Abstract
Information regarding the relationship between fertilization, mycorrhizas, and plant growth is scattered for non-conventional productive plant species. We evaluated the effect of different substrates and fertilization treatments on growth and colonization by arbuscular mycorrhizas of young Berberis microphylla plants, a native Patagonian shrub with edible fruits. We conducted a greenhouse experiment based on two factors: substrate (conventional or native soil) and fertilization (no fertilization, organic fertilization, or inorganic fertilization). When plants were grown in conventional substrate, both fertilizers promoted growth, having the inorganic fertilizer a greater effect. The effect of both fertilizers was similar when plants were cultivated in native soil, and lesser than in conventional substrate. Plants grown in native soil were larger than those in conventional substrate when organic fertilizer or no fertilizer was applied, but this was reversed when inorganic fertilizer was applied. There was no mycorrhization on plants grown in conventional substrate. In native soil, mycorrhization was highest for non-fertilized plants (60.1%), followed by those with organic fertilization (40.4%), and lowest when inorganic fertilizer was applied (29.9%). The relative abundances of both vesicles and arbuscules showed the opposite tendency, having both their highest values in treatments with inorganic fertilizer. Mycorrhization was positively correlated with plant size, but only when fertilizers were applied. Based on our results, we hypothesized that fertilization reduce mycorrhization but select more beneficial mycorrhizal fungi. We concluded that organic fertilizers have a comparable effect to inorganic fertilizers in terms of promoting plant growth, accompanied by a lesser reduction of mycorrhization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, there has been a growing recognition of the need to improve plant production, while simultaneously mitigating the negative environmental impacts associated with conventional agricultural practices (Vitousek et al. 2009; Gregory and George 2011). In the pursuit of a more sustainable and environmentally friendly production, it has been suggested that a change in soil fertility management is crucial (Verma et al. 2020). The standard practice of relying on the use of inorganic fertilizers to improve soil fertility has shown significant short-term benefits, such as enhanced crop yields through increased nutrient availability. However, this practice has also been associated with unintended consequences, including soil and water pollution, greenhouse gas emissions, and biodiversity loss (Gundersen et al. 2006; Vitousek et al. 2009; Sharma 2017). Organic fertilizers (such as manure and compost) represent a potential alternative to inorganic fertilizers (Gregory and George 2011). Organic fertilizers usually require to be applied in higher doses to provide the same amount of nutrients as inorganic ones, and have a slower nutrient release rate. However, they can provide several benefits, such as improving soil texture, and water retention capacity, and organic matter content, while also promoting soil microorganisms communities beneficial to plants (Masood et al. 2014; Sharma 2017; Liu et al. 2021).
Soil microorganisms play a crucial role in sustainable plant production. For example, there are many soil fungi that form mutualistic symbiotic associations with plant roots. These relationships are known as mycorrhizas and they usually enhance plant’s capacity to absorb nutrients and water, as well as to tolerate various types of stress, in exchange for photosynthates required by the fungi as carbon source (Smith and Read 2008; Thirkell et al. 2017). However, under certain circumstances, mycorrhizas might not provide any benefits, or even occasionally present a parasitic behavior (Johnson et al. 1997; Purin and Rillig 2008; Hoeksema et al. 2010; Rúa et al. 2016). The negative impact of the application of inorganic fertilizer on these symbiotic associations has been proven multiple times (Treseder 2004; Han et al. 2020; Trejo et al. 2020; Alsharmani et al. 2023). Increases in nutrients such as N and P not only decrease mycorrhizal colonization and diversity (Treseder 2004; Ma et al. 2021), but also affect negatively the benefits mycorrhizal fungi provide, such as the mitigation of soil N and P loss (Qiu et al. 2022) as well as soil carbon storage and structure (Yang et al. 2018). In contrast, organic fertilizers have shown lesser effect on them, and even a positive impact on mycorrhizal colonization, propagules and diversity (Gryndler et al. 2006; Qin et al. 2015; Jiang et al. 2021).
The relationship between plant production, the application of different types of fertilizers and mycorrhizal dynamics has been previously explored for different crops (Qin et al. 2015; Bilalis et al. 2015). However, this type of information in relation to native species of emerging economic and ecological importance is limited, despite being essential for the implementation of sustainable production practices. The aim of this study was to evaluate in a greenhouse experiment the effect of different fertilization treatments (application of organic fertilizer, inorganic fertilizer, or no fertilization) on Berberis microphylla G. Forst. (Berberidaceae) young plants growth and association with arbuscular mycorrhizas (AM) when cultivated in different substrates (conventional cultivation substrate or native soil). We used this species as a model since it is a native shrub of great ecological importance in Patagonian woodlands that produces high quality nutritional fruits and there is a strong and emerging interest in the market for their national and international commercialization (Bustamante et al. 2020; Cofré Guerrero and Jofre Vásquez 2021). We hypothesized that: (1) The application of fertilizers negatively affects the colonization by AM because these associations are mainly involved in plant nutrition, needs that are mostly met by fertilizers applications. This effect is particularly strong where nutrient availability is higher (inorganic fertilizer), and the availability of natural AM inoculum is lower (conventional substrate). (2) The application of inorganic fertilizer, by providing a more readily available nutrients input to plants, has a greater effect on plant growth and mycorrhization than organic fertilizer. (3) Plants in treatments with higher percentages of colonization by AM are sturdier, as these symbiotic associations promote not only plant growth but also their sturdiness.
2 Materials and methods
2.1 Experimental design and soil collection site
We grew B. microphylla plants in 3-liter pots over a complete cultivation cycle from November 2021 (spring) to June 2022 (winter) at the experimental greenhouse of the Universidad Nacional de Río Negro, in San Carlos de Bariloche (Argentina). The seedlings were germinated from fruits harvested during 2021–2022 summer in San Carlos de Bariloche (Argentina). At the end of November 2021, when the seedlings were three months old, we transplanted them into their corresponding pots. At that point, seedlings shoot length was 3.5 cm on average, and although all of them already had true leaves, some of them still retained their cotyledons.
Our study tested two factors: (1) the type of substrate, with two levels, based on either native shrubland soil (hereinafter referred to as “native soil”) or based on commercial products (referred to as “conventional substrate”); and (2) the type of fertilizer applied, with three levels including no fertilizer, organic fertilizer, and inorganic fertilizer. A completely randomized factorial design was implemented, combining the levels of all factors (six treatments in total), and fifteen replicates (n = 15) for each of them, resulting in a total of 90 pots.
The native soil was collected from “Conciencia” property (a project that focuses on biodiversity conservation, biodiversity education, and provides space for the development of scientific projects in collaboration with universities, public agencies, and NGOs) in El Foyel, Río Negro (Argentina). Soils are young Andisols classified as Hapludands (Soil Survey Staff 2022), which usually retain P and are limited by N. Mycorrhizal plants growing in this type of soil are mostly limited by N (Diehl et al. 2008). The climate is temperate-cold, with an average temperature of approximately 9 °C, and an annual rainfall of 920 mm (Reque et al. 2007). The site is a native mixed shrubland, dominated by species such as Nothofagus antarctica (G. Forst.), Lomatia hirsuta (Lam.) Diels subsp. obliqua (Ruiz & Pav.) R.T. Penn, B. microphylla, and Fabiana imbricata (Ruiz & Pav.). Numerous studies have been conducted in the property before, including an analysis of mycorrhization of adult B. microphylla plants where it was observed that they are naturally colonized by AM (approximately 20% of root length colonized; Fioroni et al. submitted). This is the reason why we decided to use this soil, since we already knew that there was available AM inoculum that successfully colonized B. microphylla plants. Furthermore, during plant production in local nurseries, it is common practice to use soil from the surrounding area, which naturally contains AM inoculum.
The “native soil” substrate consisted of three parts of soil obtained from a native mixed shrubland and one part of perlite, while the “conventional substrate” was composed of four parts of Sphagnum sp. peat, two parts of perlite, and one part of vermiculite. Chemical characteristics of both substrates are summarized in Table 1. The pots were completely filled with each of the substrates and, two weeks after seedlings´ transplantation, when plants were already well established, we applied the fertilization treatment. For organic fertilization we added Guanito from Italpollina (containing NPK, 4 N – 4 P2O5 – 4 K2) at a dose of 5 g per plant, and for inorganic fertilization we used Basacote Plus 6 M 16-8-12(+ 2 + TE) at a dose of 1.25 g per plant. The doses have been calculated so that both fertilizers deliver the same amount of nitrogen (0.2 g per plant). Fertilization was repeated in early February 2022. During the experiment, the plants were irrigated three times a day through aspersion for 8 min each time.
2.2 Morphological measurements of the cultivated plants
After the active growth period, the plants were transported to the laboratory, where we carefully removed them from their containers. We washed their roots with tap water to remove any adhered substrate without damaging the root system. We recorded the number of axes, collar diameter, and length of the longest axis for each plant (hereinafter referred to as “shoot length”). We considered length as related to the photosynthetic capacity and the competitive advantage against weeds, while the diameter is regarded as the best predictor of growth and survival. We also calculated the shoot length: diameter ratio, which is considered an indicator of plants´ sturdiness and survival capacity (Haase 2008).
For biomass estimation, we separated the aerial fraction of each plant from the roots. Prior to drying the plant material, we preserved a small fraction of the root system (approximately 20% of the fresh fine roots) to analyze AM colonization, which was conserved in alcohol 70% until staining. We recorded the fresh weight of the complete root system and the weight of the remaining roots after separating a fraction for AM analysis. Next, we dried each fraction of the plant at 60 °C until constant weight, and measured the dry weight of each part separately (aerial and roots dry biomass). The roots dry fraction was used to estimate the dry weight of the complete root system based on the following formula (Rydlová and Püschel 2020):
where, CR: weight of the complete root system, FR: fraction of the root system not used for AM analyses, F and D: fresh and dry samples, respectively. The measurement of these variables was considered important as aerial biomass is an indicator of photosynthetic capacity and growth potential, while radical biomass is an indicator of survival capacity. The shoot: root dry weight ratio was also calculated as an indicator of the quality of the plants (Haase 2008). Finally, we calculated the Dickson Quality Index (DQI), since it is a comprehensive quality index based on an integration of some of the quality indicators previously mentioned, and it’s calculated as follows (Dickson et al. 1960; Lin et al. 2018):
2.3 Mycorrhizal colonization
To analyze root colonization by AM, the technique described by Phillips and Hayman (1970) was used for staining the fungal structures present inside the roots. The technique was modified for this study and involved the following steps: (a) Clarification: the roots were removed from the alcohol and rinsed with tap water, then immersed in a 10% w/v KOH solution and boiled for 30 min. After boiling, the KOH solution was removed, and the samples were washed three times with tap water. The samples were left in water for three days to ensure the thorough removal of excess KOH and cellular content before the next step; (b) Acidification: roots were immersed in a 1% v/v HCl solution for 4–5 min. After that time, the acid was discarded, without rinsing the roots; (c) Staining: the samples were submerged in a 0.05% Trypan-Blue solution (0.05% w/v Trypan-Blue, 31% v/v glycerol, 31% v/v lactic acid) until fully covered and then left for 10 min in boiling water. Then, the dye was removed and the roots were rinsed carefully under running water to remove any excess of dye.
To estimate the percentage of roots colonized by AM, eight portions of stained roots, approximately 3.5 cm long each, were randomly chosen and arranged parallel to each other on a slide. Two slides per sample were prepared, and were observed under an optical microscope (Olympus BX40). At least 150 fields were examined in each slide at 200X magnification (approximately 300 fields per sample). The percentage of AM root colonization was estimated by counting the number of fields in which structures corresponding to AM fungi were observed and comparing them with the total number of quantified fields (McGonigle et al. 1990). The structures corresponding to AM were discriminated into hyphae, arbuscules, and vesicles to evaluate the relative abundance of each of these structures. Therefore, the evaluated variables were: total AM colonization, relative abundance of vesicles and relative abundance of arbuscules. The structures observed were photographically documented as brightfield images using a Sony ExwaveHAD camera and the TCapture software for Windows (v. 4.3.0.605, Tucsen Photonics Co., Ltd. 2019).
2.4 Data analysis
To evaluate the effect of substrate type and fertilization, we performed linear models for shoot length, shoot length: diameter ratio, and shoot: root dry weight ratio; generalized linear models (GLM) for collar diameter, number of axes, shoot dry weight, root dry weight, and DQI; and generalized linear mixed-effects models (GLMM) for total mycorrhizal colonization, relative abundance of arbuscules and relative abundance of vesicles. Substrate type, fertilization, and their interaction were included as explanatory variables in the models for morphological variables, and only fertilization was included in mycorrhizal colonization models, since we found AM colonization only in one of the evaluated substrates. For mycorrhizal colonization variables we decided to utilize the cbind function (as follows: cbind(number of positive fields, total number of fields - number of positive fields), to ensure that the model adequately represent the nature of the data. Plant ID was included as a random effect for mycorrhizal colonization to account for the fact that the fields counted within the same plant are not independent of each other, as they are with respect to those from another plant. All GLMs were fitted using a Gamma distribution, and a Binomial distribution for GLMMs. We performed Tukey’s post hoc pairwise comparisons. Finally, we carried out a correlation analysis between morphological variables and AM colonization, to provide insights into the relationship between plants’ growth and their symbiosis. All statistical analyses were conducted in R software version 4.2.2 (R Core Team 2022). We used the lme4 package version 1.1–31 (Bates et al. 2015) to estimate model parameters, the DHARMa package version 0.4.6 (Hartig 2020) to verify the models’ assumptions, the emmeans package version 1.8.4-1 for post hoc comparisons (Lenth et al. 2018), and the corrplot package version 0.92 (Wei and Simko 2021) to carry out the correlation analysis.
3 Results
3.1 Morphological variables
We observed an interactive effect between substrate type and fertilization on all the evaluated morphological variables (p < 0.001 in all cases; Table 2; Fig. 1, illustrated and summarized in Supp. Fig. S1). Regarding the effect of the fertilizers for each cultivation substrate, we observed that when plants were cultivated in a conventional substrate, the application of fertilizers improved plant growth, leading to higher values in all the morphological variables evaluated (shoot length − 256.5% higher-, collar diameter − 96.4% higher-, number of axes − 308.9% higher-, as well as shoot and root dry weight − 583.3% and 198.1% higher respectively-; Fig. 1). Besides, the effect of the inorganic fertilizer was more pronounced than that of the organic fertilizer (67.1% higher for shoot length, 72.7% higher for collar diameter, 105.2% higher for number of axes, and 212.1% and 169.9% higher for shoot and root dry weight, respectively; p < 0.01 in all cases; Fig. 1; Table 3). Additionally, both fertilizers resulted in equally higher shoot length: diameter (80.3%) and shoot: root dry weight (121.2%) ratios with respect to the no fertilized treatment (p < 0.001 in all cases). For plants grown in conventional substrate, the addition of inorganic fertilizer increased the DQI by a 225.9% in comparison with the remaining two fertilization treatments (p < 0.001 in both cases), while plants treated with organic fertilizer did not differ significantly with non-fertilized plants (p = 0.388). On the other hand, when grown in native soil both organic and inorganic fertilizers enhanced only shoot (69.5% higher) and root (56.8% higher) dry weight of B. microphylla young plants (p < 0.05 in all cases), while only the inorganic fertilizer significantly increased collar diameter (33.3% higher, Fig. 1b). No significant differences were observed between the plants treated with one fertilizer in comparison with the other. When the plants were cultivated in the native soil, fertilization had no significant impact on shoot length, number of axes, and the shoot: root dry weight ratio, but the addition of the inorganic fertilizer decreased the stem length: diameter ratio by a 18.2% (p = 0.019). Finally, the DQI was higher for plants when inorganic fertilizer was added in comparison with non-fertilized plants by a 113.3% (p < 0.001; Fig. 1h), having intermediate values the treatment with organic fertilizer.
Morphometric measures of Berberis microphylla plants. a Stem length (SL), b Collar diameter (CD), c Stem length: diameter ratio (SL: CD), d Number of axes (NAx), e Shoot dry weight (SDW), f Root dry weight (RDW), g Shoot: root dry weight ratio (SDW: RDW), h Dickson Quality Index (DQI). F-: no fertilization, OF: organic fertilization, IF: inorganic fertilization. Means and standard error are represented by the bars
In terms of comparisons between substrates, we observed that when no fertilizer or organic fertilizer was applied, plants cultivated in the native soil exhibited significantly greater shoot length (302.1% or 80.2% higher), collar diameter (85.3% or 48.6% higher), and shoot (233.4% or 60.5% higher) and root (163.8% or 137.9% higher) dry weight compared to those grown in a conventional substrate (p < 0.001 in all cases; Table 4). It is worth noting that, when compared with the conventional substrate, the application of inorganic fertilizer had a negative impact on the number of axes (47.9% less) and shoot dry weight (42.2% lower) when plants were cultivated in the native soil (p < 0.01 in all cases). The stem collar: diameter ratio was greater in plants grown in native soil when no fertilizer (110.7% higher) or organic fertilizer (20.2% higher) was applied, whereas the shoot: root dry weight ratio was lower in native soil by a 38.9% when either fertilizer was used (p < 0.01 in all cases). Finally, the DQI was higher for plants in native soil than in conventional substrate by a 42.7% when no fertilizer and by a 69.4% when organic fertilizer was added (p = 0.038 and p = 0.003, respectively), but there were no differences between these substrates when inorganic fertilizer was added (p = 0.27).
3.2 Mycorrhizal colonization
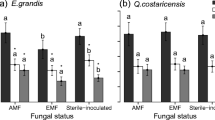
We did not find any evidence of mycorrhizal colonization in the roots of plants grown in conventional substrate, while plants grown in native soil successfully established associations with AM fungi in all the treatments (Fig. 2). However, we observed a detrimental effect of inorganic fertilizer on mycorrhizal colonization, compared to non-fertilized plants (p < 0.001, Table 3; Fig. 3a). Plants treated with organic fertilizer exhibited higher colonization than those treated with inorganic fertilizers by a 48.3%, but lower than non-fertilized plants by a 26.2%, although the differences were only marginal (p = 0.075 and 0.066, respectively; Table 3; Fig. 3a). Interestingly, we observed the opposite tendency for both the relative abundance of arbuscules and the relative abundance of vesicles. That is, we found a lower abundance of both these structures in non-fertilized plants than in those treated with inorganic fertilizers (45.4% lower for vesicles and 59.3% lower for arbuscules; p = 0.011 in both cases; Table 3; Fig. 3b), while plants treated with organic fertilizer showed intermediate values (Table 3; Fig. 3b).
Mycorrhization of Berberis microphylla plants. a Total mycorrhizal colonization. b Relative abundance of vesicles and arbuscules. F- indicates no fertilization, OF organic fertilization, and IF inorganic fertilization. Means and standard error are represented by the bars. Different letters indicate significant differences
3.3 Correlation between morphological characteristics and mycorrhization
In plants colonized by AM fungi, we did not find any correlation between morphological characteristics and mycorrhization when no fertilizer was added to the pots, but several correlations were found when fertilizers were applied (Fig. 4). When plants were treated with organic fertilizer, total mycorrhizal colonization and the relative abundance of vesicles showed a positive correlation with collar diameter, number of axes, and root and shoot dry weight. Additionally, the relative abundance of vesicles showed a positive correlation with shoot length (Fig. 4). The relative arbuscule abundance was negatively correlated with shoot length and the shoot length: diameter ratio under this fertilization treatment (Fig. 4).
Correlogram of Berberis microphylla morphological measures and mycorrhization (overall colonization and relative abundance of vesicles and arbuscules). Non-significant correlations are denoted with an “X”, numbers in white indicate correlation coefficient. Results are separated by the fertilization treatment
Similarly, when plants were treated with inorganic fertilizer our results showed a positive correlation between total mycorrhization and collar diameter, number of axes, root and shoot dry weight, and shoot: root dry weight ratio. However, and in contrast to the tendencies observed for the organic fertilizer, in this case we did not find any correlation between the relative abundance of arbuscules and morphometric measurements, and the relative abundance of vesicles was only positively correlated with the number of axes (Fig. 4).
For both fertilizers, a positive and significant correlation between total mycorrhization and DQI was registered (Fig. 4). In the case of the organic fertilizer, this was also true for the relative abundance of vesicles (Fig. 4).
4 Discussion
4.1 Substrate and fertilization type effect on morphological measurements
In general, plants that received either organic or inorganic fertilizers exhibited increased growth, a well-documented phenomenon supported by numerous previous studies, both in controlled greenhouse experiments and natural field conditions (Obsa 2017; Ji et al. 2017; Moe et al. 2019; Bergstrand 2022). The enhanced growth can be attributed to the nutrients supplied by fertilizers, which are essential for optimal plant development (Havlin et al. 2017; Ahmadi et al. 2020).
However, this fertilization effect diminished when we cultivated plants in native soil. In this substrate, differences in plant growth between the fertilization treatments were smaller, and in some cases, negligible. For example, the increase in aerial biomass for fertilized plants was 584.3% (average effect of organic and inorganic fertilizers) in the conventional substrate but only 69.5% in native soil, and we found no differences between organic and inorganic fertilizers in plants grown in native soil. There are several factors that might contribute to this differential effect of the fertilizers, depending on the cultivation substrate. On the one hand, non-fertilized plants grown in conventional substrate were already smaller than those grown in native soil, possibly due to the lower nutrient availability of the substrate, as forest soils generally have a higher nutrient content than non-fertilized inert substrates (Hussain et al. 2014). Even though conventional substrate had higher percentages of total nitrogen, since is mostly vegetal organic matter, the nitrogen available for plants (nitrates, among other forms of nitrogen) were higher for native soil. On the other hand, conventional growth substrates are optimal for retaining nutrients in a manner that makes them readily available for plant uptake (Wilkinson et al. 2014). This can be attributed to the inherent difficulty that plants encounter in acquiring nutrients from natural soils, as contrary to conventional substrates. For instance, phosphorus availability is severely limited in Andisols by the extremely high phosphorus retention capacity of the andic materials (Brady and Weil 2008). Consequently, and depending on its proportion of sand and clay, the use of soil can lead to nutrient immobilization or the outright leaching of nutrients through irrigation (Wilkinson et al. 2014).
While analyzing these results as a whole, it can be observed that the disparities in plant size between fertilized and unfertilized plants are more pronounced in conventional substrate, with unfertilized plants being considerably smaller and fertilized ones notably larger. In contrast, in native soil, the differences are less dramatic, with unfertilized plants being moderately smaller (but still larger than their counterparts in conventional substrate) and fertilized plants reaching a larger size. For instance, when we added inorganic fertilizer, plants grown in conventional substrate had more axes in comparison with native soil, which translated to a greater shoot dry weight. This highlights the significant impact of substrate type on the fertilization effect, with conventional substrate accentuating differences and native soil moderating them.
In addition, we found that inorganic fertilizer resulted in greater plant growth compared to organic fertilizer when growing plants in conventional substrate. The greater effect of inorganic fertilizer may be due to its faster release and more readily attainable acquisition of its nutrients input by plants in the short term, when compared to organic fertilizer (Sharma 2017; Niedziński et al. 2021). However, we did not observe differences between both types of fertilizers in plants grown in native soil. It is possible that the physical and chemical characteristics of native soil previously mentioned made it more difficult for plants to access the nutrients provided by inorganic fertilizers (Wilkinson et al. 2014). Instead, greater colonization by mycorrhizae (as seen in plants treated with organic fertilizer in comparison with those treated with inorganic fertilizer) could have facilitated the absorption of nutrients supplied by organic fertilizer (Hodge 2017). However, it is noteworthy that the higher nutritional quality and greater availability of mycorrhizal inoculum in the native soil than in conventional substrate, makes it difficult to discern the effect of each factor.
4.2 Effect of substrate and fertilization type on mycorrhization
We did not find any evidence of mycorrhizal colonization in plants grown in conventional substrate. This may be attributed to the absence of inoculum in this substrate, in contrast to the natural availability of spores and hyphae in the native soil, where plants successfully formed AM. In native soil, we found strong effects of fertilization on mycorrhization. When no fertilizer was applied, the mycorrhizal colonization in plants grown in native soil was found to be 60.1%. However, when we applied inorganic fertilizer, the colonization percentages were significantly lower (29.9%), whereas the application of organic fertilizer resulted in an intermediate level of mycorrhization (44.4%). These findings are consistent with similar studies conducted in other systems ( El Kinany et al. 2019; Ikoyi et al. 2020; Liu et al. 2020; Yazici et al. 2021). It is known that plants establish mycorrhizal associations to obtain nutrients, among other reasons. The application of inorganic fertilizer, which provides a greater amount of available nutrients, could reduce the need for plants to associate with mycorrhizal fungi (Nagy et al. 2009; Balzergue et al. 2011; Amir et al. 2021). This could allow plants to allocate less carbon to maintain mycorrhizal associations and use it instead for vegetative growth (Konvalinková et al. 2017; Andrino et al. 2021; Salmeron-Santiago et al. 2021). On the other hand, nutrients from organic fertilizers are less accessible to plants, and so mycorrhizal fungi could still play an important role in nutrient uptake (Hodge 2017).
Surprisingly, even though the overall percentage of mycorrhizal colonization decreased with fertilizers application, we observed the opposite tendency for both the relative abundance of arbuscules and the relative abundance of vesicles. We found that plants that were not fertilized had lower abundance of both these structures, while higher abundances were found when an inorganic fertilizer was used. Arbuscules are responsible for the exchange of water, nutrients, and sugars between the plant and the mycorrhizal fungus (Smith and Read 2008). When the abundance of arbuscules is high, it generally means that there is a greater benefit to the plant (Gange and Ayres 1999). One possible explanation for our observations is that when fertilizers are applied to the soil, the plant’s need to associate with mycorrhizal fungi decreases. Thus, plants growing in nutrient-rich soils are able to selectively associate with mycorrhizal fungi that offer higher benefits, unlike plants growing in environments where mycorrhizal associations are more crucial (i.e. nutrient-poor soils), which cannot afford to be as selective in their fungal partnerships. These results are surprising considering the vast amount of studies reporting the reduction in benefits for plants provided by mycorrhizal fungi after fertilization (Hoeksema et al. 2010) and, furthermore, that mycorrhizal fungi could turn parasitic when fertilizers are applied (Johnson 1993; Peng et al. 2023) These disparities could indicate that the role of fertilizer as “filter agent” is dependent on the context, including plant and fungal species, as shown by Li et al. (2016) meta-analysis. In addition, it has been suggested that plants could discriminate mycorrhizal fungi in terms of the benefits they provide, and allocate more carbon to their preferred fungal partners (Wang et al. 2017). This could explain the observed increase in the abundance of vesicles, which are considered to be storage structures of the fungi, and tend to be more abundant when plants allocate more carbon to the mycorrhiza (Smith and Read 2008).
4.3 Correlation between mycorrhization and morphological measurements
When analyzing the relationship between the levels of mycorrhization and plant growth responses, we found that mycorrhization had a positive correlation with morphological variables only in the fertilized treatments. These results were mainly observed in terms of total mycorrhization percentages and relative abundance of vesicles. The relative abundance of arbuscules had a negative correlation with shoot length and the shoot length: diameter ratio. Although this behavior could suggest that a higher abundance of arbuscules is associated with less plant growth, we hypothesize that this is not the case since no significant correlations were found with other morphological variables. On the contrary, we believe that this correlation could indicate greater plant sturdiness when there is a higher relative abundance of arbuscules (a decrease in shoot length but not in collar diameter translates to a lower shoot length: diameter ratio), as reported in several studies (Urgiles et al. 2014; Corsini et al. 2022; Tapwal et al. 2022). The correlations found in this study could also support the aforementioned selective symbiosis hypothesis, where the use of fertilizers could lead to the selection of more beneficial mycorrhizal fungi, as we only observed these tendencies in the fertilized treatments. It is noteworthy to mention that this hypothesis remains untested and it should be addressed in future research.
4.4 Future perspectives
Our results indicate that growing plants on shrubland soil supplied with organic fertilizer could result in high quality plants, possibly with a greater capacity for survival upon transplanting for establishment in field production systems (Haase 2008). Even though plants growing on conventional substrate had a greater shoot dry weight (as a consequence of a higher number of axes), it is important to take into consideration that only plants grown in native soil presented associations with AM. Whether the benefits of having young plants with a higher number of axes (which normally are pruned to enhance fructification; Albert et al. 2010) surpasses or not those of AM colonization related to an increase in plants survival capacity during transplant, yield and fruit quality, is yet to be studied. Additionally, it is noteworthy that when we calculated the cost of the production of young plants of B. microphylla in our study, we found that cultivation with native soil and organic fertilization was 18.5% cheaper than the production with conventional substrate and inorganic fertilizer (Supp. Table S1). However, further research is necessary to evaluate field performance. It is also necessary to consider that the discussions in this study do not aim to promote the extraction of soils from native forests for massive greenhouse plant cultivation, since it could significantly harm natural systems. This approach could deplete the forest’s soil resources, which would disrupt the delicate balance of these ecosystems. Nevertheless, it is important to note that when plants are produced using soil as part of the substrate, the application of organic fertilizers allows for a similar growth than inorganic fertilizers, while not severely impacting the association of plants with mycorrhizal fungi.
5 Conclusion
This study was the first one to analyze the role of mycorrhization on a Patagonian native fruit plant species growth, shedding light to the production of not only this but also other native and economic relevant fruiting shrubs. Our results revealed that the plants grown in conventional substrate and treated with inorganic fertilizer had a significant growth, but with a low colonization by mutualistic fungi. On the other hand, the plants grown in native soil and treated with organic fertilizer showed a similar growth, but higher plant quality and mycorrhization rates. These results provide support for our first two hypotheses. We also found that overall higher percentages of mycorrhization are correlated with plants sturdiness and quality, which supports our third hypothesis. Additionally, we observed that fertilization may lead to a selective process of specific mycorrhizal fungi that provide more benefits for the plants, even though this is a hypothesis derived from our results and needs to be approached in future studies. While further research is necessary to provide cultivation recommendations that improve production and reduce environmental impacts, the results of this study demonstrate the potential for implementing alternatives that are more sustainable than conventional methods of production without compromising plant quality.
Data availability
The datasets generated during and analyzed during the current study are available in the Figshare repository, https://figshare.com/s/b31f7c7fff9d391e0583.
References
Ahmadi F, Samadi A, Rahimi A (2020) Improving growth properties and phytochemical compounds of Echinacea purpurea (L.) medicinal plant using novel nitrogen slow release fertilizer under greenhouse conditions. Sci Rep 10:13842. https://doi.org/10.1038/s41598-020-70949-4
Albert T, Karp K, Starast M, Paal T (2010) The effect of mulching and pruning on the vegetative growth and yield of the half-high blueberry. Agron Res 8:759–768
Alsharmani AR, Solaiman ZM, Leopold M et al (2023) Impacts of Rock Mineral and traditional phosphate fertilizers on mycorrhizal communities in pasture plants. Microorganisms 11:1051. https://doi.org/10.3390/microorganisms11041051
Amir H, Gensous S, Cavaloc Y et al (2021) Phosphorus fertilization of an Ultramafic Soil reduced effects of Arbuscular Mycorrhizal Fungi but not mycorrhizal colonization. J Soil Sci Plant Nutr 21:3544–3554. https://doi.org/10.1007/s42729-021-00626-6
Andrino A, Guggenberger G, Sauheitl L et al (2021) Carbon investment into mobilization of mineral and organic phosphorus by arbuscular mycorrhiza. Biol Fertil Soils 57:47–64. https://doi.org/10.1007/s00374-020-01505-5
Balzergue C, Puech-Pagès V, Bécard G, Rochange SF (2011) The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J Exp Bot 62:1049–1060. https://doi.org/10.1093/jxb/erq335
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear mixed-effects models using lme4. J Stat Soft 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Bergstrand K-J (2022) Organic fertilizers in greenhouse production systems – a review. Scientia Hortic 295:110855. https://doi.org/10.1016/j.scienta.2021.110855
Bilalis D, Karkanis A, Angelopoulou F et al (2015) Effect of Organic and Mineral fertilization on Root Growth and Mycorrhizal colonization of pea crops (Pisum sativum L). BUASVMCN-HORT 72:288–294. https://doi.org/10.15835/buasvmcn-hort:11497
Brady NC, Weil RR (2008) The Nature and Properties of soils, 14th edn. Pearson Prentice Hall, New Jersey
Bustamante GN, Soler R, Blazina AP, Arena ME (2020) Fruit provision from Berberis microphylla shrubs as ecosystem service in Nothofagus forest of Tierra Del Fuego. Heliyon 6:e05206. https://doi.org/10.1016/j.heliyon.2020.e05206
Cofré Guerrero PI, Jofre Vásquez MPPG (2021) Berberis microphylla G. Forst (calafate). Una revisión de la actualidad, producción, calidad nutracéutica y sus perspectivas agronómicas. Bachelor thesis, Universidad Adventista de Chile
Corsini D, Vigevani I, Oggioni SD et al (2022) Effects of controlled mycorrhization and deficit irrigation in the nursery on post-transplant growth and physiology of Acer campestre L. and Tilia cordata Mill. Forests 13:658. https://doi.org/10.3390/f13050658
Dickson A, Leaf AL, Hosner JF (1960) Quality appraisal of white spruce and white pine seedlings stock in nurseries. Chron 36:10–13. https://doi.org/10.5558/tfc36010-1
Diehl P, Mazzarino MJ, Fontenla S (2008) Plant limiting nutrients in Andean-Patagonian woody species: effects of interannual rainfall variation, soil fertility and mycorrhizal infection. Ecol Manag 255:2973–2980. https://doi.org/10.1016/j.foreco.2008.02.003
El Kinany S, Achbani E, Faggroud M et al (2019) Effect of organic fertilizer and commercial arbuscular mycorrhizal fungi on the growth of micropropagated date palm cv. Feggouss J Saudi Soc Agric Sci 18:411–417. https://doi.org/10.1016/j.jssas.2018.01.004
Gange AC, Ayres RL (1999) On the relation between Arbuscular Mycorrhizal Colonization and Plant Benefit. Oikos 87:615. https://doi.org/10.2307/3546829
Gregory PJ, George TS (2011) Feeding nine billion: the challenge to sustainable crop production. J Exp Bot 62:5233–5239. https://doi.org/10.1093/jxb/err232
Gryndler M, Larsen J, Hršelová H et al (2006) Organic and mineral fertilization, respectively, increase and decrease the development of external mycelium of arbuscular mycorrhizal fungi in a long-term field experiment. Mycorrhiza 16:159–166. https://doi.org/10.1007/s00572-005-0027-4
Gundersen P, Schmidt IK, Raulund-Rasmussen K (2006) Leaching of nitrate from temperate forests effects of air pollution and forest management. Environ Rev 14:1–57. https://doi.org/10.1139/a05-015
Haase DL (2008) Understanding forest seedling quality: measurements and interpretation. TPN 52:24–30
Han Y, Feng J, Han M, Zhu B (2020) Responses of arbuscular mycorrhizal fungi to nitrogen addition: a meta-analysis. Glob Change Biol 26:7229–7241. https://doi.org/10.1111/gcb.15369
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package, version 0.4.6. http://florianhartig.github.io/DHARMa/
Havlin JL, Tisdale SL, Nelson WL, Beaton JD (2017) Soil fertility and fertilizers. An introduction to nutrient management, 8th edn. Pearson, India
Hodge A (2017) Accessibility of Inorganic and Organic nutrients for Mycorrhizas. Mycorrhizal mediation of Soil. Elsevier, pp 129–148
Hoeksema JD, Chaudhary VB, Gehring CA et al (2010) A meta-analysis of context dependency in plan response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407. https://doi.org/10.1111/j.1461-0248.2009.01430.x
Hussain A, Iqbal K, Aziem S et al (2014) A review on the science of growing crops without soil (soilless culture)-a novel alternative for growing crops. Int J Agric Crop Sci 7:833
Ikoyi I, Egeter B, Chaves C et al (2020) Responses of soil microbiota and nematodes to application of organic and inorganic fertilizers in grassland columns. Biol Fertil Soils 56:647–662. https://doi.org/10.1007/s00374-020-01440-5
Ji R, Dong G, Shi W, Min J (2017) Effects of Liquid Organic fertilizers on Plant Growth and Rhizosphere Soil characteristics of Chrysanthemum. Sustainability 9:841. https://doi.org/10.3390/su9050841
Jiang S, An X, Shao Y et al (2021) Responses of Arbuscular Mycorrhizal Fungi occurrence to Organic Fertilizer: a meta-analysis of field studies. Plant Soil 469:89–105. https://doi.org/10.1007/s11104-021-05153-y
Johnson NC (1993) Can fertilization of Soil Select Less Mutualistic. Mycorrhizae? Ecol Appl 3:749–757. https://doi.org/10.2307/1942106
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–585. https://doi.org/10.1046/j.1469-8137.1997.00729.x
Konvalinková T, Püschel D, Řezáčová V et al (2017) Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 419:319–333. https://doi.org/10.1007/s11104-017-3350-6
Lenth R, Singmann H, Love J et al (2018) emmeans: Estimated marginal means, aka least-squares means. https://github.com/rvlenth/emmeans
Li M, Jordan NR, Koide RT et al (2016) Meta-analysis of crop and weed growth responses to Arbuscular Mycorrhizal Fungi: implications for Integrated Weed Management. Weed Sci 64:642–652. https://doi.org/10.1614/WS-D-16-00050.1
Lin KH, Wu CW, Chang YS (2018) Applying Dickson Quality Index, Chlorophyll fluorescence, and Leaf Area Index for assessing Plant Quality of Pentas lanceolata. Not Bot Horti Agrobo 47:169–176. https://doi.org/10.15835/nbha47111312
Liu J, Zhang J, Li D, Xu C, Xiang X (2020) Differential responses of arbuscular mycorrhizal fungal communities to mineral and organic fertilization. Microbiologyopen 9:e00920. https://doi.org/10.1002/mbo3.920
Liu J, Shu A, Song W et al (2021) Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 404:115287. https://doi.org/10.1016/j.geoderma.2021.115287
Ma X, Geng Q, Zhang H et al (2020) Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytol 229:2957–2969. https://doi.org/10.1111/nph.17077
Masood S, Naz T, Javed MT et al (2014) Effect of short-term supply of farmyard manure on maize growth and soil parameters in pot culture. Arch Agron Soil Sci 60:337–347. https://doi.org/10.1080/03650340.2013.792990
McGonigle TP, Miller MH, Evans DG et al (1990) A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Moe K, Moh SM, Htwe AZ et al (2019) Effects of Integrated Organic and Inorganic fertilizers on yield and growth parameters of Rice varieties. Rice Sci 26:309–318. https://doi.org/10.1016/j.rsci.2019.08.005
Nagy R, Drissner D, Amrhein N et al (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol 181:950–959. https://doi.org/10.1111/j.1469-8137.2008.02721.x
Niedziński T, Sierra MJ, Łabętowicz J et al (2021) Release of Nitrogen from Granulate Mineral and Organic fertilizers and its effect on selected Chemical parameters of Soil. https://doi.org/10.3390/agronomy11101981. Agronomy 11:1981
Obsa GCZ (2017) Effect of organic and inorganic fertilizers on growth, yield and yield components of chick pea (Cicerarietinum) and enhancing soil chemical properties on vertisols at ginchi, central highlands of Ethiopia. J Biology Agric Healthc 7:28–34
Peng Z, Johnson NC, Jansa J et al (2023) Mycorrhizal effects on crop yield and soil ecosystem functions in a long-term tillage and fertilization experiment. New Phytol Early view. https://doi.org/10.1111/nph.19493
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. TBMS 55:158–IN18. https://doi.org/10.1016/S0007-1536(70)80110-3
Purin S, Rillig MC (2008) Parasitism of arbuscular mycorrhizal fungi: reviewing the evidence. FEMS Microbiol Lett 279:8–14. https://doi.org/10.1111/j.1574-6968.2007.01007.x
Qin H, Lu K, Strong PJ et al (2015) Long-term fertilizer application effects on the soil, root arbuscular mycorrhizal fungi and community composition in rotation agriculture. Appl Soil Ecol 89:35–43. https://doi.org/10.1016/j.apsoil.2015.01.008
Qiu Q, Bender SF, Said Mgelwa A, Hu Y (2022) Arbuscular mycorrhizal fungi mitigate soil nitrogen and phosphorus losses: a meta-analysis. Sci Total Environ 807:150857. https://doi.org/10.1016/j.scitotenv.2021.150857
R Core Team (2022) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Reque JA, Sarasola M, Gyenge J, Fernández ME (2007) Caracterización silvícola de ñirantales del norte de la Patagonia para la gestión forestal sostenible. Bosque (Valdivia) 28. https://doi.org/10.4067/S0717-92002007000100006
Rúa MA, Antoninka A, Antunes PM et al (2016) Home-field advantage? Evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis. BMC Evol Biol 16:122. https://doi.org/10.1186/s12862-016-0698-9
Rydlová J, Püschel D (2020) Arbuscular mycorrhiza, but not hydrogel, alleviates drought stress of ornamental plants in peat-based substrate. Appl Soil Ecol 146:103394. https://doi.org/10.1016/j.apsoil.2019.103394
Salmeron-Santiago IA, Martínez-Trujillo M, Valdez-Alarcón JJ et al (2021) An updated review on the modulation of carbon partitioning and allocation in arbuscular mycorrhizal plants. Microorganisms 10:75. https://doi.org/10.3390/microorganisms10010075
Sharma A (2017) A review on the Effect of Organic and Chemical fertilizers on plants. IJRASET V 677–680. https://doi.org/10.22214/ijraset.2017.2103
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Elsevier, New York, p 787. https://doi.org/10.1016/B978-0-12-370526-6.X5001-6
Soil Survey Staff (2022) Keys to Soil Taxonomy, 13th edn. USDA-Natural Resources Conservation Service
Tapwal A, Kapoor KS, Thakur Y (2022) Growth enhancement in containerized Pinus gerardiana seedlings inoculated with ectomycorrhizal fungi. Arch Microbiol 204:724. https://doi.org/10.1007/s00203-022-03328-4
Thirkell TJ, Charters MD, Elliott AJ et al (2017) Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J Ecol 105:921–929. https://doi.org/10.1111/1365-2745.12788
Trejo D, Bañuelos J, Gavito ME, Sangabriel-Conde W (2020) High phosphorus fertilization reduces mycorrhizal colonization and plant biomass of three cultivars of pineapple. Terra 38:853–858. https://doi.org/10.28940/terra.v38i4.701
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355. https://doi.org/10.1111/j.1469-8137.2004.01159.x
Tucsen Photonics Co.,Ltd (2019) TCapture. China. www.tucsen.com
Urgiles N, Strauß A, Loján P, Schüßler A (2014) Cultured arbuscular mycorrhizal fungi and native soil inocula improve seedling development of two pioneer trees in the Andean region. New for 45:859–874. https://doi.org/10.1007/s11056-014-9442-8
Verma BC, Pramanik P, Bhaduri D (2020) Organic fertilizers for sustainable soil and Environmental Management. In: Meena RS (ed) Nutrient Dynamics for sustainable crop production. Springer Singapore, Singapore, pp 289–313
Vitousek PM, Naylor R, Crews T et al (2009) Nutrient imbalances in Agricultural Development. Science 324:1519–1520. https://doi.org/10.1126/science.1170261
Wang W, Shi J, Xie Q et al (2017) Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol Plant 10:1147–1158. https://doi.org/10.1016/j.molp.2017.07.012
Wei T, Simko V (2021) R package corrplot: visualization of a correlation matrix. Version 0.92. https://github.com/taiyun/corrplot
Wilkinson KM, Landis TD, Haase DL et al (2014) Tropical Nursery Manual: a guide to starting and operating a nursery for native and traditional plants, vol 732. Department of Agriculture, Forest Service, Washington, DC: U.S
Yang H, Schroeder-Moreno M, Giri B, Hu S (2018) Arbuscular Mycorrhizal Fungi and their responses to Nutrient Enrichment. In: Giri B, Prasad R, Varma A (eds) Root Biology. Soil Biol, vol 52. Springer, Cham. https://doi.org/10.1007/978-3-319-75910-4_17
Yazici MA, Asif M, Tutus Y et al (2021) Reduced root mycorrhizal colonization as affected by phosphorus fertilization is responsible for high cadmium accumulation in wheat. Plant Soil 468:19–35. https://doi.org/10.1007/s11104-021-05041-5
Acknowledgements
To Dr. Ramón A. Martínez and Dr. Ayelén I. Carrón for their invaluable contribution on the substrate’s chemical analyses. To Dr. Javier G. Puntieri and Dr. Paula F. Zermoglio for their insights and reviewing this research. To Gustavo Sánchez and Claudio D’Ambrosio for having donated the Berberis microphylla plants.
Funding
This work was supported by grants from FONCYT (Fondo para la Investigación Científica y Tecnológica, PICT 2018 − 941, PICT 2018–4029, PICT 2019 − 0393), and Universidad Nacional de Río Negro (PI UNRN 2019 40-B-804).
Author information
Authors and Affiliations
Contributions
Conceptualization: FF, SN, NVF and LAG; Methodology: FF, SN and NVF; Investigation: FF, SN and NVF; Formal Analysis: FF; Writing - original draft preparation: FF; Writing - review and editing: SN, NVF and LAG; Funding acquisition: NVF and LAG; Supervision: NVF and LAG.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fioroni, F., Naón, S., Fernández, N.V. et al. The growth and mycorrhization of young Berberis microphylla G. Forst. plants are differently affected by organic and inorganic fertilizers, depending on the substrate. Symbiosis 93, 69–80 (2024). https://doi.org/10.1007/s13199-024-00990-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-024-00990-8