zhang et al. 2019).
A lack of soil nutrients is a critical signal for sexual reproduction in sporocarp-forming fungi (Sakamoto 2018). Soil nitrogen (N) availability has been reported to significantly affect ECM fungal sporocarp production, depending on the amount and duration of N addition (Velmala 2014). Previous studies have reported that N fertilization reduced sporocarp production in Suillus hirtellus in Pinus taeda plantation (Menge and Grand 1978), and reduced Laccaria bicolor colonization in container-grown jack pine seedlings (Godbout and Fortin 1992). Generally, ECM fungal sporocarp production is more sensitive to increased N supply than ECM colonization of root tips or the growth of extramatrical ECM hyphae (Lilleskov et al. 2011, 2018). Numerous field studies have shown that N addition and deposition reduce photosynthate allocation from the host plant to ECM fungi (Kjøller et al. 2012; Hasselquist and Högberg 2014; Franklin et al. 2014). Host plants may selectively allocate photosynthate to roots under limited nutrient absorption conditions to form beneficial fungal partnerships; these relationships may change with shifting environmental conditions (Victoroff 2020). ECM fungi that produce medium-distance, fringe exploration hyphae, including some Tricholoma and Cortinarius species, show greater reductions in abundance with N addition (Hobbie and Agerer 2010; Victoroff 2020). Therefore, ECM fungal genera that require greater C allocation from their host plants may be more significantly affected by N addition or deposition (Victoroff 2020). However, Trappe et al. (2009) reported that three Cortinarius species [Cortinarius depressus Fr., Cortinarius gentilis (Fr.) Fr., and Cortinarius montanus Kauffman] appear to favor sites with higher levels of total C and N, suggesting that the responses of ECM fungal sporocarps to N application may depend on whether the ECM taxa are ‘nitrophobic’ or ‘nitrophilic’ (Lilleskov et al. 2001; Karst et al. 2021; Wang et al. 2021). Many recent studies on the responses of ECM fungi to N application have focused on ECM fungal abundance, conservation, community composition, and the mechanism by which ECM fungi obtain N (Albarracín et al. 2013; Lilleskov et al. 2018; Karst et al. 2021), whereas studies examining how N application affects ECM fungal sporocarp formation are rare. It remains unclear whether the sporocarp formation of nitrophilic species requires more N, or whether nitrophobic species require lower N concentrations. In addition, relative to N, the effects of other soil nutrients, such as phosphorus and potassium, on ECM fungal productivity have been poorly studied, especially with respect to sporocarp production (Victoroff 2020; Frank and Garcia 2021).
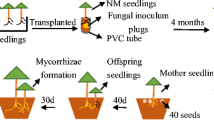
Laccaria species widely distribute in the world except for tropical South America and sub-Saharan Africa (Wilson et al. 2017). Nine edible Laccaria spp. have been reported from 17 countries and consumed worldwide, including L. amethystina, L. amethysteo-occidentalis, L. bicolor, L. edulis, L. farinacea, L. laccata, L. proxima, L. vinaceoavellanea and L. scrobiculatus (Flores et al. 2002; Zhang et al. 2015; Kaliyaperumal et al. 2018). Laccaria japonica is symbiotically associated with deciduous and coniferous trees in the Pinaceae, Fagaceae, and Salicaceae families in Japan (Vincenot et al. 2012, 2017). In our laboratory, the mycorrhizas of L. japonica were easily formed within one week after inoculation and the sporocarps were produced within three months under controlled conditions (Zhang et al. 2019). Thus, L. japonica was used to study the effect of nitrogen application on the sporocarp formation in this study.
Most edible ECM fungi cannot be cultivated artificially; as such, the effects of soil nutrients on sporocarp formation and yield remain largely unknown. Therefore, the establishment of suitable soil nutrient content guidelines for rapid prediction of sporocarp formation is crucial for cultivating ECM fungi and improving the sporocarp yield in the wild. The main objective of this study was to evaluate how N supply influences mycelial growth in L. japonica, as well as sporocarp formation in L. japonica-colonized seedlings of P. densiflora under controlled conditions. We investigated the effects of different N levels on L. japonica mycelial growth in vitro, L. japonica mycorrhizal formation and sporocarp formation in L. japonica-colonized Pinus densiflora seedlings under controlled conditions.
Materials and methods
Preparation of seedlings colonized by L. japonica
In this study, we used the ECM fungus L. japonica, an ideal model material of studing the mechanism of sporocarp formation, and its host plant P. densiflora (Zhang et al. 2019). We maintained L. japonica on modified Melin–Norkrans (MMN) agar medium (Marx 1969). Seeds of P. densiflora were collected in Iwate Prefecture, Japan and surface-sterilized as previously described (Zhang et al. 2019).
At 45 days after germination, non-mycorrhizal P. densiflora seedlings were inoculated with fresh L. japonica–agar plugs (ca. 2 cm × 2 cm) containing mycelia. Seedlings that were not inoculated with L. japonica were also prepared as the control. All seedlings were cultivated in a temperature-controlled greenhouse with a 16-h day (25 °C)/8-h night (20 °C) cycle.
During cultivation, a few seedlings were randomly sampled and their root tips were observed under a stereomicroscope (SZX7; Olympus, Tokyo, Japan). Further experiments were performed when the L. japonica mycorrhizal colonization rate reached > 90%.
Experimental setup
Experiment 1: In vitro L. japonica mycelial growth at different N concentrations
Laccaria japonica mycelia were cultured on MMN medium at 25 °C in the dark. After 4 weeks, a 9-mm-diameter agar plug was cut from the actively growing margin of an L. japonica colony and transferred to a petri dish (diameter: 9 cm) containing a new 20-mL MMN agar media covered with cellophane film (Bio-Rad Laboratories, Hercules, CA, USA) with different N concentrations (0, 5, 25, 50, 100, or 200 mg/L), using NH4NO3 as the N source. All treatments had three biological replicates.
After 1 month, the growth area of L. japonica mycelia was measured using a planimeter (X-plan 380F; Kantum Ushikata, Yokohama, Japan). The mycelia were collected using tweezers and washed gently with deionized water to remove the residual solution. The mycelia were patted dry using absorbent paper and then oven-dried at 60 °C for 48 h prior to dry weight measurements.
Experiment 2: Effects of N application on mycorrhizal colonization and sporocarp formation in L. japonica colonizing P. densiflora seedlings
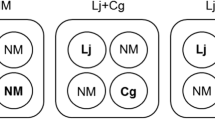
Seedlings were transplanted into polypropylene pots (volume: 600 mL; bottom diameter × upper diameter × depth = 6 cm × 9 cm × 14 cm), which were surface-sterilized with 70% v/v ethanol and filled with an autoclaved soil mixture composed of 30 g of vermiculite (Nittai, Osaka, Japan) and 360 g of pumice (diameter: 3–6 mm; Komeri, Niigata, Japan) per pot. We transplanted three non-mycorrhizal seedlings into each control pot, and one seedling inoculated with L. japonica and two non-inoculated seedlings into each treatment pot (Table 1). The seedlings were watered with tap water until 1 week after transplanting. Subsequently, we weekly added 30 mL of the nutrient solutions (50 μM KH2PO4, 100 μM K2SO4, 100 μM CaCl2·2H2O, 125 μM MgSO4·7H2O, 10 μM FeNa-EDTA, 12.5 μM H3BO3, 1 μM MnCl2·4H2O, 1 μM ZnSO4·7H2O, 0.25 μM CuSO4·5H2O, 0.25 μM Na2MoO4·2H2O, 0.25 μM CoCl2·6H2O) (Cumming and Weinstein 1990) with different N concentrations (0, 5, 25, 50, or 100 mg N/L) into each pot during the experimental period. Nitrogen was supplied with NH4NO3 and the N/P (weight) ratio in the nutrient solution with each N concentration ranged from 0 to 64.3 (Table 1). Finally, the total N amounts added into each pot with the concentrations of 0, 5, 25, 50, or 100 mg N/L were 0, 3.15, 15.8, 31.5 and 63.5 mg, respectively (Table 1). The solution pH was adjusted to 4.8 with H2SO4. Each N treatment had 15 replicates and the control seedlings had 9 biological replicates. The total amounts of N added to each treatment at different sampling periods in experiment 2 are listed in Supplementary Table S1.
Samples were harvested at three time points, as described in our previous study (Zhang et al. 2019). Briefly, these time points were the first appearance of L. japonica primordia (45 days after transplanting), the first appearance of L. japonica sporocarps (65 days after transplanting), and 4 months after transplanting. Seedlings were harvested from three control pots and five treatment pots per N treatment in each sampling period.
In situ seedling photosynthetic rates
Seedling photosynthetic rates were measured immediately before each sampling period, as described previously (Zhang et al. 2019). Briefly, all seedling needles were placed inside the conifer chamber of an LI-6400 photosynthetic system under natural light with 400 ppm CO2 at 25 °C. Three control pots and five treatment pots were randomly selected, and photosynthetic rates were measured in one control seedling, one non-inoculated treatment seedling, and one inoculated treatment seedling.
Tracking the appearance of primordia and sporocarps in L. japonica
Structures with and without a distinct cap and stipe were defined as sporocarps and primordia, respectively (Zhang et al. 2019). Once the L. japonica sporocarp emerged at the soil surface, we began to record sporocarp numbers in each pot. Sporocarps longer than 5 mm were collected every 3–4 days until the end of the experiment. The collected sporocarps were oven-dried at 60 °C for 48 h and then weighed.
Quantification of ECM colonization rates and seedling biomass
Among the collected seedlings, we randomly selected two non-mycorrhizal seedlings in each control pot and two formerly non-inoculated seedlings in the treatment pots to determine mycorrhizal colonization rates. All root tips were observed under a stereomicroscope and the mycorrhizal colonization rate was calculated as follows:
Colonization rate (%) = (Number of mycorrhizal root tips/Total number of root tips per seedling) × 100.
After mycorrhizal colonization was observed, all sampled seedlings were divided into shoot and root parts, dried at 60 °C for 48 h, and weighed. Prior to the experiment, we randomly selected six control and L. japonica-inoculated seedlings to calculate their initial dry weights, as follows:
Increase in dry weight (mg/plant) = Measured dry weight – Initial dry weight.
Quantification of total N and C content in L. japonica mycelia and sporocarps and P. densiflora seedlings
In experiment 1, after dry weight measurements, pure mycelia of L. japonica were thoroughly ground using a grinder (MS-100; TOMY Seiko, Tokyo, Japan). About 6–10 mg of ground mycelium powder was used to analyze the concentrations (%, dry weight) of total C and N using an NC analyzer (Sumigraph NC-22F; Sumika Chemical Analysis Service, Osaka, Japan).
In experiment 2, after dry weight measurements, seedling samples at 4 months after transplanting and L. japonica sporocarps were used to determine total C and N concentrations. We thoroughly ground two seedlings from each of three randomly selected control pots and two formerly non-inoculated seedlings from each of three randomly selected treatment pots using a mortar and pestle (shoots and roots were separated). Approximately 15 mg of ground powder was used to determine total C and N using an NC analyzer.
Statistical analyses
We evaluated the effects of N application levels on mycelial growth, total N content, total C content, and the C/N ratio of L. japonica in vitro using paired t-tests. Treatment pots containing dead seedlings at sampling were not used for further analysis, except for the 0 mg N/L treatment. Two-way analysis of variance (ANOVA), with five levels of N treatment × control or L. japonica treatment, was performed to determine significant differences in growth (dry weight and photosynthetic rate), total N content, total C content, and the C/N ratio of P. densiflora seedlings. We evaluated the effects of N concentrations on P. densiflora seedling biomass, photosynthetic rate, mycorrhizal colonization rate, total N content, total C content, and the C/N ratio in control and L. japonica treatment plants using paired t-tests; significance was evaluated at P < 0.05 following adjustment using the Benjamini–Hochberg method.
Correlations among biological parameters (dry weight, photosynthetic rate and mycorrhizal colonization rate) in P. densiflora seedlings, and the numbers and dry weight of L. japonica sporocarps, were evaluated using Spearman’s rank correlation. All statistical analyses were performed using R 3.5.1 software (R Core Team 2018).
Results
In vitro L. japonica mycelial growth at different N concentrations
After 1 month, the color of cultured L. japonica mycelia varied among N treatments (Fig. 1). In petri dishes treated with 0 and 5 mg N/L, the mycelial color changed from white to light grey, and remained constant over 1 month of culture. In petri dishes treated with 25 mg N/L, mycelial color changed gradually from purple to white, light grey, and finally brown (Fig. 1). In petri dishes treated with 50, 100, and 200 mg N/L, the mycelia remained purple over 1 month of culture after the color change (Fig. 1).
The area of L. japonica mycelial growth was significantly larger in petri dishes treated with 50 mg/L than in all other treatments and was significantly inhibited at N concentrations higher than 100 mg/L (n = 3; P < 0.05; paired t-test; Fig. 2a). Mycelial dry weight also increased as the N concentration increased to 50 mg/L, remained constant at N concentrations of 50–100 mg/L, and then markedly decreased at 200 mg N/L (Fig. 2b).
Mycelial extension area (a) and dry weight (b) of Laccaria japonica after 1 month of growth on MMN media culture under different N treatments (0, 5, 25, 50, 100, and 200 mg N/L). Values are means ± standard deviations (SD; n = 3). Different letters indicate significant differences among different treatments (P < 0.05 after Benjamini–Hochberg adjustment; paired t-test)
Mycelial total C content reached a maximum at 25 mg N/L (n = 3; P < 0.05; paired t-test; Table 2), whereas total N increased as the N treatment concentration increased to 50 mg N/L. The C/N ratio decreased as N levels increased to 50 mg/L (Table 2).
Laccaria japonica primordium and sporocarp formation
Laccaria japonica primordia were first observed in pots treated with 5 and 25 mg N/L at 45 days after transplanting. Thereafter, primordia were observed at 47 and 49 days in the 50 and 100 mg N/L treatments, respectively. Sporocarps first appeared at 65 days after transplanting in the 25 and 50 mg N/L treatments, and then at 70 and 87 days after transplanting in the 100 and 5 mg N/L treatments, respectively. Primordia and sporocarps were not observed in L. japonica + formerly non-inoculated seedling pots receiving no N treatment, or in control pots throughout the study period (Table 3).
The total number of sporocarps increased with N supply (Fig. 3a). Sporocarp dry weight was highest in the 50 mg N/L treatment (Fig. 3b). The average dry weight of sporocarps ranged from 6.16 mg (25 mg N/L treatment) to 1.15 mg (100 mg N/L treatment) (Fig. 3c). Most sporocarps formed in the 100 mg N/L treatment were small, with lengths within 2–3 mm after pileus formation (Fig. 3c). Immature small sporocarps changed in color from purple to white within 3–4 days and wilted at 1 week after formation.
Total number (a), corresponding dry weight (b) and average dry weight (c) of sporocarps formed under different N treatments (0, 5, 25, 50, and 100 mg N/L) at 4 months after Pinus densiflora seedling transplantation. Each control pot contained three non-mycorrhizal (NM) seedlings, and each treatment pot contained one seedling inoculated with Laccaria japonica (Lj) and two formerly non-inoculated seedlings. Values are means ± standard deviations (SD; n = 3–5). Different letters indicate significant differences among different treatments (P < 0.05 after Benjamini–Hochberg adjustment; paired t-test)
Mycorrhizal colonization of seedlings
Mycorrhizal roots were not observed in control seedlings throughout the experimental period. In pots containing L. japonica-inoculated and formerly non-inoculated seedlings without N treatment (0 mg N/L), L. japonica mycorrhizal root tips were not observed in formerly non-inoculated seedlings at 45 or 65 days after transplanting, and only a few mycorrhizal root tips were observed (colonization rates < 1%) at 4 months after transplanting (Fig. 4). In the 5 mg N/L treatment, mycorrhizal colonization rates gradually increased from 45 days (5%) to 4 months (73%) after seedling transplantation (Fig. 4). In the remaining treatments (25, 50, and 100 mg N/L), higher mycorrhizal colonization rates (90%, 81%, and 69%, respectively) were observed at 45 days after transplanting. The mycorrhizal colonization rate was significantly lower in the 100 mg N/L treatment than 25 and 50 mg N/L treatments at 4 months after transplantation (P < 0.05; Fig. 4).
Laccaria japonica mycorrhizal rates in two formerly non-inoculated Pinus densiflora seedlings under different N treatments at 45 days, 65 days, and 4 months after transplanting. Values are means ± standard deviations (SD; n = 3–5). Different letters indicate significant differences among different treatments (P < 0.05 after Benjamini–Hochberg adjustment; paired t-test)
Growth and photosynthetic rates of P. densiflora seedlings
At 4 months after transplanting, reddish discoloration of the needles of control seedlings was observed in all N treatments (Supplementary Figure S1). In pots of L. japonica + non-inoculation seedlings, reddish discoloration was observed at low N treatment concentrations (0 and 5 mg /L) (Supplementary Figure S1), but not at higher concentrations (25, 50, and 100 mg N/L) (Supplementary Figure S1).
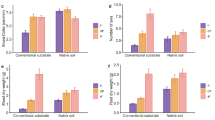
N supply, L. japonica colonization, and the interaction of these factors significantly affected P. densiflora seedling growth in both the control and L. japonica + non-inoculated seedling pots (P < 0.05; two-way ANOVA; Supplementary Table S2). Seedlings colonized by L. japonica had higher biomass than non-mycorrhizal seedlings in control pots at 4 months after transplantation (Fig. 5; Supplementary Table S2). Control seedlings showed no significant differences in shoot biomass according to N treatment (Fig. 5). In pots of L. japonica-inoculated + non-inoculated seedlings, shoot biomass showed no differences according to N treatment at 45 or 65 days after transplantation. However, shoot and root biomass were significantly higher in the 50 and 100 mg N/L treatments than all other treatments at 4 months after transplanting (Fig. 5).
Total increases in shoot, root, and net photosynthetic rates in control and treatment Pinus densiflora seedlings in different N treatments (0, 5, 25, 50, and 100 mg N/L) at 45 days (a–c), 65 days (d–f), and 4 months (g–j) after transplanting. Primordia and sporocarps were first observed at 45 and 65 days after transplanting, respectively. Each control pot contained three non-mycorrhizal (NM) seedlings and each treatment pot contained one seedling inoculated with Laccaria japonica (Lj) and two formerly non-inoculated seedlings. Values are means ± standard deviations (SD; n = 3–5). Different letters indicate significant differences among different treatments (P < 0.05 after Benjamini–Hochberg adjustment; paired t-test)
Photosynthetic rates (per dry needle weight) of non-mycorrhizal seedlings did not differ among control pots except at 65 days after transplanting, when those of the 50 and 100 mg N/L treatments were significantly higher than those of the 0 and 5 mg N/L treatments (P < 0.05; Fig. 5). In pots of L. japonica-inoculated + non-inoculated seedlings, photosynthetic rates increased significantly in formerly non-inoculated compared to non-mycorrhizal seedlings in the control pots at 65 days and 4 months after transplantation, in a manner dependent on N supply (Fig. 5; Supplementary Table S2).
Total N and C content of P. densiflora seedlings and L. japonica sporocarps
In both the control and L. japonica-inoculated + non-inoculated seedling pots, N supply rates were associated with increasing total C content (%) in shoots, but not in roots (Fig. 6). Total N content and the C/N ratio in shoots and roots of P. densiflora seedlings significantly increased with N supply up to 50 mg N/L (P < 0.05; Fig. 6). We observed no difference in seedling C/N ratios between the 50 and 100 mg N/L treatments (Fig. 6).
Total C and N content, and C/N ratios, in shoots and roots of Pinus densiflora seedlings in different N treatments (0, 5, 25, 50, and 100 mg N/L) at 4 months after transplanting. Each control pot contained three non-mycorrhizal (NM) seedlings and each treatment pot contained one seedling inoculated with Laccaria japonica (Lj) and two formerly non-inoculated seedlings. Values are means ± standard deviations (SD; n = 3). Different letters indicate significant differences among different treatments (P < 0.05 after Benjamini–Hochberg adjustment; paired t-test)
Laccaria japonica sporocarps had significantly higher total C content in the 5 mg N/L treatment than 25 and 100 mg N/L treatments (P < 0.05; Table 4). Sporocarp total N content was significantly higher in the 5–50 mg N/L treatments and stabilized at higher N concentrations. Sporocarp C/N ratios were significantly lower at N concentrations of 5–50 mg/L, with no significant differences between the 50 and 100 mg N/L treatments (Table 4). The total N contents in sporocarps ranged from 2.8% to 5.1% that were significantly higher than those in the seedlings (0.5–3.8%) (Fig. 6, Table 4).
Correlation between L. japonica sporocarp biomass and P. densiflora seedling growth
Total sporocarp numbers were positively correlated with the dry weight of shoots (R = 0.83; P < 0.001) and roots (R = 0.85; P < 0.001), photosynthetic rates in formerly non-inoculated seedlings (R = 0.74; P = 0.002), and total N content in shoots (R = 0.94; P < 0.001) and roots (R = 0.94; P < 0.001) at 4 months after transplantation (Table 5). Total sporocarp numbers were negatively correlated with C/N in shoots (R = −0.93; P < 0.001) and roots (R = −0.93, P < 0.001) (Table 5). A similar relationship was observed between sporocarp dry weight and P. densiflora seedling growth (Table 5).
Discussion
This study provides the first evidence that the most appropriate N supply for L. japonica mycelium growth in vitro (50 mg N/L) is the same as that for L. japonica sporocarp formation. This finding suggests that suitable N concentrations for ECM fungus sporocarp formation may easily be estimated by determining the optimal concentrations of corresponding nutrients for mycelial growth in vitro. To our knowledge, this is the first study to investigate the relationship between the effects of N concentration on mycelium growth in vitro and sporocarp formation in an ECM fungus colonizing P. densiflora seedlings under controlled conditions.
In this study, N concentration clearly affected mycelium growth, in terms of both growth area and dry weight. Mycelial biomass increased with increasing N supply, from 50 to 100 mg N/L, and significantly decreased at higher N concentrations (200 mg N/L; Fig. 2). Growth suppression through high N application was previously reported in some ECM fungal mycelia. For example, Wallander and Nylund (1992) demonstrated that the growth of extramatrical mycelia of L. bicolor and Suillus bovinus was completely restrained at high N supply, with mycelial death observed under excess N supply (200 mg/L). Similar results were reported in a study of Paxillus involutus and S. bovinus (Arnebrant 1994). Therefore, in N-deficient soils, L. japonica mycelia growth can be enhanced by suitable N supply, but inhibited by excess N.
The growth of our seedlings colonized by L. japonica was significantly higher than that of control seedlings, which remained non-mycorrhizal 4 months after transplantation; there was a significant increase in total dry weight among shoots (F = 27.15; P < 0.001) and roots (F = 52.12; P < 0.001) (Fig. 5; Supplementary Table S2; Supplementary Figure S1). These results are consistent with those of previous studies (Lilleskov et al. 2001; Smith and Read 2008; Pena and Polle 2014; Zhang et al. 2019), indicating that ECM fungi improve the nutrient acquisition ability of the host tree and enhance tree growth. Total sporocarp numbers were positively correlated with the seedling total biomass and photosynthetic rate (Table 5). Thus, N fertilization improved host tree growth and increased the amount of photosynthate allocated to ECM fungi. Previous studies found that host tree growth was strongly affected by sporocarp formation (Högberg et al. 2001; Kuikka et al. 2003). Godbout and Fortin (1992) reported a positive correlation between jack pine (Pinus banksiana) biomass and Laccaria bicolor sporocarp biomass in an N fertilization experiment. The photosynthetic rate of P. strobus seedlings colonizing L. bicolor and L. bicolor sporocarp growth rate was also found to be positively correlated (Lamhamedi et al. 1994). These findings suggest that the effect of N on ECM fungus growth and development is mediated by host tree growth, and that healthy host tree growth is beneficial for sporocarp formation and development. Therefore, to increase the production of edible ectomycorrhizal fungi, the first step is to ensure healthy growth of the host plant. However, soil fertilization also affects the growth of soil extraradical mycelia, and excessive fertilization inhibits extraradical mycelial growth, which presents a major challenge to ECM fungus production. In this study, P. densiflora seedling biomass peaked at 50 and 100 mg N/L, so the additional 50 mg N/L had no effect on seedling growth. Our results also showed that 50 mg N/L is a favorable N concentration for mycelial growth in vitro (Fig. 2). Previous studies reported that ECM fungal mycelial growth was directly related to the N status of the host tree (Nilsson and Wallander 2003). We speculate that L. japonica and P. densiflora may have reached a state of balance in terms of their mutual benefits, and that similar ranges of N concentration may be suitable for the growth of both organisms. Whether this phenomenon occurs in other ECM fungi requires further study.
Mycorrhizal colonization was significantly inhibited under the no N application (0 mg/L) and limited N application (5 mg/L) conditions (Fig. 4). This restriction of mycorrhizal colonization was alleviated by N accumulation (3.15 mg/pot) in the 5 mg N/L treatment, whereas excess N accumulation (100 mg N/L, 63 mg/pot) depressed mycorrhizal colonization after 4 months of N application (Supplementary Table S1; Fig. 4). Similarly, ECM colonization was reduced at high N application rates in forest and pot experiments (Newton and Pigott 1991; Termorshuizen and Ket 1991; Arnebrant and Söderström 1992; Wallander 1995), indicating that N is required for mycorrhizal formation, but that mycorrhizal colonization may depend on the N supply. In this study, sporocarp numbers were weakly positively correlated with mycorrhizal colonization rates following N application (Table 5). Mycorrhizal colonization rates appeared to have an indirect influence on sporocarp formation, suggesting that sporocarp yield did not depend on the number of ECM fungal root tips. These results are consistent with those of our previous study (Zhang et al. 2019) and earlier reports, in which high colonization rates were not necessarily required for high sporocarp fruiting in Laccaria species (Nylund 1988; Godbout and Fortin 1992). Some studies have also demonstrated that the amount and activity of ectomycorrhizae do not reflect sporocarp formation (Wallander 1995; Nara et al. 2003).
In this study, L. japonica sporocarp formation occurred earlier with lower N supply (5 and 25 mg N/L) than in other treatments (Table 3). Similarly, a previous study found that initial sporocarp formation in the ECM fungus Laccaria proxima colonizing P. banksiana seedlings generally occurred at low fertilization levels (30 mg N/L), but rarely at higher levels (60 and 120 mg N/L) (Danielson et al. 1984). N starvation may also be an important factor for inducing sporocarp formation among saprophytic and ECM fungi (Sakamoto 2018). The highest N concentration applied in the present study was 100 mg N/L, corresponding to a total N application amount of ca. 63 mg/pot (ca. 21 mg/seedling) at 4 months after transplanting (Table 1). Godbout and Fortin (1990) showed that the sporocarp formation in L. bicolor associated with container-grown P. banksiana seedlings was enhanced by a short photoperiod and low fertilization rate (6.6 mg N/seedling); Godbout and Fortin (1992) also observed that L. bicolor sporocarps occurred at 5–6 mg N/seedling but decreased with lower or higher N supply under both short and long photoperiods. Numerous studies have concluded that Laccaria is a nitrophilic taxon (Lilleskov et al. 2001, 2002, 2011; Corrales et al. 2017). Laccaria japonica appears to be more nitrophilic than L. bicolor, suggesting that the responses of ECM sporocarps to N supply may be dependent on the ECM fungal taxon (Corrales et al. 2017).
In this study, sporocarp numbers were enhanced by N application; however, L. japonica sporocarp dry weight was not linearly related to sporocarp number (Fig. 3). Interestingly, the 100 mg N/L treatment was associated with more sporocarps, but lowest average dry weight of sporocarps (1.15 mg) (Fig. 3c). Most sporocarps in this treatment stopped growing after formation, suggesting that carbon supply per primordium was much less at 100 mg/L than at 50 mg/L. Similar L. japonica sporocarp performance was observed by Teramoto et al. (2012), perhaps due to a decrease in C flux to sporocarps. Although Teramoto et al. (2012) did not focus on the effects of N application on sporocarp formation, they demonstrated that recently produced photosynthates were mainly transferred to sporocarps of L. japonica (referred to as L. amethystina). Previous studies have reported a strong correlation between C and N dynamics in symbiosis, as well as host tree regulation of photosynthate allocation to ECM fungi, depending on plant N status (Högberg et al. 2010; Hasselquist et al. 2012; Franklin et al. 2014). Lilleskov et al. (2002) reported that the sporocarp δ15N of ECM fungi became more enriched relative to the foliage of host trees with increasing soil inorganic N in a field investigation. The similar trend also was observed in our study that the N enrichment of sporocarps of L. japonica was much larger than those in shoots and roots of the plant hosts with the increasing of N concentration in the soils under controlled conditions (Fig. 6, Tables 2 and 4). Although the mycelia and sporocarps of L. japonica enriched higher N with the increasing of N concentration, the change of N contents and C/N ratios in mycelia and sporocarps had a much narrower range compared to the N/P ratios of nutrient solutions supplied (Fig. 6, Tables 1, 2, and 4). Phosphorus how to affect the N absorption by mycelia and sporocarps of L. japonica needs to be further investigated. High N fertilization decreases C flux to ECM fungi, which may result in decreased C flux to sporocarps (Hobbie et al. 2019). Marx et al. (1977) found that high levels of soil N decreased the sucrose content of short roots in P. taeda, thereby decreasing their susceptibility to ectomycorrhizal development by Pisolithus tinctorius; they speculated that this was caused by the stress of high N concentrations, which can induce mass sporocarp formation. High N levels can disrupt continuous transport of carbohydrate from the host to ECM fungi, preventing the development of initial sporocarps (Victoroff 2020). The mechanisms driving these phenomena require further study. Field study also shown that increasing N availability significantly reduced ECM sporocarp production, however, these general responses were strongly dependent on the application rate and duration of N additions (Hasselquist and Högberg 2014). In contrast to the high N treatment (100 kg N ha−1 yr−1), which almost eliminated the production of sporocarps of ECM fungi, the production of ECM sporocarps in the low N (20 kg N ha−1 yr−1) plot was roughly 20% greater compared to the unfertilized control plot (Hasselquist et al. 2012), suggesting that moderately low concentration of nitrogen fertilizer can improve ECM fungal sporocarp production in the field.
Thus, our findings indicate that excessive N application does not increase sporocarp yield but inhibits sporocarp development. We conclude that determination of the most appropriate N supply is essential for the cultivation of edible ECM fungi. Our in vitro and pot experiments yielded the same optimal N concentration (50 mg N/L) for L. japonica sporocarp formation (Figs. 2 and 3). Lilleskov et al. (2002) also reported that ECM fungal taxa with different responses to nitrogen deposition in the field study also differed in pure culture organic and inorganic nitrogen utilization. However, the physiology and growth of an ECM fungus is quite different in a symbiotic association as compared with a non-symbiotic phase of the fungus. Whether these patterns are common in other ECM fungi needs to be further confirmed. Moreover, our study of L. japonica mycelial growth in vitro had several limitations, including the lack of a field host plant experiment, which did not allow us to examine the effects of complex environmental factors. Although until now the similar studies have not been reported, our findings may be applicable to the management of soil N to increase the production of economically important edible sporocarps of ECM fungi that cannot be artificially cultivated at present, and instead are collected in natural forests.
N fertilization may also increase substrate pH and influence the uptake of other soil nutrients, such as phosphorus and potassium, by both the host tree and ECM fungus (Kües and Liu 2000; Nilsson and Wallander 2003; Trappe et al. 2009; Kennedy 2010). These factors also may influence sporocarp formation and development (Guerin-Laguette 2021).
Conclusions
In this study, we determined that N nutrition plays key roles in mycelial growth and mycorrhizal and sporocarp formation in the ECM fungus L. japonica. An N supply of 50 mg N/L was favorable for mycelial growth in vitro and was also suitable for the sporocarp formation of L. japonica in pot experiments. This study is the first to demonstrate that the same range of N supply was optimal for sporocarp formation, vegetative growth, and mycorrhizal formation in L. japonica. Although these trends are common in other ECM fungi needs to be further confirmed, our study provides insights into the production management of unartificial edible ECM mushrooms by controlling the nutrients in the soils.
References
Albarracín MV, Six J, Houlton BZ, Bledsoe CS (2013) A nitrogen fertilization field study of carbon-13 and nitrogen-15 transfers in ectomycorrhizas of Pinus sabiniana. Oecologia 173:1439–1450
Ambra R, Grimaldi B, Zamboni S, Filetici P, Macino G, Ballario P (2004) Photomorphogenesis in the hypogeous fungus Tuber borchii: isolation and characterization of Tbwc-1, the homologue of the blue-light photoreceptor of Neurospora crassa. Fungal Genet Biol 41:688–697
Arnebrant K (1994) Nitrogen amendments reduce the growth of extramatrical ectomycorrhizal mycelium. Mycorrhiza 5:7–15
Arnebrant K, Söderström B (1992) Effects of different fertilizer treatments on ectomycorrhizal colonization potential in two Scots pine forests in Sweden. For Ecol Manage 53:77–89
Aslani F, Tedersoo L, Põlme S, Knox O, Bahram M (2020) Global patterns and determinants of bacterial communities associated with ectomycorrhizal root tips of Alnus species. Soil Biol Biochem 148:107923
Corrales A, Turner BL, Tedersoo L, Anslan S, Dalling JW (2017) Nitrogen addition alters ectomycorrhizal fungal communities and soil enzyme activities in a tropical montane forest. Fungal Ecol 27:14–23
Cumming JR, Weinstein LH (1990) Aluminum-mycorrhizal interactions in the physiology of pitch pine seedlings. Plant Soil 125:7–18
Danielson RM, Griffiths CL, Parkinson D (1984) Effects of fertilization on the growth and mycorrhizal development of container-grown jack pine seedlings. Forest Science 30:828–835
Flores R, Bran MD, Honrubia M (2002) Edible mycorrhizal mushrooms of the west highland of Guatemala. In: Edible mycorrhizal mushrooms and their cultivation. Proceedings of the Second International Conference on Edible Mycorrhizal Mushrooms, Christchurch, New Zealand, 3–6 July, 2001, 2002. Crop & Food Research. pp 0–5
Frank HE, Garcia K (2021) Benefits provided by four ectomycorrhizal fungi to Pinus taeda under different external potassium availabilities. Mycorrhiza: 1–12
Franklin O, Näsholm T, Högberg P, Högberg MN (2014) Forests trapped in nitrogen limitation–an ecological market perspective on ectomycorrhizal symbiosis. New Phytol 203:657–666
Genre A, Lanfranco L, Perotto S, Bonfante P (2020) Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol 18:649–660
Godbout C, Fortin JA (1990) Cultural control of basidiome formation in Laccaria bicolor with container-grown white pine seedlings. Mycol Res 94:1051–1058
Godbout C, Fortin JA (1992) Effects of nitrogen fertilization and photoperiod on basidiome formation of Laccaria bicolor associated with container-grown jack pine seedlings. Can J Bot 70:181–185
Gorka S, Dietrich M, Mayerhofer W, Gabriel R, Wiesenbauer J, Martin V, Zheng Q, Imai B, Prommer J, Weidinger M, Schweiger P, Eichorst SA, Wagner M, Richter A, Schintlmeister A, Woebken D, Kaiser C (2019) Rapid transfer of plant photosynthates to soil bacteria via ectomycorrhizal hyphae and its interaction with nitrogen availability. Front Microbiol 10:168
Guerin-Laguette A (2021) Successes and challenges in the sustainable cultivation of edible mycorrhizal fungi–furthering the dream. Mycoscience 62:10–28
Hasselquist NJ, Högberg P (2014) Dosage and duration effects of nitrogen additions on ectomycorrhizal sporocarp production and functioning: an example from two N-limited boreal forests. Ecol Evol 4:3015–3026
Hasselquist NJ, Metcalfe DB, Högberg P (2012) Contrasting effects of low and high nitrogen additions on soil CO2 flux components and ectomycorrhizal fungal sporocarp production in a boreal forest. Glob Change Biol 18:3596–3605
Hobbie EA, Agerer R (2010) Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 327(1):71–83
Hobbie EA, Chen J, Hasselquist NJ (2019) Fertilization alters nitrogen isotopes and concentrations in ectomycorrhizal fungi and soil in pine forests. Fungal Ecol 39:267–275
Högberg MN, Briones MJ, Keel SG, Metcalfe DB, Campbell C, Midwood AJ, Thornton B, Hurry V, Linder S, Näsholm T, Högberg P (2010) Quantification of effects of season and nitrogen supply on tree below-ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest. New Phytol 187:485–493
Högberg P, Nordgren A, Buchmann N, Taylor AF, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Kaliyaperumal M, Kezo K, Gunaseelan S (2018) A Global Overview of Edible Mushrooms. In: Passari AK (ed) Singh BP, Lallawmsanga. Biology of Macrofungi. Springer International Publishing, Cham, pp 15–56
Karst J, Wasyliw J, Birch JD, Franklin J, Chang SX, Erbilgin N (2021) Long-term nitrogen addition does not sustain host tree stem radial growth but doubles the abundance of high-biomass ectomycorrhizal fungi. Glob Change Biol 27:4125–4138
Kennedy P (2010) Ectomycorrhizal fungi and interspecific competition: species interactions, community structure, coexistence mechanisms, and future research directions. New Phytol 187:895–910
Kjøller R, Nilsson LO, Hansen K, Schmidt IK, Vesterdal L, Gundersen P (2012) Dramatic changes in ectomycorrhizal community composition, root tip abundance and mycelial production along a stand-scale nitrogen deposition gradient. New Phytol 194:278–286
Kües U, Liu Y (2000) Fruiting body production in basidiomycetes. Appl Microbiol Biotechnol 54:141–152
Kuikka K, Härmä E, Markkola A, Rautio P, Roitto M, Saikkonen K, Ahonen-Jonnarth U, Finlay R, Tuomi J (2003) Severe defoliation of Scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology 84:2051–2061
Lamhamedi MS, Godbout C, Fortin JA (1994) Dependence of Laccaria bicolor basidiome development on current photosynthesis of Pinus strobus seedlings. Can J for Res 24:1797–1804
Lilleskov EA, Fahey TJ, Lovett GM (2001) Ectomycorrhizal fungal aboveground community change over an atmospheric nitrogen deposition gradient. Ecol Appl 11:397–410
Lilleskov EA, Hobbie EA, Horton TR (2011) Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol 4:174–183
Lilleskov EA, Hobbie EA, Fahey TJ (2002) Ectomycorrhizal fungal taxa differing in response to nitrogen deposition also differ in pure culture organic nitrogen use and natural abundance of nitrogen isotopes. New Phytol 154(1):219–231
Lilleskov EA, Kuyper TW, Bidartondo MI, Hobbie EA (2018) Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: a review. Environ Pollut 246:148–162
Marx DH (1969) The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59:153–163
Marx DH, Hatch AB, Mendicino JF (1977) High soil fertility decreases sucrose content and susceptibility of loblolly pine roots to ectomycorrhizal infection by Pisolithus tinctorius. Can J Bot 55:1569–1574
Menge JA, Grand LF (1978) Effect of fertilization on production of epigeous basidiocarps by mycorrhizal fungi in loblolly pine plantations. Can J Bot 56:2357–2362
Nara K, Nakaya H, Hogetsu T (2003) Ectomycorrhizal sporocarp succession and production during early primary succession on Mount Fuji. New Phytol 158:193–206
Newton AC, Pigott CD (1991) Mineral nutrition and mycorrhizal infection of seedling oak and birch: I. Nutrient uptake and the development of mycorrhizal infection during seedling establishment. New Phytol 117:37–44
Nilsson LO, Wallander H (2003) Production of external mycelium by ectomycorrhizal fungi in a Norway spruce forest was reduced in response to nitrogen fertilization. New Phytol 158(2):409–416
Nylund JE (1988) The regulation of mycorrhiza formation-carbohydrate and hormone theories reviewed. Scand J for Res 3:465–479
Obase K (2020) Effects of bacterial strains isolated from the ectomycorrhizal roots of Laccaria parva on sporocarp production by the fungus in vitro. Mycoscience 61(1):9–15
Pena R, Polle A (2014) Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J 8:321–330
R Core Team B (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/. Version 3.5.1. Released July 2, 2018. Accessed 3 Oct 2018
Sakamoto Y (2018) Influences of environmental factors on fruiting body induction, development and maturation in mushroom-forming fungi. Fungal Biol Rev 32:236–248
Sharma R (2017) Ectomycorrhizal mushrooms: their diversity, ecology and practical applications. In: Mycorrhiza-Function, Diversity, State of the Art. Springer. pp 99–131
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic press, New York, pp 191–268
Teramoto M, Wu B, Hogetsu T (2012) Transfer of 14 C-photosynthate to the sporocarp of an ectomycorrhizal fungus Laccaria amethystina. Mycorrhiza 22:219–225
Termorshuizen A, Ket PC (1991) Effects of ammonium and nitrate on mycorrhizal seedlings of Pinus sylvestris 1. Eur J for Pathol 21:404–413
Trappe MJ, Cromack K, Trappe JM, Wilson J, Rasmussen MC, Castellano MA, Miller SL (2009) Relationships of current and past anthropogenic disturbance to mycorrhizal sporocarp fruiting patterns at Crater Lake National Park, Oregon. Can J for Res 39:1662–1676
Velmala S (2014) Genetic control of susceptibility to fungal symbionts of juvenile Norway spruce (Picea abies (L.) H. Karsten) in relation to long-term growth performance. University of Helsinki, Finland
Victoroff C (2020) Response of ectomycorrhizal fungal fruiting to nitrogen and phosphorus additions in bartlett experimental forest, New Hampshire. Dissertations and Theses, State University of New York, New York
Vincenot L, Nara K, Sthultz C, Labbe J, Dubois M, Tedersoo L, Martin F, Selosse M (2012) Extensive gene flow over Europe and possible speciation over Eurasia in the ectomycorrhizal basidiomycete Laccaria amethystina complex. Mol Ecol 21:281–299
Vincenot L, Popa F, Laso F, Donges K, Rexer KH, Kost G, Yang ZL, Nara K, Selosse MA (2017) Out of Asia: Biogeography of fungal populations reveals Asian origin of diversification of the Laccaria amethystina complex, and two new species of violet Laccaria. Fungal Biol 121:939–955
Wallander H (1995) A new hypothesis to explain allocation of dry matter between mycorrhizal fungi and pine seedlings in relation to nutrient supply. In: Nilsson LO, Hüttl RF, Johansson UT (eds) Nutrient uptake and cycling in forest ecosystems. Springer, pp 243–248
Wallander H, Nylund JE (1992) Effects of excess nitrogen and phosphorus starvation on the extramatrical mycelium of ectomycorrhizas of Pinus sylvestris L. New Phytol 120:495–503
Wang T, Persson P, Tunlid A (2021) A widespread mechanism in ectomycorrhizal fungi to access nitrogen from mineral-associated proteins. Environ Microbiol 23(10):5837–5849
Wang Y, Cummings N, Guerin-Laguette A (2012) Cultivation of basidiomycete edible ectomycorrhizal mushrooms: Tricholoma, Lactarius, and Rhizopogon. In: Edible Ectomycorrhizal Mushrooms. Springer. pp 281–304
Wang Y, Hall IR, Evans LA (1997) Ectomycorrhizal fungi with edible fruiting bodies 1. Tricholoma matsutake and related fungi. Econ Bot 51:311–327
Wilson AW, Hosaka K, Mueller GM (2017) Evolution of ectomycorrhizas as a driver of diversification and biogeographic patterns in the model mycorrhizal mushroom genus Laccaria. New Phytol 213:1862–1873
Zhang J, Li T, Yang Y-L, Liu H-G, Wang Y-Z (2015) Arsenic concentrations and associated health risks in Laccaria mushrooms from Yunnan (SW China). Biol Trace Elem Res 164(2):261–266
Zhang S, Vaario L-M, Xia Y, Matsushita N, Geng Q, Tsuruta M, Kurokochi H, Lian C (2019) The effects of co-colonising ectomycorrhizal fungi on mycorrhizal colonisation and sporocarp formation in Laccaria japonica colonising seedlings of Pinus densiflora. Mycorrhiza 29:207–218
Acknowledgements
We are grateful to Mitsuko Goto, Yang Liu, Suguru Tanaka, Masayuki Kubota, Huayong Wang for technical assistance, the staff of the University of Tokyo Tanashi Forest for providing the forest soils, Kazuhide Nara for providing the culture of ectomycorrhizal fungus and Maki Narimatsu for providing the seeds of P. densiflora. We thank two anonymous reviewers for revising and useful comments on the manuscript.
Funding
This work was supported in part by JSPS KAKENHI (17H03824).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, S., Tsuruta, M., Li, C. et al. Estimation of the most suitable nitrogen concentration for sporocarp formation in Laccaria japonica colonizing Pinus densiflora seedlings through in vitro mycelial culture. Mycorrhiza 32, 451–464 (2022). https://doi.org/10.1007/s00572-022-01085-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-022-01085-2