Abstract
Ambispora, the only genus in Ambisporaceae and one of three deeply rooted families in Archaeosporales, Glomeromycetes, is amended. Analysis of the morphology of specimens from types and living cultures and 28S ribosomal DNA (rDNA; LSU) sequences resulted in two major changes that redefined Ambispora to include only species with the potential for spore dimorphism (acaulosporoid and glomoid). First, species described as producing only glomoid spores (Ambispora leptoticha, Ambispora fecundispora, and Ambispora callosa), only acaulosporoid spores (Ambispora jimgerdemannii), or both spore morphotypes (Ambispora appendicula) were synonymized with a redefined dimorphic species, A. leptoticha. LSU sequences and more conserved SSU gene data indicated little divergence between genotypes formerly classified as separate species. Second, Ambispora fennica was synonymized with Ambispora gerdemannii based on morphological and LSU sequence variation equivalent to that measured in the sister clade A. leptoticha. With this analysis, Ambispora was reduced to three species: A. leptoticha, A. gerdemannii, and Ambispora granatensis. Morphological and molecular characters were given equal treatment in this study, as each data set informed and clarified grouping and ranking decisions. The two inner layers of the acaulosporoid spore wall were the only structural characters uniquely defining each of these three species; all other characters were shared. Phenotypes of glomoid spores were indistinguishable between species, and thus were informative only at the genus level. Distinct subclade structure of the LSU gene tree suggests fixation of discrete variants typical of clonal reproduction and possible retention of polymorphisms in rDNA repeats, so that not all discrete genetic variants are indicative of speciation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classification of arbuscular mycorrhizal fungi in the phylum Glomeromycota has undergone numerous changes during the past decade as application of sequence data from ribosomal RNA and beta-tubulin genes, together with some input from morphology, inferred more natural phylogenetic relationships (Redecker and Raab 2006; Msiska and Morton 2009; Oehl et al. 2011; Krüger et al. 2012; Redecker et al. 2013). Some clades were well resolved and highly supported by both morphological and molecular data, so they evoked little controversy. Others were more problematic because of conflicts between traditional morphological characters and ribosomal DNA (rDNA) sequence data. Fungal species in the order Archaeosporales (Schüßler et al. 2001) exemplified some of these problems and the difficulties in resolving them. Dimorphic species, with spore phenotypes bridging two families, created nomenclatural and phylogenetic confusion. Complicating this story were misleading interpretations of degraded type specimens and treating any perceived morphological difference as a species-defining character. Also, use of molecular data to provide independent verification of morphological interpretations was not possible for species lacking living material.
Archaeosporales presently consists of three monogeneric families. Geosiphonaceae contains one nonmycorrhizal species Geosiphon pyriformis (Schüßler et al. 2001; Schüßler 2002). Archaeosporaceae consists of Archaeospora trappei (Morton and Redecker 2001), described first as Acaulospora trappei (Ames and Linderman 1976) and Archaeospora schenckii (Krüger et al. 2012), described first as Entrophospora schenckii (Sieverding and Toro 1987). Ambisporaceae contains eight species in the genus Ambispora (Walker et al. 2007a; Walker 2008), a group that is the focus of this study. Erection, synonymizations, resurrections, and nomenclatural modifications of species in Ambispora are summarized in Fig. 1 to clarify events in a complex timeline.
Species in Ambispora were classified initially by mode of spore formation. Species forming glomoid spores with a subtending hypha were placed in Glomus, and species with acaulosporoid spores attached to a terminal saccule were placed in Acaulospora (Gerdemann and Trappe 1974). Protologue descriptions of Acaulospora gerdemannii (Nicolson and Schenck 1979), Glomus leptotichum (Schenck and Smith 1982), and Glomus fecundisporum (Schenck and Smith 1982) were based on only one spore morphotype. Acaulospora appendicula (Schenck et al. 1984) also was described as monomorphic even though glomoid spores, described as being chlamydospore-like, were mentioned in the protologue. For strains deposited in the International Culture Collection of Vesicular Mycorrhizal Fungi (INVAM) by N.C. Schenck, glomoid and acaulosporoid spores in Acaulospora appendicula FL130A were indistinguishable from those in a dimorphic culture of G. leptotichum FL184B propagated from a single spore of each spore type (Morton et al. 1997). Examination of Oregon State University Herbarium (OSC) holotype and University of Florida (FLAS) isotype specimens revealed a similar result. Morton et al. (1997) then synonymized both Acaulospora and both Glomus species into a dimorphic species classified as Acaulospora gerdemannii to satisfy the criterion of nomenclatural priority. 18S ribosomal RNA (rRNA) gene (SSU) sequences verified dimorphism and also showed that this species was basal to most other glomeromycotan clades (Sawaki et al. 1998; Redecker et al. 2000).
Rose et al. (1979) described Glomus gerdemannii from field-collected spores that were interpreted as possessing glomoid features. Similar novel ornamentations of spore wall layers and subtending hypha were observed on acaulosporoid spores of a dimorphic culture of INVAM accession AU215. This fungus also formed smaller glomoid spores that were phenotypically similar to those of G. leptotichum and type specimens of G. fecundisporum (Morton and Redecker 2001). A small sampling of SSU sequences grouped this species with Acaulospora gerdemannii (Redecker et al. 2000).
Analysis of SSU sequences also showed that Acaulospora trappei was more closely related to the two dimorphic species than to other Acaulospora species, and so Morton and Redecker (2001) placed all three species in a new genus Archaeospora and in a new family Archaeosporaceae. Since G. gerdemannii and Acaulospora gerdemannii shared a common specific epithet, the former was renamed Archaeospora gerdemannii and the latter Archaeospora leptoticha.
Schüßler et al. (2001) used a broader sampling of SSU sequences that included the nonmycorrhizal species G. pyriformis to resolve three distinct monophyletic clades. Geosiphon was placed in its own family, Geosiphonaceae in a new order Archaeosporales. The dimorphic species (Archaeospora leptoticha and Archaeospora gerdemannii) grouped into one clade and Archaeospora trappei into the other. Spain et al. (2006) focused on the dimorphic clade and placed them in a new genus, Appendicispora. At the same time, these workers reversed all four synonymizations by Morton et al. (1997) based on their interpretations of morphological evidence. The dimorphic Archaeospora leptoticha was synonymized as the monomorphic G. leptotichum, and Acaulospora appendicula was resurrected as the dimorphic species Appendicispora appendicula. Acaulospora gerdemannii was restored as a monomorphic acaulosporoid species and renamed Appendicispora jimgerdemannii. G. fecundisporum was resurrected based on perceived differences in type specimens (spore wall outer layer). The dimorphic species Archaeospora gerdemannii was renamed Appendicispora gerdemannii.
Independently, Walker et al. (2007a) erected a new genus Ambispora typified by Ambispora fennica, a dimorphic fungus with SSU and ITS sequences that diverged from those of the other dimorphic species. The acaulosporoid spore was of similar phenotype to that of Archaeospora gerdemannii, but a single, short SSU and an ITS sequence of Archaeospora gerdemannii published by Redecker et al. (2000) did not cluster with Ambispora fennica sequences. These data, then, were used to discriminate the latter as a distinct species. They also transferred Glomus callosum (Sieverding 1988) to Ambispora callosa, relying on two glomoid strains from Japan (OK1, MAFF520057 and V1, MAFF520058) annotated as G. leptotichum in the National Institute of Agricultural Sciences (NIAS) Genebank databases. ITS sequence divergence was used as the basis for separating A. callosa from Ambispora leptoticha.
Two publications with classifications involving the same taxa (Spain et al. 2006; Walker et al. 2007a) created a confusing nomenclature. Walker et al. (2007b) resolved this problem by assigning priority to Appendicispora, erecting a new family Appendicisporaceae, and transferring all Ambispora species to this genus. Appendicispora was determined later to be a homonym, so Ambispora was resurrected and Appendicisporaceae was synonymized with a new family Ambisporaceae (Walker 2008). Palenzuela et al. (2011) then described a new dimorphic species, Ambispora granatensis, based on a combination of morphological and SSU and ITS sequence data. In this study, both comparative morphology and sequences of a 700–750-bp region of the 5′ end of the 28S rRNA (LSU) gene were used to reexamine the eight species currently circumscribed in Ambispora (Fig. 1). Ambispora reticulata has been added more recently (Oehl et al. 2012), but its taxonomic status will be discussed in a separate analysis because there is no evidence it is even a mycorrhizal fungus.
Materials and methods
Specimens
Type materials of Ambispora species consisted of Acaulospora appendicula (OSC 41495, FLAS F53673), Acaulospora gerdemannii (OSC 37514, FLAS F51804), Ambispora fennica (EPITYPE Att550-30 from M. Vestberg), G. fecundisporum (OSC 40250, FLAS F52579), G. gerdemannii (OSC 39476), and G. leptotichum (OSC 40249, FLAS F52577). Spores of G. callosum (OSC 147148) preserved in 5 % formalin were used for both morphological and molecular analyses. Only slide vouchers were available for analysis of A. granatensis (OSC 134712, Z + ZT 1626).
Ambispora leptoticha strains consisted of INVAM accessions used by Morton et al. (1997) and Morton and Redecker (2001) for comparison with type specimens of Acaulospora appendicula, Acaulospora gerdemannii, G. fecundisporum, and G. leptotichum. Living cultures consisted of strains FL130A, FL184B, MX982A, NC169, NC171, NC176, WV109C, and VZ856B. Accessions FL184B and FL130A were identified as reference strains for G. leptotichum and Acaulospora appendicula, respectively, because they were deposited by Schenck, an author of both protologues. Neither strain is designated here as ex-types because provenance with the type pot cultures could not be established unequivocally. Cultures of Ambispora gerdemannii strains consisted of INVAM accessions MT106 and ON205A. The reference strain for Ambispora gerdemannii AU215A used by Redecker et al. (2000) and Morton and Redecker (2001) died before this study began, so only slide vouchers were available for morphological reevaluation.
The plant host used to culture all fungi was Sorghum sudanense (Staph.) Piper, according to protocols described by Morton et al. (1993). Voucher specimens consisted of spores from each culture mounted permanently on glass slides in polyvinyl alcohol-lactic acid-glycerol (PVLG; Koske and Tessier 1983) and PVLG mixed with Melzer’s reagent (1:1, v/v). Specimens were examined with a Nikon Eclipse E600 microscope and photographed with a Nikon DS-Ri1 digital camera. All slides are stored in the INVAM voucher library.
DNA extraction
DNA was extracted from single spores of Ambispora leptoticha and Ambispora gerdemannii crushed in a 0.2-mL microcentrifuge tube containing 14 μL 10× Taq polymerase buffer (New England Biolabs, Ipswich, MA) with a ultraviolet (UV)-sterilized micropestle. Tube contents were transferred to 94 °C water bath for 4 min, snap chilled, and stored on ice until amplification.
Two rounds of PCR amplification were performed to generate enough product of the LSU gene. For the first round of amplification, primer pair ITS1 and NDL22 was used, followed by a nested amplification using primers LR1 and FLR2 (White et al. 1990; van Tuinen et al. 1998; Trouvelet et al. 1999). PCR was performed in 50 μL volumes containing 4 μL spore DNA, 3 μL 10× PCR buffer (New England BioLabs, Ipswich, MA), 0.2 mmol of each dNTP, 1.5 mmol MgCl2, 5 pmol of each primer, and 0.1 μL of Taq DNA polymerase (New England BioLabs, Ipswich, MA). The second round of PCR was similar to the first but used 1.0 μL of first amplification product as template with 7 μL 10× PCR buffer. Cycling parameters for both rounds of amplification entailed initial denaturation at 94 °C for 4 min, 30 cycles at 94 °C for 30 s, annealing at 58 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. PCR products (8 μL) were stained with ethidium bromide and electrophoresed on a 1.5 % agarose gel and visualized by UV illumination. Amplification products were purified with QIAquick PCR Purification kit (Qiagen USA, Valencia, CA) and cloned with pCR®4-TOPO plasmid vector and transformed using One Shot® TOP10 chemically competent cells (Invitrogen, Carlsbad, CA). Positive transformants were verified with colony PCR using LR1/FLR2 primers. Plasmid DNA containing the insert was purified using QIAprep Miniprep kit and DNA sequenced at Davis Sequencing (Davis, CA, USA) using M13 primers. New LSU sequences were deposited in NCBI as accession numbers KC166251–KC166283. Sequence alignment is available at INVAM (http://invam.wvu.edu/). Published LSU sequences of Ambispora appendicula and Ambispora fennica were obtained from the National Center for Biotechnology Information (NCBI) database.
Phylogenetic analyses
Sequence chromatographs were inspected in Biological Sequence Alignment Editor (BioEdit, www.mbio.ncsu.edu/BioEdit/bioedit.html) and subjected to a search on the NCBI nucleotide Basic Local Alignment Search Tool (nBLAST) to verify homology with other glomeromycotan fungal sequences. Nucleotide sequences were aligned using MUSCLE (Edgar 2004). Partial 28S (LSU) rDNA phylogenetic trees were reconstructed using both Bayesian and maximum likelihood (ML) methods. MEGA6 was used to determine the best evolutionary model and nucleotide substitution pattern. The Kimura 2 parameter model plus gamma (K2P + G) had the lowest Bayesian inference criteria (BIC) score and was selected for analyses (Nei and Kumar 2000) with four discrete gamma categories. Bayesian phylogeny reconstruction was carried out in MrBayes 3.2.2 (Ronquist et al. 2011; http://mrbayes.net). Settings included the 4 × 4 general-type DNA model, general time reversible (GTR) substitution model converted to K2P by fixing stationary state frequencies to equal (http://mrbayes.sourceforge.net/wiki), and ploidy set to haploid. The analysis included two runs of 10,000,000 generations with a burn-in of 2,500,000 generations. MrBayes tree with posterior probabilities was visualized in FigTree v1.3.1 (http://tree.bio.ed.ac.uk/software/figtrees). ML method was implemented in MEGA6 with 1000 replications to assess bootstrap support (Tamura et al. 2013).
Results
Molecular analysis
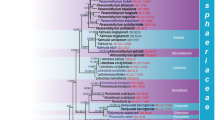
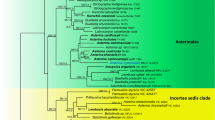
LSU sequences grouped acaulosporoid and glomoid spores of Ambispora leptoticha and Ambispora appendicula strains in a highly supported monophyletic clade, thus providing evidence of conspecificity (Fig. 2). MrBayes consensus tree and ML phylogenetic tree reconstructions had identical topologies. In the absence of living source material, LSU sequences could not be obtained from G. fecundisporum, A. callosa, or A. granatensis.
MrBayes phylogenetic tree reconstructed from partial 28S (LSU) rDNA gene sequences using the Kimura 2 parameter plus gamma (K2P + G) model. Maximum likelihood (ML) tree had identical topology. Bayesian posterior probabilities greater than 0.97 are depicted by thickened branches, and ML bootstrap values greater than 70 % are designated above the branches. Branch labels sequentially specify: spore morphotype (ac acaulosporoid, gl glomoid), source material (spore1, spore2, spore3), INVAM accession, and NCBI accession code. Asterisks, a third party sequence submitted to GenBank and erroneously annotated as Glomus gerdemannii
LSU sequences from the one representative strain of Ambispora fennica grouped with those of two North American strains of Ambispora gerdemannii in a highly supported clade (Fig. 2), but they also formed a distinct subclade. When equivalence in branching pattern and sequence variation within sister clades is used as the criterion to determine rank at the molecular level (Redecker et al. 2013), then Ambispora fennica and Ambispora gerdemannii are conspecific. A third-party LSU sequence of G. gerdemannii AU215 (GenBank accession AJ510233) grouped with Ambispora leptoticha, verifying that this sequence was annotated incorrectly when it was submitted to NCBI (Kaonongbua et al. 2010).
Morphological analyses
All of the Ambispora species described in this paper are potentially dimorphic. Holotype and isotype specimens of many species were parasitized or degraded in a lactophenol storage medium, thus altering color and dimensions of spore wall layers so they no longer matched protologue descriptions. Important diagnostic features still were identifiable for comparative purposes. Range of spore diameters has been reviewed elsewhere (Walker et al. 2007a and references therein).
Glomoid spores
Type specimens (Fig. 3a, b) and living reference culture G. leptotichum FL184B (Fig. 3c, d) were indistinguishable from the phenotypes of glomoid spores from type specimens of G. fecundisporum (Figs. 3e), Acaulospora gerdemannii (Fig. 3f, g), Acaulospora appendicula (Fig. 3h), A. gerdemannii (Fig. 3i, j), and Ambispora granatensis (Fig. 3k, l). Color images of Fig. 3 can be viewed in Online resource 1. Glomoid spores of all Ambispora species described to date, whether examined as type or fresh specimens, possessed a similar bilayered spore wall continuous with a bilayered subtending hyphal wall (Figs. 3 and 4). The outer layer (L1) often thins with age and then appears granular, flaky, or is absent from sloughing. An “alveolate reticulum” of shallow ridges was rarely observed on the surface of the spore wall outer layer of G. leptotichum (Fig. 3a, b), a trait used by Schenck and Smith (1982) and Spain et al. (2006) to distinguish this species from G. fecundisporum (Fig. 3e). The spore wall inner layer (L2) is permanent and was semi-pliable even in preserved specimens because it changed shape with applied pressure in all species and sometimes had a wrinkled inner surface, all of which likely contributed to observed phenotypic variability. Spores also varied in degree of browning (Fig. 3e–g), which is likely an artifact because spores were hyaline to pale yellow in all healthy living cultures. Glomoid spores from Ambispora gerdemannii MT106, and those from isotype specimens of A. granatensis were indistinguishable from glomoid spores of other Ambispora species except that they were smaller, and thus had thinner spore wall layers (Fig. 3i–l). The wide range in spore size observed in a culture of G. leptotichum FL184B (50–280 μm) also was found in vials of glomoid type specimens of G. leptotichum, G. fecundisporum, Acaulospora gerdemannii, and Acaulospora appendicula. Glomoid spores of an A. granatensis in pot culture (Palenzuela et al. 2011) have a smaller size range (40–70 μm) than those of Ambispora gerdemannii MT106/ON205A (40–190 μm), which overlaps into the range of Ambispora leptoticha spores.
Comparative morphology of glomoid spores produced by Ambispora species. All form a bilayered spore wall consisting of a somewhat friable hyaline to pale brown outer layer (L1) and a hyaline semi-pliable inner layer (L2) that is continuous with a bilayered (L1–L2) subtending hyphal wall. a Small specimen of Glomus leptotichum holotype OSC40249. b Typical specimen from G. leptotichum OSC40249. c Spore of Ambispora leptoticha from active culture FL184B. d Spore and subtending hypha of A. leptoticha FL184B. e Spore and subtending hypha of Glomus fecundisporum OSC40250. f Spores and attached hyphae from Acaulospora gerdemannii holotype OSC37514. g Small spore from A. gerdemannii OSC37514. h Transition between spore and subtending hyphal wall of Acaulospora appendicula isotype FLAS F53673. i Spore of Ambispora gerdemannii MT106 with transition between bilayered spore wall and subtending hyphal wall. j Spore of A. gerdemannii ON205. k-l Spores from Ambispora granatensis isotype Z + ZT isotype 55-5503. Bar = 10 μm
Comparative morphology of whole and broken spores of Glomus callosum holotype OSC147148 (a–c) and glomoid spores of Ambispora leptoticha FL184B after 9 months in refrigerated dry storage (d–f). a Whole spores in 5 % formalin (size range, 60–310 μm). b Typical spore with a friable outer layer (L1) and thicker semi-pliable inner layer (L2). c Thinner-walled spore. d Whole spores in water (size range, 72–290 μm). e Typical spore showing semi-pliable phenotype of inner spore wall layer (L2). f Thinner-walled spore. Bar for (a), d = 250 μm, all others = 10 μm
Glomus callosum was reclassified as A. callosa because of SSU relatedness and ITS clade structure (Walker et al. 2007a). Glomoid spores of holotype G. callosum (Fig. 4a–c) did not possess any traits that distinguished this species from fresh spores of G. leptotichum FL184B (Fig. 4d–f), even though type spores had been preserved in 5 % formalin for 15 years. The range in spore size overlapped between species, and all were within the range reported by Walker et al. (2007a). As with other Ambispora species (Fig. 3), the hyaline to pale yellow spore wall of both species was bilayered, with a somewhat friable outer layer and a thicker semi-pliable inner layer (Fig. 4b,c, e–f). Color images of Fig. 4 can be viewed in Online resource 2. In particular, the continuity of bilayered organization in spore and attached hyphal walls was a consistent phenotype shared among glomoid spores of all species in Ambispora. The absence of distinctive morphological traits renders this spore synanamorph taxonomically uninformative by itself at the rank of species.
Acaulosporoid spores
All Ambispora species form a glomoid-like subtending hypha (pedicel) that branches from the neck of a sporiferous saccule, hence the term “acaulosporoid” (Fig. 5a, b). The spore wall in all taxa consists of three discrete layers and a developmentally separate semi-pliable hyaline inner wall (Fig. 5a–c). Only the two inner layers of the spore wall (L2–L3) diverge as discrete and stable phenotypes, and so they are the taxonomically significant morphological traits at the species level. The L2 and L3 layers of the spore wall form hemispherical protrusions and depressions, respectively, in Ambispora leptoticha (Fig. 5a). Both layers are smooth and rigid, but L3 is brittle and breaks into shards that are birefringent in polarized light when crushed in Ambispora gerdemannii (Fig. 5b). Both L2 and L3 are rigid, but L2 is quite thin (<2 μm) with a faintly wrinkled surface in A. granatensis (Fig. 5c). All other characters vary somewhat, but much of that variation is encompassed within and between strains of any given species. The semi-flexible hyaline inner wall (iw) is indistinguishable between species (Fig. 5a–c). Color images of Fig. 5 can be viewed in Online resource 3.
Comparative morphology of acaulosporoid spores produced by known Ambispora species. All form a friable and sloughing outer spore wall layer (L1), with divergence between species expressed in phenotypes of the second (L2), and inner (L3) layers of the spore wall. The middle spore wall layer (L2) is continuous with the wall of the pedicel (P) which branches from the neck of a sporiferous saccule. a A. leptoticha FL130A, b A. gerdemannii ON205A. c A. granatensis Z + ZT isotype 55-5504. A thick hyaline semi-pliable inner wall that forms separately from the spore wall (iw) varies in number and thickness of separable layers, but this phenotypic variation is shared by all three species. Bar = 5 μm
Some traits are overlapping and therefore are not sufficiently informative to separate species. Spain et al. (2006) distinguished Acaulospora gerdemannii (reclassified as Ambispora jimgerdemannii) from Acaulospora appendicula (reclassified as Ambispora appendicula) based on two phenotypes of the outer spore wall layer that tend to degrade and slough readily (Fig. 6a): (i) “cerebriform” folds consisting of prominent ridges that resembled convolutions of a mammalian brain (e.g., Fig. 6b) and (ii) a “crazed” surface consisting of fine friable cracks and fissures that easily crumbled (e.g., Fig. 6c). The cerebriform phenotype documented in the protologue and type specimens of Ambispora jimgerdemannii (Fig. 6b) also was found on spores from Acaulospora appendicula FL130A (Fig. 6d) and G. leptotichum FL184B. From a population of spores from an active culture of FL130A (Fig. 6f), phenotypes from crazed to cerebriform phenotypes were present in a continuum (Fig. 6d–f). Color images of Fig. 6 can be viewed in Online resource 4. The crazed phenotype used to distinguish Acaulospora appendicula also predominated in fresh spores extracted from a culture of Ambispora leptoticha WV109. Moreover, the same continuum of phenotypes chronicled above for FL130A also was present in this pot culture. Clearly, the character states of the outer layer of the spore wall can co-occur as a range of phenotypes because of friability and susceptibility to decomposition. Even absence of the outer two layers of the acaulosporoid spore wall (Fig. 6a) is not unusual because it has been observed in all 19 INVAM accessions of Ambispora leptoticha. Since these character states are neither stable nor consistent, they have no taxonomic significance or relevance at the species level.
Comparative morphology of the acaulosporoid spores of Ambispora species. a Spores of A. leptoticha FL130A in water showing various stages of degradation/sloughing of the outer spore wall layer (OL) exposing the bright white ornamented inner spore wall layer (L3). b Cerebriform phenotype of spore wall outer layer from Acaulospora gerdemannii OSC37514. c Crazed phenotype of spore wall outer layer from Acaulospora appendicula OSC41495. d–f Spores sampled at the same time from an active culture of A. leptoticha FL130A showing variation in phenotypes of the outer spore wall layers. d Crazed phenotype. e Crazed and cerebriform regions of the outer layer on the same spore. f Cerebriform phenotype. Bar for a = 200 μm, for all others = 25 μm
Spore morphology is indistinguishable between Ambispora fennica, Ambispora gerdemannii, and the holotype of G. gerdemannii when population level variation, age, degradation, and preservation artifacts are considered together (Fig. 7; Online resource 5). In all three species, the acaulosporoid spore consists of a three-layered spore wall and a semi-pliable inner wall of separate origin during development (Morton and Redecker 2001). The outer two layers of the spore wall in specimens of both Ambispora fennica (Fig. 7a–c) and Ambispora gerdemannii MT106 (Fig. 7d–f) are adherent and friable, breaking apart readily when crushed, and produce a dark dextrinoid staining reaction in Melzer’s reagent. These two layers vary greatly in appearance depending on degree of degradation or sloughing and can be partly to mostly sloughed or absent in some spores. The inner layer of the spore wall (L3) is novel, with a fracture pattern that can produce sharp-edged shards that are birefringent in polarized light. The thick semi-pliable inner hyaline wall may appear as only one layer, but sometimes separates into with very thin layers on either or both proximal and distal surfaces (Fig. 7c, f).
Equivalence in comparative morphology of acaulosporoid spores of Ambispora fennica and Ambispora gerdemannii. Spores of Ambispora fennica spores mounted on slides labeled Att550-30 from J. Blaszkowski (a–c). a In Melzer’s reagent, showing the dextrinoid reaction of a friable outer spore wall layer (L1), a more permanent second layer (L2), a fracturing rigid inner layer (L3), and a semi-flexible inner wall (iw). b In PVLG, with the outer layer of the spore wall (L1) mostly sloughed. c Spore with all layers of the spore wall (L1–L3) and inner wall (iw) present. Spores of Ambispora gerdemannii strain MT106 (d–f). d In Melzer’s reagent. e In PVLG with only remnants of the outer spore wall layer (L1) present. f All layers of the spore wall (L1–L3) and inner wall (iw) present. Bar = 10 μm.
Analysis of type specimens and phylogenetic analysis by Palenzuela et al. (2011) support A. granatensis as a discrete species in the Ambispora. No LSU sequences were available for analysis of A. granatensis in this study, but SSU phylogeny positioned this species as a monophyletic clade in the genus (Palenzuela et al. 2011). Phylogenetic tree branch lengths reported in their analysis suggest greater sequence variation than has been measured in other Ambispora species. Type material verified that A. granatensis is a dimorphic species with glomoid spore types (Fig. 3k, l) similar in phenotype to those produced by other Ambispora species and with acaulosporoid spores having a unique phenotype of the L2 and L3 spore wall layers (Fig. 5c).
Revised classification
Ambisporaceae C. Walker, Vestberg & Schüßler emend. R.J. Bills & J.B. Morton
Synonym: Appendicisporaceae C. Walker, Vestberg & Schüßler, Mycol. Res. 111:254 (2007); nom. illegit. (Arts 53.1, 53.3)
Typus: Ambispora (C. Walker, Vestberg & Schüßler) R.J. Bills & J.B. Morton
Arbuscular mycorrhizal fungi producing dimorphic propagules of both glomoid and acaulosporoid spores, although fungal genotypes and environment impact on expression of either or both spore types. Spores formed singly or in clusters, from the terminal tip of a sporiferous hypha (glomoid snynanamorph) or subtending an acaulosporoid spore (acaulosporoid synanamorph). Glomoid spores are hyaline with bilayered spore and hyphal walls lacking any discrete species-level characters. Acaulosporoid spores formed from a pedicel branching from the neck of a hyaline sporiferous saccule. Acaulosporoid spores possess a three-layered spore wall and a separate semi-pliable hyaline inner wall with layers of variable number and thickness. Spore wall outer layer tends to degrade and slough with age, and the middle and inner layers of the spore wall express unique phenotypes that differentiate species. Distinguished from other families in the Archaeosporales by potential dimorphism, unique acaulosporoid spore morphology, and monophyly derived from rDNA sequence variation.
Ambispora C. Walker, Vestberg & Schüßler emend. R. Bills & J.B. Morton
Type species: Ambispora gerdemannii (S.L. Rose, B.A. Daniels & Trappe) R. Bills & J.B. Morton, comb. nov.
Dimorphic arbuscular mycorrhizal fungi capable of producing either or both glomoid and acaulosporoid spores. Glomoid spores hyaline with bilayered spore and subtending hyphal walls lacking discrete species-level characters; formed singly or in clusters, from the terminal tip of a sporiferous hypha or hyphae subtending an acaulosporoid spore. Acaulosporoid spores formed from a pedicel branching from the neck of a hyaline sporiferous saccule, each with a spore wall consisting of three layers: a degradable and sloughing outer layer, a middle layer developing from a bilayered pedicel, and a rigid inner layer. The inner two layers of the acaulosporoid spore wall are unique in each species. An inner semi-pliable multi-layered hyaline wall forms independent of the spore wall and is indistinguishable among species.
Ambispora leptoticha (N.C. Schenck & G.S. Sm.) R.J. Bills & J.B. Morton comb. nov. (Figs. 2, 3a–h, 4, 5a, and 6)
The species is described in Morton and Redecker (2001). It is distinct from other species in the genus Ambispora by its rDNA characteristics (Fig. 2) and acaulosporoid spore wall morphology (Figs. 5a and 6).
Basionym: Acaulospora gerdemannii N.C. Schenck & T.H. Nicolson, Mycologia 71:193 (1979).
Acaulospora appendicula Spain, Sieverd. & N.C. Schenck, Mycologia 76:686 (1984).
Glomus leptotichum N.C. Schenck & G.S. Sm., Mycologia 74:82–83 (1982).
Glomus fecundisporum N.C. Schenck & G.S. Sm., Mycologia 74:81 (1982).
Synonyms: Archaeospora leptoticha (N.C. Schenck & G.S. Sm.) J.B. Morton & D. Redecker, Mycologia 93:184 (2001).
Appendicispora leptoticha (N.C. Schenck & G.S. Sm.) C. Walker, Vestberg & Schüβler, Mycol. Res. 111:255 (2007).
Pseudoglomus leptotichum (N.C. Schenck & G.S. Sm.) S.P. Gautam & U.S. Patel, The Mycorrhizae, Diversity, Ecology and Applications (Delhi):5 (2007).
Ambispora leptoticha (N.C. Schenck & G.S. Sm.) C. Walker, Mycol. Res. 112:297 (2008).
Appendicispora jimgerdemannii (N.C. Schenck & T.H. Nicolson) Spain, Oehl & Sieverd., Mycotaxon 97:176 (2006).
Ambispora jimgerdemannii (Spain, Oehl & Sieverd.) C. Walker, Mycol. Res. 112:298 (2008).
Appendicispora fecundispora (N.C. Schenck & G.S. Sm.) C. Walker, Vestberg & Schüßler, Mycol. Res. 111:254 (2007).
Ambispora fecundispora (N.C. Schenck & G.S. Sm.) C. Walker, Mycol. Res. 112:298 (2008).
Glomus callosum Sieverd., Angew. Botanik 62:374 (1988).
Appendicispora callosa (Sieverd.) C. Walker, Vestberg & Schüßler, Mycol. Res. 111:254 (2007).
Ambispora callosa (Sieverd.) C. Walker Mycol. Res. 112:298 (2008).
Appendicispora appendicula (Spain, Sieverd. & N.C. Schenck) Spain, Oehl & Sieverd., Mycotaxon 97:170 (2006).
Paracaulospora appendicula (Spain, Sieverd. & N.C. Schenck) S.P. Gautam & U.S. Patel, The Mycorrhizae, Diversity, Ecology and Applications (Delhi):5 (2007).
Ambispora appendicula (Spain, Sieverd. & N.C. Schenck) C. Walker Mycol. Res. 112:298 (2008).
Ambispora gerdemannii (S.L. Rose, B.A. Daniels & Trappe) R.J. Bills & J.B. Morton comb. nov. (Figs. 2, 3i, j, 5b, and 7).
LSU sequence data (Fig. 2) and acaulosporoid spore wall morphological (Figs. 5b, 7) together provide strong support that Ambispora fennica and Ambispora gerdemannii are conspecific and distinct from other species in Ambispora. Both Ambispora fennica and Ambispora gerdemannii are dimorphic in living cultures (Morton and Redecker 2001; Spain et al. 2006; Walker et al. 2007a).
Basionym: Glomus gerdemannii S.L. Rose, B.A. Daniels & Trappe, Mycotaxon 8:297 (1979).
Synonyms: Archaeospora gerdemannii (S.L. Rose, B.A. Daniels & Trappe) J.B. Morton & D. Redecker, Mycologia 93:186 (2001).
Appendicispora gerdemannii (S.L. Rose, B.A. Daniels & Trappe) Spain, Oehl & Sieverd., Mycotaxon 97:174 (2006).
Ambispora gerdemannii (S.L. Rose, B.A. Daniels & Trappe) C. Walker, Vestberg & Schüßler, Mycol. Res. 111:148 (2007).
Ambispora gerdemannii (S.L. Rose, B.A. Daniels & Trappe) C. Walker, Mycol. Res. 112:298 (2008).
Ambispora fennica C. Walker, Vestberg & Schüßler, Mycol. Res. 111:148 (2007).
Appendicispora fennica (C. Walker, Vestberg & Schüßler) C. Walker, Vestberg & Schüßler, Mycol. Res. 111:254 (2007).
Ambispora fennica (C. Walker, Vestberg & Schüßler) C. Walker, Mycol. Res. 112:298 (2008).
Epitype: Ambispora gerdemannii INVAM accession MT106 submitted by C. Rosier, University of Montana, Missoula, MT, March 26, 2003.
Discussion
The proliferation of species based on weak or selective evidence jeopardizes the information content of a classification and how that information may be utilized in comparative studies. The taxonomic history of taxa that now comprise Ambispora, the only genus in the family Ambisporaceae, exemplifies this issue. As an outcome of this study, eight species classified in Ambispora have been reduced to three species based on a congruent combination of mutually supportive morphological and rDNA evidence.
The redefinition of Ambispora leptoticha to include Ambispora fecundispora, Ambispora appendicula, and Ambispora jimgerdemannii comes full circle, representing a return to the original species hypothesis of Morton and Redecker (2001). In addition, a reinterpretation of type material and published SSU/ITS sequences of A. callosa indicates conspecificity with Ambispora leptoticha. The four species resurrected from Archaeospora leptoticha by Spain et al. (2006) relied on their interpretations of morphology, which were based on three assumptions shown in this study to be incorrect. First, acaulosporoid spores of putatively monomorphic Ambispora jimgerdemannii were distinguished from dimorphic Ambispora appendicula based on the perception that “cerebriform” and “crazed” phenotypes in type material were novel, stable, and discrete enough to differentiate species. Comparative analysis of these spores in 19 INVAM accessions of Ambispora leptoticha propagated over a 20-year period indicated that both phenotypes are extremes in a continuum of variation. These characters, therefore, are not informative either taxonomically or phylogenetically. Instead, they represent population-level variation associated with age, senescence, or environmental degradation. A similar perception was used to separate Ambispora fecundispora (as G. fecundisporum) from Ambispora leptoticha (as G. leptotichum), based on whether the surface appearance of the spore wall outer layer was “reticulate” (former) or not (latter). This layer is somewhat friable and undergoes some degradation, so a range of variation is expressed that include both phenotypes. The synonymization of each pair of species remains strictly morphological in the study here, because ex-type materials of Ambispora jimgerdemannii and Ambispora fecundispora have never been available to test hypotheses of gene phylogeny. Records indicate that no cultures were established in INVAM (as Acaulospora gerdemannii, G. fecundisporum) when the collection was curated by N.C. Schenck, one of the authors of both protologues.
Secondly, Spain et al. (2006) viewed consistent sporulation of only one spore morphotype as indicative of a strict monomorphic habit. The absence of the acaulosporoid morphotypes in cultures of G. fecundisporum and G. leptotichum is not proof, however, that this behavior does not exist because expression of dimorphism is both unpredictable and highly variable. For example, Acaulospora appendicula FL130A has produced varying frequencies of both morphotypes over 17 propagation cycles (Morton et al. 1997; Redecker et al. 2000; Morton and Redecker 2001). Another strain, Ambispora leptoticha VZ856, sporulates only as the acaulosporoid morph (Morton et al. 1997; Morton and Redecker 2001). In contrast, G. leptotichum FL184B has sporulated predominantly, and sometimes exclusively, as the glomoid morph over 16 propagation cycles spanning 22 years (Morton et al. 1997; Morton and Redecker 2001). Moreover, several acaulosporoid spores were present in the type specimen of G. leptotichum that provides physical evidence of a dimorphic habit.
Similar problems were encountered in interpreting the taxonomy of A. callosa, which has had a confusing history. When Sieverding (1988) described G. callosum, dimorphism in Glomeromycota had not yet been discovered. Therefore, if acaulosporoid spores were present, they likely were interpreted as a different species rather than as part of the same organism. Subsequent cultures (Kojima et al. 2004; Walker et al. 2007a) were exclusively monomorphic, but as stated earlier, presence or absence of dimorphism alone cannot reliably discriminate this species because unidentified developmental or environmental variables may suppress one morphotype or the other. Walker et al. (2007a) relied on two glomoid strains from Japan (OK1, MAFF520057 and V1, MAFF520058) annotated as G. leptotichum in the National Institute of Agricultural Sciences (NIAS) GenBank databases. Kojima et al. (2004) were the first to report that SSU sequences from these strains were identical to a dimorphic Archaeospora leptoticha (=Ambispora leptoticha) isolate F3b. Yet, prior to this study, all revisions of this species from its erection by Sieverding (1988) as G. callosum and later transfer to A. callosa were based on SSU and ITS sequences (Stockinger et al. 2010; Palenzuela et al. 2011). However, these data are not congruent because SSU sequences grouped the species with Ambispora leptoticha and ITS sequences grouped the species separately (Walker et al. 2007a). The ITS region alone sometimes provides poor resolution of species-level relationships in Glomeromycota because of its variability, as evidenced in this group when equivalence in clade structure is used as the ranking criterion (Krüger et al. 2012).
Thirdly, Spain et al. (2006) perceived any morphological differences between sampled populations as sufficient criteria to group and rank species. Some phenotypic differences (novelties), no matter how stable or discrete they might be, do not reflect speciation events at all but instead indicate fixation of character variants that evolved as disjunct clonal populations (Morton and Msiska 2010a, b; vanKuren et al. 2013). One example is intercalary spore formation, which was considered unique to G. fecundisporum. However, this trait is an example of a minor character so rare that it isn’t even represented in type material.
Analysis of G. fecundisporum posed difficulties. Even though the species was described from cultures by Schenck and Smith (1982), no representative living cultures exist. Records indicate this species was never deposited as a coded accession in Florida-INVAM or in any other lab. Hence, comparative morphological evidence rested only with the type material, which was in poor condition. Still, phenotypic variation in glomoid spores overlapped with that of spores from other merged species.
The revision of Ambispora gerdemannii in this study corrects the interpretation of Ambispora fennica as a separate species. Walker et al. (2007a) described Ambispora fennica as being “very close morphologically” to Ambispora gerdemannii and the comparative evidence provided in this study goes further and concludes they are indistinguishable. Dimorphism is well established, and the glomoid morph shares all of the traits of the glomoid spores of Ambispora leptoticha except that the size range is smaller.
The separation of Ambispora fennica from Ambispora gerdemannii by Walker et al. (2007a, b) and Walker (2008) was based on SSU sequence data. This action warrants discussion because it highlights two common problems associated with molecular data: (i) misannotations in public databases and (ii) undersampling of taxa. These workers relied on a single divergent SSU sequence annotated erroneously in NCBI as Ambispora gerdemannii. INVAM accession AU215 produced spores that matched the phenotype of Ambispora gerdemannii, but the representative sequences submitted to NCBI were actually from Ambispora leptoticha. Cultures of this accession died before the discrepancy could be investigated. Second, sample size greatly influences tree topology and thus ranking decisions (Pollock et al. 2002; Heath et al. 2008), and sequences from Ambispora fennica were limited to a spore from one strain (Walker et al. 2007a; Walker 2008; Krüger et al. 2012). When additional SSU sequences from Ambispora gerdemannii INVAM MT106 and Chinese strain n8_9 (JF439210) were added in an analysis by Krüger et al. (2012), these strains grouped with Ambispora fennica. In this study, Ambispora gerdemannii LSU sequences and those from one population of Ambispora fennica placed both taxa in a monophyletic clade and as an equivalent sister group to Ambispora leptoticha. The topology of Ambispora fennica as a distinct subclade is likely an artifact of limited sample size (both in number of targeted populations and the number of specimens sampled). All sequences from Ambispora fennica were obtained from transformant clones of a single spore (Krüger et al. 2012), and thus may not even represent the scope of genetic variation in the source population. A similar distinct topology could be generated within Ambispora leptoticha when only selected strains were analyzed (result not shown). The genetic distance observed in the Ambispora gerdemannii clade is low, being much less than that of strains representing species clades in other genera such as Paraglomus occultum (this study), Acaulospora paulinae (Kaonongbua et al. 2010), Claroideoglomus etunicatum (van Kuren et al. 2013), and Dentiscutata heterogama (Morton and Msiska 2010b).
Although no LSU sequence analysis was performed on A. granatensis, enough molecular and morphological evidence exists to support the retention of A. granatensis as a phylogenetically discrete species in Ambispora. An SSU gene tree grouped A. granatensis in a monophyletic clade with greater sequence variation than clades that grouped sequences from Ambispora fennica and the A. callosa/Ambispora appendicula clade (Palenzuela et al. 2011). At the organismal level, A. granatensis is dimorphic in pot cultures (Palenzuela et al. 2011). Glomoid spores possess the same bilayered spore wall and subtending hyphae phenotype shared by other species in Ambispora. Acaulosporoid spores shared the same three-layered spore wall and hyaline multilayered semi-pliable inner wall, and uniqueness was expressed in predictable divergent inner layers of the spore wall.
Phylogenetic analysis of morphology relies on shared derived characters (synapomorphies) (Hillis 1987; Davis and Nixon 1992) of discrete and stable spore phenotypes (Morton 1990; Morton and Msiska 2010a). Results of this study indicate that novel synapomorphies separating species described to date reside in divergent phenotypes of the inner two layers of the acaulosporoid spore wall. This pattern follows a more general one in Glomeromycota, where essential species-level traits of AMF, regardless of clade, are found mostly in spore wall characters (Morton 1995; Stürmer and Morton 1997, 1999). Unlike other glomeromycotan clades, however, Ambispora species have a capacity for dimorphism and the glomoid morphotype appears to lack any capacity for divergence as discrete and stable traits. Possibly, genes involved specifically in glomoid spore formation are historically constrained, and there is little pressure to select for any emergent variants or alternatively, the phenotypic space is too narrow for expression of new and distinctive variation.
This study reveals some of the inherent difficulties in systematic interpretations of evidence at all levels. Comparative morphology relies on a broad enough sampling of specimens to distinguish variation between populations versus species so that the former are not mistaken for the latter (Morton and Msiska 2010b; Redecker et al. 2013). That was not feasible by Spain et al. (2006) in resurrecting four species from Archaeospora leptoticha because of a limited range of specimens. Equally important, however, are analyses that identify those morphological characters which provide unique and consistent markers of speciation. Other phenotypic differences that are the product of mutation events fixed readily because of clonal reproduction in populations must be excluded, and nowadays gene sequence analysis contribute to exposing these traits (Kaonongbua et al. 2010; Morton and Msiska 2010b).
Similar considerations apply to comparative molecular data, either for rDNA gene repeats where concerted evolution is a critical process in homogenizing variants or where duplicate copies of protein-encoding genes exist (Morton 2009; Msiska and Morton 2009). Linked rRNA gene polymorphisms are present in C. etunicatum strains that form a distinct clade, but they do not disrupt monphyly of the species (VanKuren et al. 2013). The polymorphisms appear to be maintained in disjunction nucleoli, with concerted evolution occurring within each localized rRNA gene array. Phylogeny of the beta-tubulin gene also reveals distinct subclade structure in D. heterogama (Msiska and Morton 2009), but there is no evidence of divergent paralogs. Rather, the discovery of this genetic diversity is attributed to extensive sampling of transformant clones among a range of fungal strains. As was done in this study, resolution of such conflicts resides in consideration of available evidence at all scales.
References
Ames RN, Linderman RG (1976) Acaulospora trappei sp. nov. Mycotaxon 3:565–569
Davis JI, Nixon KC (1992) Populations, genetic variation, and the delimitation of phylogenetic species. Syst Biol 41:421–435
Edgar RC (2004) MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:1–19
Gautam SP, Patel US (2007) Rhizoendomutualmycota (REMM): a new phylum for the farmers’ friend number one. In: Tiwari M, Sati SC (eds) The Mycorrhizae: diversity, ecology and applications. Daya Pub. House, Delhi, pp pp. 1–pp. 13
Gerdemann JW, Trappe J (1974) The Endogonaceae in the Pacific Northwest. Mycol Mem 5:1–76
Heath TA, Hedtke SM, Hillis DM (2008) Taxon sampling and the accuracy of phylogenetic analyses. J Syst Evol 46:239–257
Hillis DM (1987) Molecular versus morphological approaches to systematics. Ann Rev Ecol Syst 18:23–42
Kaonongbua W, Morton JB, Bever JD (2010) Taxonomic revision transferring species in Kuklospora to Acaulospora (Glomeromycota) and a description of Acaulospora colliculosa sp. nov. from field collected spores. Mycologia 102:1497–1509
Kojima T, Sawaki H, Saito M (2004) Detection of arbuscular mycorrhizal fungi, Archaeospora leptoticha, and related species colonizing plant roots by specific PCR primer. Soil Sci Plant Nutr 50:95–101
Koske RE, Tessier B (1983) A convenient, permanent slide mounting medium. Mycol Soc Amer Newslett 34:59
Krüger M, Krüger C, Walker C, Stockinger H, Schüssler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984
Morton JB (1990) Evolutionary relationships among arbuscular mycorrhizal fungi in the Endogonaceae. Mycologia 82:192–207
Morton JB (1995) Taxonomic and phylogenetic divergence among five Scutellospora species (Glomales, Zygomycetes) based on comparative developmental sequences. Mycologia 87:127–137
Morton JB (2009) Reconciliation of conflicting morphological and rRNA gene phylogenies of fungi in Glomeromycota based on underlying patterns and processes. In: Azcon-Aguilar C, Barea JM, Gianinazzi S, Gianinazzi-Pearson V (eds) Mycorrhizas—functional processes and ecological impact. Springer-Verlag, Berlin, pp 137–154
Morton JB, Msiska Z (2010a) Phylogenies from genetic and morphological characters do not support a revision of Gigasporaceae (Glomeromycota) into four families and five genera. Mycorrhiza 19:501–513
Morton JB, Msiska Z (2010b) Ontogeny and phylogeny of Scutellospora heterogama mutant, with implications for morphological recognition of species in Glomeromycota. Fungal Biol 114:410–420
Morton JB, Redecker D (2001) Two new families of Glomales, Archaeosporaceae and Paraglomeraceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93:181–195
Morton JB, Bentivenga SP, Wheeler WW (1993) Germ plasm in the International Collection of Arbuscular and Vesicular-arbuscular Mycorrhizal Fungi (INVAM) and procedures for culture and development, documentation and storage. Mycotaxon 48:491–528
Morton JB, Bever JD, Pfleger FL (1997) Taxonomy of Acaulospora gerdemannii and Glomus leptotichum, synamorphs of an arbuscular mycorrhizal fungus in Glomales. Mycol Res 101:625–631
Msiska Z, Morton JB (2009) Phylogenetic analysis of the Glomeromycota by a partial β-tubulin gene. Mycorrhiza 19:247–254
Nei M, Kumar S (2000) Molecular Evolution and Phylogenetics. New York, Oxford University Press
Nicolson TH, Schenck NC (1979) Endogonaceous mycorrhizal endophytes in Florida. Mycologia 71:178–198
Oehl F, Sieverding E, Palenzuela J, Ineichen K, da Silva GA (2011) Advances in Glomeromycota taxonomy and classification. IMA Fungus 2:191–199
Oehl F, Castillo C, Schneider D, Säle V, Sieverding E (2012) Ambispora reticulata, a new species in the Glomeromycota from mountainous areas in Switzerland and Chile. J Appl Bot Food Qual 85:129–133
Palenzuela J, Barea J, Ferrol N, Oehl F (2011) Ambispora granatensis, a new arbuscular mycorrhizal fungus, associated with Asparagus officinalis in Andalucia (Spain). Mycologia 103:333–340
Pollock DD, Zwickl DJ, McGuire JA, Hillis DM (2002) Increased taxon sampling is advantageous for phylogenetic inference. Syst Biol 51:664–671
Redecker D, Raab P (2006) Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): Recent developments and new gene markers. Mycologia 98:885–895
Redecker D, Morton JB, Bruns TD (2000) Ancestral lineages of arbuscular mycorrhizal fungi (Glomales). Mol Phylogenet Evol 14:276–284
Redecker D, Schüßler A, Stockinger H, Stürmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model across a large model space. Syst Biol 61:539--542
Rose S, Daniels BA, Trappe JM (1979) Glomus gerdemannii sp. nov. Mycotaxon 8:297–301
Sawaki H, Sugawara K, Saito M (1998) Phylogenetic position of an arbuscular mycorrhizal fungus, Acaulospora gerdemannii, and its synamorph Glomus leptotichum, based on 18S rRNA gene sequence. Mycoscience 39:477–480
Schenck NC, Smith GS (1982) Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 74:77–92
Schenck NC, Spain JL, Sieverding E, Howeler RH (1984) Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76:685–699
Schüßler A (2002) Molecular phylogeny, taxonomy, and evolution of Geosiphon pyriformis and arbuscular mycorrhizal fungi. Plant Soil 244:75–83
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: evolution and phylogeny. Mycol Res 105:1413–1421
Sieverding E (1988) Two new species of vesicular arbuscular mycorrhizal fungi in the Endogonaceae from tropical highlands of Africa. Angew Botanik 62:373–380
Sieverding E, Toro S (1987) Entrophospora schenckii, a new species in the Endogonaceae from Colombia. Mycotaxon 28:209–295
Spain JL, Sieverding E, Oehl F (2006) Appendicispora: a new genus in the arbuscular mycorrhiza-forming Glomeromycetes, with a discussion of the genus Archaeospora. Mycotaxon 97:163–182
Stockinger H, Krüger M, Schüßler A (2010) DNA barcoding of arbuscular mycorrhizal fungi. New Phytol 187:461–474
Stürmer SL, Morton JB (1997) Developmental patterns defining morphological characters in spores of species in Glomus (Glomales, Zygomycetes). Mycologia 89:72–81
Stürmer SL, Morton JB (1999) Scutellospora rubra, a new arbuscular mycorrhizal species from Brazil. Mycol Res 103:949–954
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetic analyses version 6.0. Mol Biol Evol 30:2725–2729
Trouvelet S, van Tuinen D, Hijri M, Gianinazzi-Pearson V (1999) Visualization of ribosomal DNA loci in spore interphasic nuclei of glomalean fungi by fluorescence in situ hybridization. Mycorrhiza 8:203–206
van Tuinen D, Jacquot E, Zhao B, Gollotte A, Gianinazzi-Pearson V (1998) Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol Ecol 7:879–887
vanKuren NW, den Bakker HC, Morton JB, Pawlowska TE (2013) Ribosomal RNA gene diversity, effective population size, and evolutionary longevity in asexual Glomeromycota. Evolution 67:207–224
Walker C (2008) Ambispora and Ambisporaceae resurrected. Mycol Res 112:297–298
Walker C, Vestberg M, Demircik F, Stockinger H, Saito M, Sawaki H, Nishmura I, Schüßler A (2007a) Molecular phylogeny and new taxa in the Archaeosporales (Glomeromycota): Ambispora fennica gen. sp. nov., Ambisporaceae fam. nov., and emendation of Archaeospora and Archaeosporaceae. Mycol Res 111:137–153
Walker C, Vestberg M, Schüßler A (2007b) Nomenclatural clarification in Glomeromycota. Mycol Res 111:253–256
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: A guide to methods and applications. Academic Press, San Diego, pp 315–322
Acknowledgments
The authors thank Bill Wheeler for assistance in growing and processing AMF cultures. We also appreciate the assistance of Dr. Donna Ford-Werntz (curator of the WVU Herbarium), Dr. Richard Halse (curator Oregon State University Herbarium), Reinhard Berndt (curator of fungus collections herbaria Z + ZT Zürich, Switzerland), Janus Blaszkowski, Jim Bever, and Wittaya Kaonongbua for providing herbarium and other specimens. Funding support was provided by National Science Foundation grants DBI0650735 and DEB0649341 to Joseph Morton.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bills, R.J., Morton, J.B. A combination of morphology and 28S rRNA gene sequences provide grouping and ranking criteria to merge eight into three Ambispora species (Ambisporaceae, Glomeromycota). Mycorrhiza 25, 485–498 (2015). https://doi.org/10.1007/s00572-015-0626-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0626-7