Abstract
The sensu stricto concept of the large and polyphyletic genus Ellisembia is revealed based on a recent collection of its type species, E. coronata, on the original host at the type locality in Germany. Phylogenetic analyses of concatenated ITS, LSU, and RPB2 sequence data suggest that the fungus belongs to Sporidesmiaceae (Sordariomycetes) where it groups together with other morphologically similar ellisembia-like taxa in a distinct monophyletic lineage distant from Sporidesmium. Ellisembia is therefore restricted to those members of this novel group having distoseptate conidia and producing none or a few percurrent conidiophore extensions. Its previous synonymy under Sporidesmium is revised, and four novel combinations are proposed including E. pseudobambusae comb. nov., recently collected on a dead branch of Arundinaria sp. (Poaceae) in Texas, USA. The holotype illustration of S. coronatum, the basionym of E. coronata, is considered ambiguous due to the depiction of eusepta instead of distosepta. Consequently, Ellisembia is epitypified with the fresh specimen from Germany after comparing it with authentic materials preserved at G and IMI. Additionally, the genus Lomaantha, typified by L. pooga, is expanded and emended to include E. brachypus and related ellisembia-like taxa grouping together in a distinct lineage within Chaetosphaeriaceae (Sordariomycetes) distant from Sporidesmiaceae. A reassessed taxonomy for members of this monophyletic clade is proposed including six new combinations, and the presence of distinct pores in their conidial distosepta was evaluated. Sporidesmiella angustobasilaris, which typifies the genus Anasporidesmiella, is reduced to synonymy of L. folliculata upon examination of its type material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The taxonomy of Sporidesmium Link has long been a contentious issue especially since Subramanian (1992) rearranged its many taxa in different genera based on the type of conidial septation, presence or absence of conidiophores and whether they extend percurrently including certain characteristics of these extensions. However, these morphological features have been repeatedly shown to be irrelevant for phylogenetically delineating these genera (Shenoy et al. 2006; Su et al. 2016; Yang et al. 2018; Wu and Diao 2022). One of these segregated genera is Ellisembia Subram. based on S. coronatum Fuckel. The genus was originally defined as having distoseptate conidia and conidiophores lacking or with rare percurrent extensions. Ellisembia is presently known to be polyphyletic and species with available molecular data are currently placed in three sordariomycetous families, Chaetosphaeriaceae, Distoseptisporaceae, Sporidesmiaceae, and the order Xylariales (Hyde et al. 2019).
The family Sporidesmiaceae was resurrected to accommodate some taxa with available molecular data and morphology similar to that of S. ehrenbergii M.B. Ellis, the lectotype species of Sporidesmium (Su et al. 2016). The clade they formed was used to define the genus; however, this concept is still a working hypothesis as DNA sequence data is missing for the lectotype (Delgado et al. 2018). Some ellisembia-like species with distoseptate conidia were revealed to belong to this lineage. Therefore, Su et al. (2016) synonymized Ellisembia under Sporidesmium and Subramanian’s concept of Sporidesmium, originally restricted to euseptate taxa, was expanded to include distoseptate species such as E. bambusicola (M.B. Ellis) J. Mena & G. Delgado and E. minigelatinosa (Matsush.) W.P. Wu. Authors such as Hyde et al. (2019), Yang et al (2021) and Wu and Diao (2022), however, preferred to keep Ellisembia as a separate genus until molecular data become available for the type species of both genera.
Chaetosphaeriaceae, on the other hand, comprises an array of morphologically diverse, mostly phialidic anamorphs but also ellisembia-like hyphomycetes (Réblová and Winka 2000; Réblová et al. 2020; Wu and Diao 2022). Members of this latter group are characterized by pigmented, distoseptate conidia, macronematous conidiophores with or without percurrent extensions and teleomorphs with multiseptate, versicolorous ascopores in asci with a non-amyloid apical annulus, persistent paraphyses, and immersed ascomata (Hyde et al. 2019). Réblová and Winka (2001) first described the teleomorph-typified ascomycete Lecythothecium (Le.) duriligni Réblová & Winka with a Sporidesmium folliculatum (Corda) E.W. Mason & S. Hughes anamorph and placed it in Chaetosphaeriaceae using LSU sequence data. Later, Shenoy et al. (2006) revealed that E. brachypus (Ellis & Everh.) Subram. clustered with Le. duriligni in a strongly supported clade within Chaetosphaeriales following a comprehensive phylogenetic assessment of sporidesmium-like fungi using nuclear ribosomal and RPB2 sequence data. Magyar et al. (2011) described a distinct anamorph isolated from grapevine and tree bark in Hungary that was placed sister to Le. duriligni based on analysis of LSU sequences. They introduced the monotypic genus Pyrigemmula D. Magyar & Shoemaker, with P. aurantiaca D. Magyar & Shoemaker as the type species, to accommodate it. Subsequent phylogenetic studies dealing with novelties or peripheral genera belonging to Chaetosphaeriaceae have consistently shown that the E. brachypus-Lecythothecium-Pyrigemmula clade is monophyletic. Hyde et al. (2019) described a second holomorphic member of this lineage, E. aurea Réblová & J. Fourn., also with an ellisembia-like anamorph. They recommended using the name Ellisembia for species placed in this clade until the systematic position of E. coronata (Fuckel) Subram. is revealed, an approach followed by Wu and Diao (2022) and Yang et al. (2023) but not Réblová and Nekvindová (2023). Hyde et al. (2019) also suggested that members of this clade are congeneric and could be accommodated into the morphologically similar Pyrigemmula once the distant position of the type species of Ellisembia is confirmed. Following the abovementioned criteria, the monotypic Le. duriligni has been treated under the name of its anamorph E. folliculata (Corda) Subram. in recent phylogenetic studies (Réblová et al. 2021a, b; Zhang et al. 2022a, b). Other authors, however, continue using its original name of Le. duriligni (Zheng et al. 2020, 2021; Yan et al. 2023). Similarly, Luo et al. (2019) collected E. brachypus on submerged decaying wood in China and retained its name in Sporidesmium following Su et al. (2016) who treated Ellisembia as its synonym. Taxonomic novelties described or reported without molecular data and phylogenetic position continue being placed in Ellisembia following the traditional morphological concept (Xia et al. 2016, 2017; Qiao et al. 2017, 2018; Kuo and Goh 2019; Ma et al. 2020; Pereira et al. 2022), whereas ellisembia-like taxa mostly resembling E. adscendens (Berk.) Subram. are placed in Distoseptisporaceae (Su et al. 2016; Luo et al. 2018, 2019; Yang et al. 2018, 2021; Zhang et al. 2022a, b; Afshari et al. 2023).
These different approaches in naming ellisembia-like taxa, particularly in Chaetosphaeriaceae, are rather confusing and reflect the need for a consensus on the taxonomy of these lineages with a relatively stable molecular phylogeny. At the center of this counterproductive situation is the absence of molecular data for the generic type, E. coronata, and the lack of information on its phylogenetic placement in Pezizomycotina. A first attempt in this direction was recently implemented by Wu and Diao (2022), who further expanded Ellisembia to include Lecythothecium and Pyrigemmula as its synonyms following the recommendation in Hyde et al. (2019). Wu and Diao also placed Lomaantha (L.) Subram. (Subramanian 1954) for the first time in Chaetosphaeriaceae based on specimens collected in China and suggested that this genus could be adopted as a potential generic name for ellisembia-like fungi in Chaetosphaeriaceae. However, two novelties recently described in this clade, E. reblovae W.P. Wu & Y.Z. Diao and E. aquirostrata J. Yang, Jian K. Liu & K.D. Hyde, were still retained in Ellisembia (Wu and Diao 2022; Yang et al. 2023).
During mycological surveys carried out in central Europe and subtropical Texas, USA, some ellisembia-like anamorphs inhabiting dead plant debris were collected. Emphasis was made on targeting the type species of the genus in its type locality of west-central Germany and other taxa for which DNA sequence data has not been generated. The aims of this paper are to characterize these collections and isolates morphologically and phylogenetically, and to provide a more effective taxonomic approach towards the problematics surrounding members of Ellisembia in Chaetosphaeriaceae and Sporidesmiaceae. Morphological and molecular evidence presented in this study support the proposal of new combinations in both Ellisembia and Lomaantha following the recommendations and data provided by Hyde et al. (2019) and Wu and Diao (2022).
Materials and methods
Morphological and cultural studies
Fresh specimens were collected on plant debris obtained in the vicinity of Wiesbaden, Germany, and southeastern Texas, USA, between the years 2020 and 2022. Materials were briefly washed under running tap water and incubated in moist chambers at room temperature (23–25 °C) for a few days. Sporulating structures were located under the stereoscope and single conidia were picked-up directly using a sterile needle for isolation. They were transferred to 2% malt extract agar (MEA) with 0.01% chloramphenicol, potato dextrose agar (PDA), and potato carrot agar (PCA) plates followed by incubation at 25 °C. Once conidia germinated in around 24 h, they were further transferred aseptically and re-incubated under similar conditions. Colony features were recorded after time periods of 2–3 weeks or longer. Fungal structures were mounted in lacto-cotton blue or lactic acid, heated to remove bubbles, and examined under an Olympus BX45 microscope or using differential interference contrast (DIC) on an Olympus BX-51 microscope with an Olympus DP72 digital camera (Olympus, Tokyo, Japan). Minimum, maximum, and 5th and 95th percentile values were calculated based on 50 measurements of each structure at 1000 × magnification with outliers given in parenthesis. Air dried pieces of specimen BPI 911246 containing colonies of E. pseudobambusae P.M. Kirk were observed under a JEOL JSM IT-200 (Jeol Ltd., Tokyo, Japan) scanning electron microscope (SEM). They were mounted on an aluminum stud and observed at 3–5 kV, with a working distance of approximately 14 mm, a probe current of 30 nA, and a pressure of 10 Pa at 23 °C after the chamber achieved local thermodynamic equilibrium. Voucher specimens are deposited in BPI or PRC and living strains in CBS or CCF. Fungaria and culture collection acronyms throughout the text follow Index Herbariorum (http://sweetgum.nybg.org/science/ih/) and the Culture Collections Information Worldwide of the WFCC-MIRCEN World Data Center for Microorganisms (http://www.wfcc.info/ccinfo/), respectively. Fungal names follow Index Fungorum (http://www.indexfungorum.org/) and host plant names are shown according to the International Plant Names Index (https://www.ipni.org).
DNA extraction, PCR amplification, and sequencing
Genomic DNA from strains of E. brachypus was extracted from 2-week-old cultures grown on MEA using a custom protocol outlined in Maciá-Vicente et al. (2022). DNA from strains of E. coronata and S. pseudobambusae was extracted using a Zymo Research Fungal/Bacterial Kit (Zymo Research, Orange, USA). Nuclear ribosomal DNA including the complete internal transcribed spacer (ITS) region and the first 900 bp of the 5′ end of the large subunit (LSU) together with fragments of the translation elongation factor 1α (TEF1) and RNA polymerase II second largest subunit (RPB2) were amplified with the primer sets ITS1F/ITS4, LR0R/LR7, 983F/2218R and RPB2-5f/fRPB2-7cR, respectively (Hopple and Vilgalys 1994; Liu et al. 1999; Rehner and Buckley 2005; White et al. 1990). The PCR products were purified and sequenced at Eurofins Genomics (Cologne, Germany) with the same primers used for amplification. Contig sequences were assembled using EMBOSS v6.6.0.0 (Rice et al. 2000) and deposited in GenBank.
Taxon sampling and phylogenetic analyses
Newly generated sequences and their closest hits in GenBank were selected and downloaded to build separate datasets for Sporidesmiaceae and Chaetosphaeriaceae, including additional sequences from Bao et al. (2021), Réblová et al. (2022), and Wu and Diao (2022) (Table 1). An unpublished ITS-LSU sequence of E. brachypus was retrieved from the NBRC website (http://www.nite.go.jp/en/nbrc/cultures/index.html), whereas an unpublished ITS sequence belonging to E. folliculata was downloaded from the NARO GenBank database (https://www.gene.affrc.go.jp/databases_en.php) and added to the Chaetosphaeriaceae dataset. Sequences from strains Buergenerula spartinae Kohlm. & R.V. Gessner ATCC 22848 and Magnaporthe salvinii (Catt.) R.A. Krause & R.K. Webster M21 were used as outgroups for the Sporidesmiaceae phylogeny, whereas Gelasinospora tetrasperma Dowding CBS 178.33 and Lasiosphaeria ovina (Pers.) Ces. & De Not. SMH 4605 served as outgroups for the Chaetosphaeriaceae phylogeny. The novel TEF1 sequences obtained from the Texas specimens of E. brachypus were used only for pairwise comparisons with those of available strains and not for phylogenetic analyses due to missing data among ellisembia-like chaetosphaeriaceous taxa. Sequences were aligned separately with MAFFT v.7.487 on the online server (https://mafft.cbrc.jp/alignment/server/index.html) (Katoh and Standley 2013; Katoh et al. 2019). Phylogenetic relationships were first inferred for individual datasets using Maximum Likelihood (ML) in RAxML v.8.2.12 (Stamatakis 2014) on the CIPRES Science Gateway server (Miller et al. 2010) and Bayesian inference (BI) using MrBayes 3.2.7a (Ronquist et al. 2012). Analyses were run following the settings outlined in Delgado et al. (2022). Separate phylogenies were topologically compatible for the most part (Supplementary Information Figs. S1–S4), and therefore alignments were concatenated in MEGA6 (Tamura et al. 2013) and run using similar settings. Best fit-substitution models were obtained also in MEGA using the corrected Akaike Information Criterion with the GTR + G + I selected for all datasets. Trees were visualized and edited in MEGA and Inkscape (https://inkscape.org). They are deposited together with alignments used for pairwise and phylogenetic analyses at figshare.com (https://doi.org/10.6084/m9.figshare.24612297).
Results
Pairwise comparison of sequences
A total of four strains of ellisembia-like hyphomycetes were obtained as a result of fieldwork. Three of them belonging to E. brachypus or E. pseudobambusae were isolated from materials collected in Texas, USA, whereas one strain was isolated from a specimen of E. coronata collected in Germany. Intraspecific pairwise comparisons with the ITS sequences of E. brachypus available in GenBank showed that the Texas strains were identical to strain NN 076460 from China. However, they differ by one C-T transition from strains MFLUCC 18–1573, NBRC 104942, and NN 050658 at position 104 along the length of their 431 aligned positions. In the case of the LSU, all new and available sequences were identical except for another C-T transition at position 353 for strain MFLUCC 18–1573 along the length of their 474 aligned positions. Interestingly, the TEF1 sequences of the Texas strains, OK383438 for CBS 147395 and OK383439 for CBS 147396, showed more variation and differed in one G-T transversion at position 306, one C-G transversion at position 336, and one C-T transition at position 703 along a length of 766 aligned positions. The only TEF1 sequence of E. brachypus available in GenBank belongs to strain MFLU 18–1615 from Thailand and differs from the TEF1 sequences of the Texas strains in five positions. The available LSU sequence of E. folliculata was considerably longer (1826 bp) compared with the one belonging to the type of Le. duriligni CBS 101317 with only 870 bp. However, they were almost identical and overlapped well for 873 bps except for a G-A transition at position 76 and gaps at positions 382, 453, and 487 of the pairwise alignment.
Phylogenetic analyses
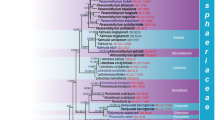
The concatenated ITS-LSU-RPB2 alignment of the Sporidesmiaceae dataset consisted of 81 taxa and 2489 characters including the outgroups, 624 from the ITS alignment, 826 from the LSU and 1039 from the RPB2. The single best scoring RAxML tree with a final ML optimization likelihood = − 28477.599526 is presented in Fig. 1. The tree is similar in topology to the 50% majority rule consensus tree of the 9978 sampled trees in the Bayesian analysis. Sporidesmiaceae was recovered as a strongly supported monophyletic clade (100% BS, 1 BPP). It consists of four distinct well-supported lineages named the Sporidesmium clade, the Lylea clade, the Ellisembia sensu lato clade, and the Ellisembia s. str. clade. The Sporidesmium clade (95% BS, 1 BPP) includes sporidesmium-like taxa such as S. aturbinatum (S. Hughes) M.B. Ellis and S. parvum (S. Hughes) M.B. Ellis among others with obpyriform or obclavate conidia together with the teleomorphic S. thailandense W. Dong, Huang Zhang & K.D. Hyde. The Lylea clade (99% BS, 1 BPP) contains strains of S. tetracoilum (Corda) G. Delgado & Koukol (formerly L. tetracoila (Corda) Hol.-Jech.) and its teleomorph along with L. dalbergiae Crous. The Ellisembia s. l. clade (81% BS, 0.99 BPP) includes ellisembia-like species such as S. appendiculatum G.N. Wang, W. Dong & H. Zhang, E. minigelatinosa and S. chiangmaiense X.D. Yu, W. Dong & H. Zhang having distoseptate conidia ending in an elongated apical cell surrounded by a gelatinous sheath or cap. The Ellisembia s. str. clade (100% BS, 1 BPP) includes E. coronata and morphologically similar taxa such as E. bambusicola and four other species currently placed within Sporidesmium, e.g., S. cangshanense Z.L. Luo & K.D. Hyde, S. melaleucae Crous, S. spiraeae Crous and S. pseudobambusae, the latter represented by a specimen recently collected in Texas. Some topological differences were observed between the individual ITS and LSU phylogenies (Supplementary Information Figs. S1 and S2). However, the Sporidesmiaceae clade consistently received strong support and the four recovered lineages were distinct and well-supported in all individual trees.
Best scoring RAxML phylogenetic tree obtained from a concatenated ITS-LSU-RPB2 dataset showing the Ellisembia s. str. clade within Sporidesmiaceae (Sordariomycetes) and the placement of E. coronata and E. pseudobambusae among related taxa. New strains are in bold and color boxes in the tree represent the four different lineages recovered within the family: Sporidesmium (blue), Lylea (yellow), Ellisembia s. l. (green), and Ellisembia s. str. (red). Bootstrap support values ≥ 70% are indicated at the nodes and thickened branches correspond to Bayesian posterior probabilities ≥ 0.95
The concatenated ITS-LSU alignment targeting Chaetosphaeriaceae consisted of 54 taxa and 1405 characters including the outgroups, 545 from the ITS alignment and 860 from the LSU. The single best scoring RAxML tree with a final ML optimization likelihood = − 9009.119585 (Fig. 7) was similar in topology to the 50% majority rule consensus tree of the 2933 sampled trees in the Bayesian analysis. The Lomaantha/Ellisembia clade formed a strongly supported monophyletic group (100% BS, 1 BPP). The Texas strains of E. brachypus grouped together with five other strains, whose sequences are available in GenBank or retrieved from NBRC with maximum support (100% BS, 1 BPP). They were sister to the only strain available of the morphologically similar E. aquirostrata J. Yang, Jian K. Liu & K.D. Hyde GZCC 20-0503a and a strain named ‘Pyrigemmula’ sp. BCC 28210 with strong support (87% BS, 0.99 BPP). The available strains of Lomaantha pooga Subram., E. aurea, and P. aurantiaca each formed strongly supported groups (100% BS, 1 BPP). A strain belonging to E. folliculata CBS 147152 and an ex-type strain of Le. duriligni CBS 101317 having an E. folliculata anamorph clustered together as expected but with moderate ML support (74% BS). The only strain available of E. reblovae NN 044776 grouped sister to the remaining six taxa. A strain named E. folliculata MAFF 240276 grouped distant from the Lomaantha/Ellisembia clade and clustered together with species of chloridium-like Gongromeriza Preuss and Chaetosphaeria Tul. & C. Tul. It formed a highly supported clade (100% BS, 1 BPP) with Ch. guttulata MFLU 18–1617. Individual ITS and LSU phylogenies recovered similar topologies despite some differences in the position of the clades Chloridium s. str. and Sporoschisma Berk. & Broome (Supplementary Information Figs. S3 and S4). They were distant from Lomaantha in the ITS tree but sister in the LSU one. However, the Lomaantha clade received strong support and identical consistency in all individual analyses.
Taxonomy
Ellisembia Subram., Proc. Indian natn Sci. Acad., Part B. Biol. Sci. 58(4): 183 (1992).
Type species: Ellisembia coronata (Fuckel) Subram., Proc. Indian natn Sci. Acad., Part B. Biol. Sci. 58(4): 183 (1992).
Descriptions and illustrations: Ellis (1958); Subramanian (1992); Wu and Zhuang (2005)
Notes: In view of the results of our phylogenetic analyses together with their shared morphological features, Ellisembia is restricted here to the members of that novel lineage within Sporidesmiaceae that includes E. coronata. The species is represented by a specimen obtained on its original host at the type locality in Germany, and the lineage also includes some other morphologically similar taxa having distoseptate conidia and conidiophores producing none or a few percurrent extensions. This is in agreement with the original generic concept of Subramanian (1992) but different from Hyde et al. (2019) and Wu and Diao (2022) who recently adopted Ellisembia for those taxa within the distant family Chaetosphaeriaceae. Therefore, we propose the following four new combinations within the genus and amend the description of E. coronata.
Ellisembia cangshanensis (Z.L. Luo & K.D. Hyde) G. Delgado, comb. nov.
MycoBank: MB#851172.
Basionym: Sporidesmium cangshanense Z.L. Luo & K.D. Hyde, Fungal Diversity 99: 506 (2019).
≡ Sporidesmium aquaticum Hong Y. Su, Z.L. Luo & K.D. Hyde, Fungal Diversity 80: 398 (2016).
≡ Sporidesmium cangshanense Z.L. Luo & K.D. Hyde, Index Fungorum 420: 1 (2019).
Description and illustration: Su et al. (2016)
Notes: This fungus was originally described as S. aquaticum on decaying wood submerged in a freshwater stream in China (Su et al. 2016). This name is a homonym of a previously described species, S. aquaticum Cabello, Mengasc. & Aramb. (Arambarri et al. 1989), and therefore invalid. Luo et al. (2019) replaced it with the name S. cangshanense but did not cite an identifier from a recognized repository in the protologue. Accordingly, a new identifier was issued in Index Fungorum. In all respects, this species matches well Ellisembia in having short conidiophores, 14–23 μm long, bearing determinate conidiogenous cells and narrowly obclavate or subcylindrical, long rostrate, 9–11-distoseptate, pale brown conidia with a pale brown to brown conico-truncate basal cell. Considering its placement and morphological similarity with related species within the Ellisembia s. str. clade, a new combination is proposed to accommodate this fungus.
Ellisembia coronata (Fuckel) Subram., Proc. Indian natn Sci. Acad., Part B. Biol. Sci. 58(4): 183 (1992) (Figs. 2–3).
Ellisembia coronata. IMI 11864. a Packet and slides. b Conidium. c Conidiophore with conidium. G00266276. d Packet containing specimen. e Herbarium material. f Original label from inside the packet with drawing. g, j Conidia. h Conidiophore with conidium. i Conidiophore with percurrent extensions. Scale bars: b, g–j 10 μm and c 20 μm
Ellisembia coronata. a Philadelphus coronarius, host plant at the type locality in Niederwalluf, Germany. b CCF 6699. Colonies on PCA and PDA after 30 days at 25 °C, surface view. PRC 9257 (epitype). c, g Conidia. d Conidiophore with conidium. Arrow indicates the delimiting septum between them. e, f Conidiophores. Scale bars: e 20 μm and c, d, f, g 10 μm
Basionym: Sporidesmium coronatum Fuckel, Jb. nassau. Ver. Naturk. 27–28: 77 (1874) [1873–74].
≡ Clasterosporium coronatum (Fuckel) Sacc., Syll. fung. (Abellini) 4: 385 (1886).
Colonies effuse, hairy. Mycelium mostly immersed in the substratum, composed of branched, septate, pale brown, smooth hyphae, 2–4 µm wide, with swollen, brown to dark brown, 5–10 μm wide cells around the base of conidiophores. Stromata absent or rudimentary and composed of a few swollen cells at the base of groups of conidiophores, brown to dark brown or blackish brown. Conidiophores macronematous, mononematous, solitary or aggregated in small groups of 2–3, loosely to somewhat densely grouped, arising from rudimentary stromata when in groups, erect, simple, straight or slightly flexuous, brown to dark reddish brown, (1–)2–5(–6)-septate, up to 66 µm long, 5–7 µm wide, 7–9 µm wide at base, with 0–2 ampulliform, lageniform or subcylindrical percurrent extensions, 0–1-septate, pale brown to brown, sometimes dark brown at the proximal end, 7–18 × 5–7 µm. Conidiogenous cells monoblastic, integrated, terminal, percurrent, brown, with truncated apex. Conidia narrowly obclavate, less often subcylindrical or subfusiform, sometimes short rostrate or long obclavate-rostrate, straight or slightly flexuous, pale brown, smooth, 7–14(–18)-distoseptate, with small pores in distosepta slightly darkened in DIC, (40–)52–92(–113) × 8–11 µm, occasionally bearing wall remnants around the basal and proximal cells as a result of aborted conidial initials which continue growing at a later time; apical cell subhyaline, rounded or tapered toward a rounded tip, with or without a mucilaginous cap; basal cell conico-truncate or short-cylindrical, brown to dark brown, 3–6 (–7) × 4–5.5 µm.
Colonies on PCA slow growing, reaching 8–10 mm diam after 30 days at 25 °C, velvety, flat, white, pale cream and slightly raised at the center, margin somewhat undulose, reverse dull white. Colonies on PDA similar to PCA but reaching 13–15 mm diam after 30 days at 25 °C, whitish cream to cream, yellow and slightly raised at the center, margin entire, reverse dull cream. Cultures sterile.
Typification: Holotype of S. coronatum: Fig. 26 (p. 77) in Fuckel, Symbolae mycologicae. Zweiter Nachtrag, Jahrbücher des Nassauischen Vereins für Naturkunde 27–28, 1874. Epitype: Germany, Hesse, Niederwalluf, by the northwest side of Sankt Johannes der Täufer Catholic church, 50° 02′ 03.7″ N, 8° 09′ 38.4″ E, 86 m a.s.l., on dead twig of Philadelphus coronarius L., 28 Sep 2022, leg. G. Delgado & M. Piepenbring (designated here, PRC 9257, ex-epitype strain CCF 6699).
Other specimens examined: Germany, Hesse, Niederwalluf, on dried twigs of P. coronarius, s.d., leg. K.W.G.L. Fuckel (G00266276); idem (IMI 11864, slides).
Notes: The description above is based on the fresh specimen collected in Germany, designated here as epitype, and the original materials deposited in G and IMI. The specimen in G consists of several twigs of P. coronarius having effuse colonies of E. coronata in some of them. The other specimen examined, originally deposited in IMI and now in K, consists of two permanent slides in poor condition but structures are still visible. A comparison between them shows they are morphologically similar although a few minor differences were noticed. Conidia in G00266276 were reddish brown, probably due to the age of the material, paler toward the apex and often long obclavate-rostrate, with a few more, up to 18-distosepta and longer conidia, up to 113 µm long compared with our collection with up to 14-distosepta and up to 100 µm long. The basal cells were more often short-cylindrical, less often conico-truncate, brown but rarely dark brown and 3–5(–6) × 4–4.5 µm in size. This is consistent with the holotype illustration (Fuckel 1874) that depicts a single long obclavate-rostrate conidium with a short-cylindrical basal cell. Conidiophores matched well and measured up to 49 µm long, 5–6 µm wide, and bear 0–2 lageniform, 0–1-septate percurrent extensions similar to our collection. Conidia in the IMI slides were mostly obclavate or subfusiform as reflected in the standardized description of Ellis (1958). He did not properly depict the brown colored conidial basal cells but they were confirmed in both the G and IMI specimens. Moreover, Ellis (1958) described shorter conidia, 35–70 µm long, whereas the original description mentioned a length of 96 µm. This is in agreement with our specimen that possesses similarly long conidia, probably as an effect of incubation in a moist chamber previous to isolation. Gelatinous caps were not detected in the original specimen or the IMI slides and most likely they are now missing due to their age and condition. Ellis (1958) did not mention this feature either, but he never referred to or depicted gelatinous caps in his descriptions of sporidesmium-like fungi. A rudimentary stroma composed of a few swollen cells was found at the base of groups of conidiophores in agreement with Ellis (1958) who reported ‘swollen, rather dark hyphal cells at the point of origin of the conidiophores.’ In general, the three specimens overlap well and, therefore, we consider our material collected on the original host plant species at the type locality, as a good representative of the fungus.
Ellisembia melaleucae (Crous) G. Delgado, comb. nov.
MycoBank: MB#851174.
Basionym: Sporidesmium melaleucae Crous, in Crous, Wingfield, Burgess, et al., Persoonia 40: 377 (2018).
Description and illustration: Crous et al. (2018)
Notes: Crous et al. (2018) described this species based on two specimens collected on Melaleuca sp. (Myrtaceae) in Australia. The fungus is characterized by short, 1–4-septate conidiophores, apparently lacking percurrent extensions, and obclavate, mid brown conidia, with conico-truncate basal cells and 5–21 distosepta having small pores. Morphologically, it resembles the remaining members of the Ellisembia s. str. clade in having similarly shaped distoseptate conidia and conidiophores lacking percurrent extensions. Based on its phylogenetic placement distant from the putative Sporidesmium s. str. clade, the fungus is transferred here to Ellisembia.
Ellisembia pseudobambusae (P.M. Kirk) G. Delgado & Koukol, comb. nov. (Figs. 4a–l, 5).
Ellisembia pseudobambusae. CCF 6709. a, b Colonies on MEA after 2 mo at 25 °C, surface and reverse views. c, d Conidiophores and conidia in culture. e Conidiophore with young conidium. f Conidiophores. BPI 911246. g Conidiophore. h, i Conidiophores with percurrent extensions and young conidium. j–l Conidia. Ellisembia carrii. AUA 1953 (holotype). m Packet containing specimen. n Groups of conidiophores on natural substrate. o, p Conidiophores with percurrent extensions and young conidium. q–s Conidia. Scale bars: a, b 1 mm; c–l, o–s 10 µm; and n 100 µm
SEM micrographs of Ellisembia pseudobambusae. BPI 911246. a Conidiophore with conidium. b Detail of the basal scar of a conidium. c Apical scars of conidiogenous cells. d, e Conidiophores with young conidia. Note the doliiform percurrent extension in e. Scale bars: a, e 10 µm; b 2 µm; c 5 µm, and d = 20 µm
MycoBank: MB#851176.
Basionym: Sporidesmium pseudobambusae P.M. Kirk, Trans. Br. mycol. Soc. 76(1): 84 (1981).
≡ Imimyces pseudobambusae (P.M. Kirk) A. Hern.-Gut. & B. Sutton, Mycol. Res. 101(2): 207 (1997).
≡ Imicles pseudobambusae (P.M. Kirk) Shoemaker & Hambl., Can. J. Bot. 79(5): 598 (2001).
Colonies effuse, hairy, black. Mycelium mostly immersed in the substratum, composed of branched, septate, smooth, pale brown to brown hyphae, 1.5–4 µm wide. Conidiophores macronematous, mononematous, solitary or aggregated in loose groups, simple, straight or flexuous, sometimes curved at the base, 3–7(–10)-septate, cylindrical, smooth, brown to dark brown, thick-walled, (39–)46–105(–117) × 4–7 µm, often swollen at their base and 6–8 µm wide, with 0–4 mostly lageniform or subcylindrical, rarely narrowly doliiform, 0- or 1-septate percurrent extensions, pale brown to brown or sometimes dark brown at the proximal end, (8–)10–22(–25) × 3–7 µm. Conidiogenous cells monoblastic, integrated, terminal, percurrent, brown to dark brown, apex truncated, sporadically bearing funnel-shape wall remnants of aborted conidia associated or not with extensions. Conidia narrowly obclavate, sometimes subfusiform or subcylindrical, often rostrate, golden brown to brown, paler toward the apex, smooth, straight or slightly flexuous, sometimes bent, 9–17-distoseptate, occasionally constricted at one septum, 61–117(–131) × 8–10 µm in the broadest part; basal cell conico-truncate, short conico-truncate or short cylindrical, brown to dark brown, 3–7 × 3–4(–5) µm, apex sometimes rounded or more often gradually tapering into an euseptate, subhyaline, subacute beak 2–3 µm wide at the tip in older, longer conidia.
Colonies on MEA very slow growing reaching 6–8 mm diam. after 2 mo at 25 °C, velvety, irregular, somewhat cerebriform, convex and raised 3–4 mm, yellowish orange at the center, creamy toward the edges, margin irregular, reverse yellowish to pale amber, cracking the medium and non-sporulating after 3-mo incubation. Colonies on PCA similar to those on MEA but sporulation was obtained after 1 mo. Conidiophores solitary or aggregated in small groups of up to 3, cylindrical, straight or slightly flexuous, smooth, 1–3-septate, pale brown to brown, 29–51 × 4–5 µm. Conidiogenous cells cylindrical or subcylindrical, determinate, apex truncate, 14–18.5(–21) × 4–5 µm. Conidia similar to those on natural substrate, obclavate, often rostrate to long obclavate rostrate, brown, paler brown to subhyaline toward the apex, smooth, straight or flexuous, 9–16-distoseptate, (80–)89–120(–136) × 7.5–10 µm, with small pores in distosepta, darkened and thickened in DIC; basal cell conico-truncate, brown.
Specimen examined: United States, Texas, Montgomery County, The Woodlands, George Mitchell Nature Preserve, along the Main Trail, 30° 08′ 49.3″ N, 95° 30′ 46.9″ W, 38 m a.s.l., on dead branch of Arundinaria sp., 8 May 2022, leg. G. Delgado (BPI 911246; CCF 6709).
Other specimen examined: Ellisembia carrii. United States, Alabama, Lee County, Auburn, on dead twigs of Buxus sempervirens var. subfruticosa L., 1 March 1977, leg. C.A. Carr (AUA 1953, holotype of Sporidesmium carrii).
Notes: The Texas specimen described above agrees well with the redescription of the holotype of S. pseudobambusae from United Kingdom made by Hernandez and Sutton (1997). They share conidia almost identical in size and shape: 65–118 × 8–10 µm and 8–18 distosepta, but percurrent extensions in the protologue were described as cylindrical to narrowly doliiform whereas those of the Texas material are more often lageniform. In culture, the fungus behaves like on natural substrate but conidiophores were shorter and conidiogenous cells were determinate, cylindrical or subcylindrical and distinctly truncate at the apex. Kirk (1981) mentioned a hyaline gelatinous cap that occasionally appeared on the conidial apices. However, this feature was not detected in our specimen on natural substrate or in culture, in which conidia are gradually tapered toward an elongated apex likely formed as an effect of incubation in moist chamber previous to isolation or due to growth on synthetic media. The illustration of Hernandez and Sutton (1997) based on the holotype shows mostly rounded but sometimes elongated conidial apices.
Ellisembia carrii (Morgan-Jones) W.P. Wu was originally considered an appropriate name for our collection and the holotype of this species was examined for comparison (Fig. 4m–s). Morgan-Jones (1977) first described E. carrii under Sporidesmium from dead twigs of B. sempervirens var. subfruticosa in Alabama, USA. This is apparently a rare species that was subsequently recollected on dead branches of a woody plant in China by Wu and Zhuang (2005), who transferred it to Ellisembia. The original description provided a conidial length of 50–120 × 9–10 µm and 8–16 distosepta. However, the type material deposited in AUA showed longer conidia, (76–)82–187(–206) µm long and 12–30-distosepta, with small pores in them. Morgan-Jones (1977) noted that once the material on natural substrate was incubated in moist chambers the conidia became distinctly rostrate but apparently, these longer conidia were not included in the original description. Ellisembia carrii can be separated from E. pseudobambusae by subtle differences consisting of shorter, 28–71(–81) µm long, and sparsely, 1–3-septate conidiophores. The protologue described mostly 1-septate conidiophores but we observed predominantly 2-septate ones. Percurrent extensions when present are also 0- or 1-septate, whereas the protologue specified them as non-septate. Our observations also show that they are mostly ampulliform or sometimes doliiform, with their apices attenuated to a 2.5–3(–4) µm wide tip, and therefore different from the truncated, slightly wider apices of E. pseudobambusae. Conidia in E. carrii are longer and more septate, whereas those of the Texas specimen of E. pseudobambusae reached a length of only 131 µm long and were 9–17-distoseptate after incubation. The conidial basal cells of E. carrii are brown and slightly long conico-truncate in the holotype, whereas those of E. pseudobambusae are consistently dark brown, short conico-truncate or short-cylindrical (Fig. 4j–l, q–s).
Ellisembia spiraeae (Crous) G. Delgado, comb. nov.
MycoBank: MB#851177.
Basionym: Sporidesmium spiraeae Crous, in Crous et al., Persoonia 47: 217 (2021).
Description and illustration: Crous et al. (2021)
Notes: This species was originally described from Spiraea japonica (Rosaceae) in The Netherlands (Crous et al. 2021). Morphologically, it is similar to our specimen of E. coronata from Germany in having obclavate, sometimes short-rostrate, pale brown conidia, (4–)6–10 distosepta bearing small pores in them and similar in size, (45–)70–85(–100) × (8–)9(–10) μm, with a dark brown, conico-truncate basal cell and short, 15–40 × 5–7 μm, 1–4-septate conidiophores, solitary or aggregated in clusters. However, conidiogenous cells were described as subcylindrical but percurrent extensions were not mentioned originally and are not visible in the illustration of the protologue. Phylogenetically, both species clustered within the Ellisembia s. str. clade but distant from each other despite their strong morphological resemblance. Considering its placement and morphological similarity with related species in Sporidesmiaceae, the fungus is transferred here to Ellisembia.
Lomaantha Subram., J. Indian Bot. Soc. 33: 31, 1954.
= Anasporidesmiella K. Zhang, R.F. Castañeda, Heredia & Jian Ma, in Zhang, Guo, Heredia, Delgado-Zúñiga, Ma & Castañeda-Ruíz, Mycotaxon 135(4): 723, 2020.
= Lecythothecium Réblová & Winka, Mycologia 93(3): 481, 2001.
= Pyrigemmula D. Magyar & Shoemaker, in Magyar, Shoemaker, Bobvos, Crous & Groenewald, Mycol. Progr. 10(3): 310, 2011.
Type species: Lomaantha pooga Subram., J. Indian Bot. Soc. 33: 32, 1954.
Illustrations: Subramanian (1954); Wu and Zhuang (2005); Ma et al. (2011); Wu and Diao (2022)
Emended description: Colonies effuse, black to brown, hairy. Mycelium mostly immersed in the substratum and composed of branched, septate, smooth, brown hyphae. Anamorph: Conidiophores macronematous, mononematous, simple, erect, cylindrical, brown, septate, or reduced to a conidiogenous cell. Conidiogenous cells monoblastic, integrated, terminal, determinate or extending percurrently a few times. Conidia acrogenous, cylindrical, obclavate or narrowly fusiform, distoseptate with cell lumina often reduced, simple, rostrate or not, with or without a filiform, simple or branched apical appendage and having distinct pores often associated with darkened and thickened distosepta. Teleomorph: Ascomata perithecial, immersed with protruding necks or becoming superficial, flask-shaped, glabrous, ostiolate. Perithecial wall leathery, consisting of two distinct layers. Ostiolar canal periphysate. Paraphyses persistent, hyaline, branched, anastomosing, septate. Asci unitunicate, 8-spored, cylindrical to clavate; ascal apex with nonamyloid, refractive apical annulus. Ascospores ellipsoidal to fusiform, transversely septate, versicolorous, central cells brown, end-cells hyaline, smooth-walled, without mucilaginous sheath or appendages.
Notes: Lomaantha as previously defined (Subramanian 1954; Wu and Zhuang 2005; Ma et al. 2011; Wu and Diao 2022) was characterized by sporidesmium-like anamorphs having conidia with hyaline, aseptate and branched apical appendages. A broader generic concept is here proposed after Wu and Diao (2022) who synonymized Pyrigemmula with anamorph- or holomorph-typified Ellisembia species placed in a distinct clade in Chaetosphaeriaceae. The expanded Lomaantha now contains novel morphological features including characters of the teleomorph, conidiophores reduced to conidiogenous cells, and conidia with or without simple or branched apical appendages and typically bearing distinct pores in the distosepta. This latter character of septal pores has been neglected in most descriptions of ellisembia-like chaetosphaeriaceous anamorphs but it is present in most species within this lineage as shown below. With the recent placement of the genus within the ellisembia-like chaetosphaeriaceous clade (Wu and Diao 2022), Lomaantha is considered here to better accommodate this group of taxa and the following six new combinations are proposed:
Lomaantha aquirostrata (J. Yang, Jian K. Liu & K.D. Hyde) G. Delgado & Koukol, comb. nov.
MycoBank: MB#851178.
Basionym: Ellisembia aquirostrata J. Yang, Jian K. Liu & K.D. Hyde, in Yang, Liu, Jones, Hyde, Liu, Bao, Liu, Li, Shen, Yu & Liu, Fungal Diversity 119: 120 (2023).
Description and illustration: Yang et al. (2023)
Notes: Yang et al. (2023) described this species based on a single specimen collected on a decaying twig submerged in a freshwater stream in China. The fungus strongly resembles E. brachypus in all respects including the presence of distinct pores in distosepta. Its cultural features, however, are different from those reported for E. brachypus. Colonies of L. aquirostrata on PDA are yellowish white, raised and pale brown in the center, with concentric rings and somewhat sulcate. They also lack the red diffuse pigment seen in the Texas isolates and other strains of E. brachypus.
Lomaantha aurantiaca (D. Magyar & Shoemaker) G. Delgado & Koukol, comb. nov. (Fig. 6g–i).
Lomaantha brachypus. CBS 147395. a, b Colonies on MEA after 3 wks at 25 °C, surface and reverse views. c Conidia. d Aberrant conidia showing septal pores. e Conidiogenous cells. BPI 926340. f Conidiophore and conidium on natural substrate. Lomaantha aurantiaca. BP 100757. g Conidia. h Conidium showing darkened basal pore. i Conidiogenous cells. Arrow indicates apical pores. Lomaantha folliculata. PRM 842728 (holotype of Sporidesmiella angustobasilaris). j, k Conidiophores. l, m Conidia. Arrow indicates a pore at the distal wall of the basal cell. PRM 842977 (holotype of Lecythothecium duriligni). n, o Conidiophores and conidia. p Conidium showing septal pores. PRM 893047. q, r Conidiophores and conidia. s Conidium. PRM 893052. t, u Conidiophore and conidia showing septal pores. PRM 893049. v Conidium. Scale bars: d, e, h–v 10 μm and c, f, g 20 μm
MycoBank: MB#851179.
Basionym: Pyrigemmula aurantiaca D. Magyar & Shoemaker, in Magyar, Shoemaker, Bobvos, Crous & Groenewald, Mycol. Progr. 10(3): 309 (2011).
≡ Ellisembia aurantiaca (D. Magyar & R. Shoemaker) W.P. Wu & Y.Z. Diao, Fungal Diversity 116: 42 (2022).
Description and illustrations: Magyar et al. (2011); this study.
Specimens examined: Hungary, Pest County, Göd, 47° 42′ 58.46″ N, 19° 08′ 03.18″ E, on bark of Platanus hybridus Brot., 27 Oct 2009, leg. D. Magyar (BP 100757); idem, Visegrád, on bark of Elaeagnus angustifolia L., 12 May 2010, leg. idem (Tk1005/1).
Notes: Compared with other members of this chaetosphaeriaceous lineage, L. aurantiaca morphologically deviates in many respects from the remaining taxa. Its conidiophores when present are micronematous, short and branched bifurcately but they are usually reduced to ovoid, pyriform, ampulliform or rarely spherical conidiogenous cells arising directly from the hyphae and ending up in a solitary pore, 2 μm in diam. They produce ellipsoidal, thin-walled, 0–5(–7)-distoseptate conidia lacking apical appendages or distinct pores in the distosepta. An examination of two representative specimens of the fungus confirmed the absence of this latter character. The inner lateral wall is thin in early stages of development but it becomes moderately thick in older conidia. Its width was found to vary somewhat depending on the conidial width, with cell lumen not distinctly reduced and squarish-shaped in wider conidia or distinctly reduced and narrowly cylindrical in narrower conidia. They often show a single basal pore which is sometimes inconspicuous as a slight depression of the outer basal wall or some other times distinct, slightly thickened and darkened or rarely somewhat protruding. These basal pores are a continuation of the solitary channel visible at the tips of the conidiogenous cells.
Lomaantha aurea (Réblová & J. Fourn.) G. Delgado, comb. nov.
MycoBank: MB#851180.
Basionym: Ellisembia aurea Réblová & J. Fourn., in Hyde et al., Fungal Diversity 96: 163 (2019).
Description and illustration: Hyde et al. (2019); Wu and Diao (2022)
Notes: This is one of two holomorphic members of this chaetosphaeriaceous lineage linked with an ellisembia-like anamorph, the other one is L. folliculata. The fungus produces obclavate to fusiform or lanceolate, 11–13(–15)-distoseptate, brown to reddish brown conidia on the natural substrate, ending in an apical extension up to 26 µm long and having a darker conico-truncate basal cell. The cell lumina are visibly reduced, distinct and cylindrical as seen in the illustrations of the protologue, and most distosepta are darkened and thickened in a fashion similar to the darkening surrounding septal pores in other species of the group. In culture, conidia are longer and present more distosepta, cell lumina are not distinctly reduced and distosepta are darkened but not as thickened as in vivo with a few visible septal pores that confirm the presence of this character in L. aurea. Wu and Diao (2022) recently reported a second specimen from China but the anamorph was not observed for comparison with the type material from France.
Lomaantha brachypus (Ellis & Everh.) G. Delgado, Koukol & Maciá-Vicente, comb. nov. (Fig. 6a–f).
MycoBank: MB#851181.
Basionym: Helminthosporium brachypus Ellis & Everh., in Millspaugh & Nuttall, Publications of the Field Columbia Museum, Bot. series 1: 92 (1896).
≡ Sporidesmium brachypus (Ellis & Everh.) S. Hughes, Can. J. Bot. 36: 807 (1958).
= Sporidesmium deightonii M.B. Ellis, Mycol. Pap. 70: 26 (1958).
≡ Ellisembia brachypus (Ellis & Everh.) Subram., Proc. Indian natn Sci. Acad., Part B. Biol. Sci. 58(4): 183 (1992).
Colonies on natural substrate effuse, hairy, black. Mycelium mostly immersed in the substratum, composed of brown, septate, smooth, branched hyphae, 2–3 µm wide. Conidiophores macronematous, mononematous, single or aggregated in groups of 2–3 at the base, simple, cylindrical, erect, straight or flexuous, smooth, 3–10-septate, brown to dark brown or dark reddish brown, 45–162(–185) × 6–9 µm, rarely with one percurrent extension, lageniform or doliiform, 6–19 × 6–7 µm, sometimes with 1–2 enteroblastic regenerative extensions unrelated to conidiation, base often blackish brown and bulbous, 8–15 µm wide. Conidiogenous cells integrated, terminal, cylindrical, determinate, smooth, brown, attenuated to a truncate apex, 3–4 µm wide, rarely percurrent. Conidia narrowly obclavate, fusiform, ellipsoidal to broadly ellipsoidal, rostrate, smooth, brown, (4–)5(–6)-distoseptate, rarely constricted at one eccentric septum, 31–48(–53) × (9–)10–15(–17) µm, with 4–6(–7) cylindrical to barrel-shaped septal pores per conidia, 2–3.5 mm wide and ending in a hyaline, aseptate, filiform appendage, up to 56 µm long, 2–3.5(–5) wide at the base, often collapsing and tapering to 1–2 µm wide at the distal end; basal cell conico-truncate, dark brown, (5–)6–8(–9) × (5.5–)6–8 µm, 3–5 µm wide at base; total length of conidial body and appendage 47–95 µm long.
Colonies on MEA moderately slow growing reaching 27–30 mm diam. after 3 wks at 25 °C, flat, somewhat convolute, pinkish whitish in color due to diffusible exudate, with gray areas of sparse to moderately dense sporulation around the center, margin irregular, reverse reddish brown, surrounded by a red soluble pigment diffused into the medium, sporulation developing around second week. Mycelium composed of hyaline, septate, smooth, branched hyphae, 1.5–4 µm wide, brown to dark brown in mass, chains or clumps of swollen cells 4–14 mm diam. present around the colony center. Conidiophores semi-macronematous, rarely macronematous, terminal or intercalary in the hyphae, mostly single but sometimes aggregated in groups of 2–4 at the base, cylindrical or subcylindrical, often reduced to the conidiogenous cell and lageniform, ampulliform or obconical, rarely bifurcating at the apex in two conidiogenous cells, smooth or verruculose, 0–3-septate, hyaline or subhyaline to brown, partly or fully pigmented, up to 38 mm long, 4–8 (–10) µm wide, attenuated to a truncate apex, 2–3.5 µm at the tip, rarely with 1–2 percurrent extensions. Conidia ellipsoidal to broadly ellipsoidal, rarely narrowly obclavate, fusiform or subcylindrical, rostrate, sometimes Y-shaped, mostly smooth, rarely verrucose, straight or curved, less often flexuous or sinuous, hyaline or subhyaline to pale brown or brown, partly or fully pigmented, with 2–6 transverse distosepta, sometimes with 1-oblique distoseptum, 2–6 pores per conidia ranging from distinct and similar to those on natural substrate to inconspicuous and not darkened or thickened, 24–52(–65) × (7–)9–12(–13) µm, ending in a hyaline or pale brown apical appendage up to 49 µm long and tapering to 1–2.5 µm at the apex, often with apical or subapical spherical cells 5–13 µm diam. and 0–3 eusepta, sometimes missing or reduced to a swollen cell, total length of conidial body and appendage 36–84 µm long.
Specimens examined: United States, Texas, Harris County, Spring, Meyer Park, 30° 00′ 15.9″ N, 95° 31′ 35.7″ W, 33 m a.s.l., on leaflets of dead leaf of Sabal minor (Jacq.) Pers., 10 Oct 2020, leg. G. Delgado (BPI 926340; CBS 147395); Houston, Bear Creek Pioneers Park, near Langham Creek, 29° 50′ 04.8″ N, 95° 37′ 29.0″ W, 30 m. a.s.l., on petiole and leaflets of dead leaf of S. minor, 22 Oct 2020, leg. idem. (BPI 926341; CBS 147396); Humble, Jesse H. Jones Park & Nature Center, around the Turtle Pond, 30° 01′ 35.4″ N, 95° 17′ 43.0″ W, 41 m a.s.l., on dead hanging vine stem, 21 May 2021, leg. idem. (BPI 911245).
Notes: Two of the specimens described above, BPI 926340 and BPI 926341, have conidia mostly ellipsoidal to broadly ellipsoidal, (11–)12–16(–17) µm wide. They are morphologically closer to some specimens described in the literature with broader, similarly shaped conidia up to 17–20 µm wide and 5–6 distosepta (Kirk 1985; Luo et al. 2019). Conidia in specimen BPI 911245, on the other hand, are narrowly obclavate or fusiform, (9–)10–13.5(–14) µm wide and closer to some other collections with narrower conidia, up to 12 µm wide and slightly more, up to 8 distosepta (Hughes and Illman 1974a; Matsushima 1975; Révay 1988). The pores associated with conidial distosepta are conspicuous but their darkening and thickening in our materials are not as distinct as those of the Canadian specimens described by Hughes and Illman (1974a) with narrower conidia and cell lumina. In our specimens, these features seem to concentrate around the pore between cells with wider cell lumina (Fig. 6f), similar to the specimens depicted by Luo et al. (2019) and Wu and Diao (2022). A gradual process of pore formation and darkening was detected and is explained in more detail in Supplementary Information Discussions 1 and Fig. S5. In culture, our strains on MEA produced a diffusible red pigment into the medium similar to the one described by Wu and Diao (2022) on PDA and the strain BCC 3466 depicted in the BIOTEC Culture Collection online catalogue (http://www1a.biotec.or.th/TNCC/dbstore/BCC_search.asp).
Lomaantha folliculata (Corda) Koukol & G. Delgado, comb. nov. (Fig. 6j–v).
MycoBank: MB#851182.
Basionym: Helminthosporium folliculatum Corda [as ‘Helmisporium’], Icon. fung. (Prague) 1: 12 (1837).
≡ Sporidesmium folliculatum (Corda) E.W. Mason & S. Hughes, in Hughes, Can. J. Bot. 31(5): 609 (1953).
≡ Ellisembia folliculata (Corda) Subram., Proc. Indian natn Sci. Acad., Part B. Biol. Sci. 58(4): 183 (1992).
= Helminthosporium brachytrichum Cooke & Ellis, Grevillea 6(no. 37): 6 (1877).
= Lecythothecium duriligni Réblová & Winka, Mycologia 93(3): 482 (2001).
= Sporidesmiella angustobasilaris Hol.-Jech., Česká Mykol. 41(1): 35 (1987).
= Anasporidesmiella angustobasilaris (Hol.-Jech.) K. Zhang, R.F. Castañeda, Heredia & Jian Ma, in Zhang, Guo, Heredia, Delgado-Zúñiga, Ma & Castañeda-Ruiz, Mycotaxon 135(4): 724 (2020).
Descriptions and illustrations: Ellis (1958); Hughes and Illman (1974b); Réblová and Winka (2001)
Specimens examined: Lecythothecium duriligni. Czech Republic, Moravia, Újezd near Moravský Krumlov, valley of the brook Rokytná, on decayed wood of Quercus sp., 3 Jul 1990, leg. V. Holubová-Jechová (PRM 842977, holotype); Sporidesmiella angustobasilaris. Cuba, Havana, Jaruco, Loma de la Coca (142 m s. m.), southeast from Campo Florido, on dead branch, 13 Feb 1981, leg. V. Holubová-Jechová (PRM 842728, holotype); Sporidesmium folliculatum. France, Massif Central, Cantal Mts., Mt. Puy Mary, on decorticated branch of Salix sp., 11 Jul 1997, leg. M. Réblová, (PRM 893047); Slovak Republic, Central Slovakia, Velká Fatra Mts., Gadierská dolina valley, on decaying wood of Fagus sylvatica L., 13 Jul 1976, leg. V. Holubová-Jechová (PRM 893049); Southern Slovakia, Pokoradz near Rimavska Sobota, on decaying wood of Quercus sp., 9 Jul 1983, leg. idem (PRM 893052). Unknown (PRM 815967).
Notes: When Réblová and Winka (2001) first connected E. folliculata with its teleomorph Le. duriligni, no mention of pores in the conidial distosepta was made. Ellis (1958) and Sinclair et al. (1990) did not describe pores either although septal darkening was illustrated. However, examination of the holotype specimen of Le. duriligni PRM 842977 confirmed their presence in mature conidia and their absence in recently formed septa (Fig. 6n–p). Moreover, the dark brown, barrel-shaped septal pores associated with thickened and darkened distosepta were as conspicuous as in the remaining collections of L. folliculata studied here. A gradual process of pore formation and darkening was also detected in these collections (Supplementary Information Discussion 1). This is similar to the one observed in L. brachypus but somewhat different as conidia of L. folliculata possess more distosepta, ranging mostly from 8–10 but up to 15, and lack an apical appendage (Hughes and Illman 1974b).
Examination of the type material of Sporidesmiella angustobasilaris (Holubová-Jechová 1987), on the other hand, revealed strong similarities with L. folliculata (Fig. 6j–m). They share in common the apically rounded, cylindrical, slightly clavate or ellipsoidal conidia, similar in size and with 3–10 distosepta, of which one or two are darkened. Basal cells are distinctly dark brown, with a marked darkening and thickening of the distal walls and a distinct pore (not mentioned or depicted in the protologue) together with inconspicuous ones at the third distosepta of several conidia. Darkening is less marked at the third distoseptum, rare at the fifth and missing in the remaining ones, but sometimes it extends to the suprabasal two cells as darkened lateral walls. This is similar to the gradual developmental pattern described for conidia of L. folliculata at their initial stages of maturity (Supplementary Information Discussion 1), and the fungus was apparently in an immature state when collected. Hughes and Illman (1974b) commented on a Canadian specimen of L. folliculata collected on wood under the bark of Ulmus sp. with shorter, 25–45 µm long, fewer septa, 4 to 9 but mostly 4 to 6, and a characteristically darker basal cell. They also described faintly septate, paler than usual conidiophores attenuated apically to a truncate apex of 1–1.5 µm similar to Holubová-Jechová (1987). Hughes and Illman (1974b) also hypothesized that this could be an aberrant collection of the fungus due to its development beneath the bark of the host tree. In a similar fashion, this could be the case of S. angustobasilaris in which particular ecological conditions present at the type locality in tropical Cuba prevented its full conidial development. Moreover, Ellis (1958) illustrated the type collection of Helminthosporium brachytrichum Cooke & Ellis, a synonym of L. folliculata, from New Jersey, USA, with conidia visibly in different developmental stages, the younger ones lacking or having incomplete septation and a marked darkening at the distal wall of their basal cells or at a single septum. Therefore, the genus Anasporidesmiella (Zhang et al. 2020), typified by S. angustobasilaris, is reduced here to synonymy of Lomaantha based on the available morphological and developmental evidence. Its second species, A. manifesta Heredia, J. Delgado, K. Zhang, R.F. Castañeda & Jian Ma, is morphologically close to S. angustobasilaris. However, we refrain to transfer this species to Lomaantha in the absence of molecular data or clues about its conidial development, and its generic position is left inconclusive.
Lomaantha reblovae (W.P. Wu & Y.Z. Diao), G. Delgado, comb. nov.
MycoBank: MB#851183.
Basionym: Ellisembia reblovae W.P. Wu & Y.Z. Diao, Fungal Diversity 116: 46 (2022).
Description and illustration: Wu and Diao (2022)
Notes: Wu and Diao (2022) recently described this fungus from a dead culm of bamboo in China and considered it distinct from related species based in the absence of an apical appendage. Its conidia are obclavate or obclavate-rostrate, brown to dark brown with a pale brown apical cell, conical or rounded at the apex and with 11–14 distosepta. No comment on the presence of septal pores was made but the illustration in the protologue shows reduced cell lumina, often long cylindrical in shape or slightly wider around the center, and distinct darkening and thickening every two distosepta, some of them with a visible pore. Wu and Diao (2022) did not observe sporulation on PDA.
Discussion
Phylogeny and epitypification of Ellisembia coronata
The recollection and sequencing of the generic type species E. coronata, on its original host and type locality in Germany, offered the possibility to refine the taxonomy of Ellisembia and circumscribe this large genus in a strict sense on the basis of combined morphological and molecular evidence. The German mycologist Karl Wilhelm Gottlieb Leopold Fuckel (1821–1876) originally collected this fungus around one hundred fifty years ago on dry, but still standing twigs or branches of P. coronarius in Niederwalluf. This is a small town west of the city of Wiesbaden and near Oestrich, where Fuckel owned a vineyard. Unfortunately, we do not know the exact collection date of the original specimen of E. coronata examined in this study. The original description (Fuckel 1874) lacks dates as Fuckel usually did not add exact dates to his collections and even years are missing (U. Braun, pers. comm.). The packet is labeled “Herbier Fuckel 1894” but this is obviously not a collection year as Fuckel died by 1876. The specimen label was probably annotated with the year when Fuckel’s Herbarium was incorporated into the Herbier Barbey-Boissier (Hennebert 2017), nowadays, Herbarium of the Conservatoire et Jardin botaniques de la Ville de Genève (G), along with several other specimens having the same label.
Fuckel (1874) described S. coronatum, the basionym of E. coronata, without designating any type specimen but cited an illustration in the protologue as Fig. 26. This is original material according to article 9.4 of the ICN (Turland et al. 2018) and it is specified under the Typification for this name in Index Fungorum. The holotype illustration consists of a single conidium upside down, narrow, long obclavate in shape, 12-euseptate, with most cells having an associated guttule and a distinct short cylindrical basal cell. The depiction of eusepta instead of distosepta make this illustration demonstrably ambiguous for the purpose of the precise application of the name Ellisembia, which was originally defined for species with distoseptate conidia. An examination of specimen G00266276 (Fig. 2) confirmed that it corresponds to the original material described by Fuckel (1874) and the fungus possesses distoseptate conidia. The packet even contains a drawing of the original picture Fig. 26 depicted in this work with the numbers 8 and 96 written on it (Fig. 2f) and matching the measurements in the original description. Ellis (1958), in his revision of the genus Sporidesmium based on specimens at IMI, provided a standardized description of the fungus and incorrectly indicated that IMI 11864 was the type of S. coronatum. An online search in MycoPortal (MycoPortal 2023) shows that there are several specimens under the name Clasterosporium coronatum (Fuckel) Sacc. mostly deposited in North American herbaria such as BPI, CUP, DAOM, MICH, MU, S and WSP. Based on their labels, they are very likely duplicates of specimen G00266276, and the written location “N.-Walluf” should be Niederwalluf and not Neu-Walluf as shown in several of these packets. Apparently, Subramanian (1992) did not examine any of these specimens when he selected E. coronata as the generic type. He probably followed the illustration in Ellis (1958) and missed the ambiguous illustration of Fuckel (1874). The fresh, sequenced specimen PRC 9257 is therefore selected as epitype to further stabilize the application of the generic name Ellisembia and to clarify any ambiguity surrounding its usage.
Ellisembia pseudobambusae, E. carrii, and related ellisembia-like fungi
Another member of the Ellisembia s. str. clade thoroughly characterized in this study is E. pseudobambusae, isolated and sequenced for the first time based on a fresh specimen collected in Texas (Figs. 4 and 5). Interestingly, our specimen and the holotype from United Kingdom share the same Arundinaria sp. host. This is a north-temperate genus of bamboos native to North America (Clark and Triplett 2023) that was probably planted and naturalized at the type locality of the fungus in the United Kingdom. A tentative identification of our material as E. carrii was considered based on the morphological similarity between the two species. Both E. carrii and E. pseudobambusae were transferred first to the genus Imimyces (Im.) A. Hern.-Gut. & B. Sutton and later to Imicles (I.) Shoemaker & Hambl. after the type species of the former, Im. densus (Sacc. & Roum.) A. Hern.-Gut. & B. Sutton, was found to be conspecific with Polydesmus elegans Durieu & Mont. (Hernández and Sutton 1997; Shoemaker and Hambleton 2001). Imicles was proposed for the remaining five species of Imimyces except Im. hollowayensis A. Hern.-Gut. & B. Sutton that was not included in this reassessment. They are characterized by conidiogenous cells producing lageniform, doliiform or ovoid percurrent extensions and distoseptate conidia. However, Wu and Zhuang (2005) reduced Imicles to a synonym of Ellisembia considering that these features were not reliable for generic delimitation, although Seifert et al. (2011) and Su et al. (2016) preferred to retain it as a separate genus. Other Imicles species including I. aquatica (Cabello, Mengasc. & Aramb.) Shoemaker & Hambl., I. bambusae (M.B. Ellis) Shoemaker & Hambl., I. heterocateniformis (Matsush.) Shoemaker & Hambl. and I. leptospora (Sacc. & Roum.) Shoemaker & Hambl., together with Im. hollowayensis and E. carrii, still lack DNA sequence data and phylogenetic placement. Therefore, it would be premature to support the synonymy of Imicles under Ellisembia following Wu and Zhuang (2005) and Wu and Diao (2022). Instead, it is recommended to wait until these species, especially the generic type I. heterocateniformis, are recollected and their systematic position using molecular data is revealed. This will also help to better separate morphologically closely related taxa such as I. leptospora and Im. hollowayensis from E. carrii and E. pseudobambusae and to achieve a more robust circumscription of this group of species.
Ellisembia, Sporidesmium, and Sporidesmiaceae
The decision to synonymize Ellisembia under Sporidesmium (Su et al. 2016) is revised here as E. coronata, along with other morphologically similar ellisembia-like taxa, forms a distinct lineage distant from the remaining Sporidesmium-like members of the family (Fig. 1). The reason behind was the lack of phylogenetic significance of the type of conidial septum for generic delimitation after species with euseptate and distoseptate conidia, particularly Ellisembia species such as E. bambusicola and E. minigelatinosa, clustered together within the resurrected Sporidesmiaceae. This decision was made regardless of the evident polyphyletic status of the genus, the poor representation of Ellisembia species available at the time for analysis and a Sporidesmium s. str. still pending definition. Similarly, the resurrection of Sporidesmiaceae based on the putative similarity of its members with S. ehrenbergii, the generic lectotype, is not supported by our results as none of the members of the recovered clades shows a close resemblance with this taxon. Ellis (1958) described S. ehrenbergii as having obclavate to subfusiform, sometimes short-rostrate, reddish brown, 7–10-euseptate conidia, with a tapered apex but rounded at the tip and a conico-truncate basal cell. In contrast, the most specious lineage within Sporidesmiaceae, called here the Sporidesmium clade, contained taxa with obpyriform conidia having fewer, mostly 3–4 eusepta and an inflated basal cell with a distinct scar. The Lylea clade, on the other hand, includes L. dalbergiae and S. tetracoilum, the latter recently transferred from Lylea to Sporidesmium based on its phylogenetic placement and remarkable morphological resemblance with the anamorph of S. lignicola (Koukol and Delgado 2021). Both species are characterized by 2–4-distoseptate, narrowly obclavate, long fusiform or subcylindrical conidia with tapering, truncate ends and in long unbranched acropetal chains. The other two clades named Ellisembia s. l. and Ellisembia s. str. include species with obclavate and distoseptate conidia which may or may not end in an elongated apical cell sometimes surrounded by a gelatinous sheath or cap. In a fashion similar to what was done during this study for E. coronata, a fresh specimen of S. ehrenbergii needs to be recollected and sequenced for a proper phylogenetic placement of this fungus and a better definition of Sporidesmiaceae and its lineages.
Lomaantha and related chaetosphaeriaceous ellisembia-like fungi
With the novel placement of E. coronata revealed in this study, the suggestion of Wu and Diao (2022) to adopt Lomaantha for those ellisembia-like taxa in Chaetosphaeriaceae was implemented here. Hyde et al (2019) previously suggested that this group of species were congeneric with Pyrigemmula (Magyar et al. 2011, Fig. 6g–i) and recommended the use of this other generic name to accommodate them once the position of E. coronata was confirmed. However, Lomaantha is older than Pyrigemmula and its generic type, L. pooga, clustered within this clade and seems to better represent its members after sharing more morphological features in common with the remaining taxa in this group. Matsushima (1975) previously commented that Sporidesmium brachypus was congeneric with Lomaantha in a far-reaching taxonomic statement later confirmed by molecular data and further evidence supporting the present taxonomic rearrangement. Moreover, the expanded concept of Ellisembia also proposed by Wu and Diao (2022), including Lecythothecium and Pyrigemmula as its synonyms, is no longer tenable as both genera are known members of the distant Chaetosphaeriaceae.
Morphology, culture characteristics, and molecular phylogenetics of L. brachypus
In the present study, the chaetosphaeriaceous anamorph Ellisembia brachypus here transferred to the genus Lomaantha, is shown to be a variable species based on morphological, cultural and molecular characters. The differences observed in conidial dimensions, width and pigmentation of distosepta between the specimens collected in Texas and those from other latitudes suggest that these characters are influenced by the disparate environmental conditions they were exposed to. The specimens BPI 926340 and BPI 926341 were collected adjacent to a pond on the palm host Sabal minor, which tend to dominate the understory of shady and humid lowland floodplain forests near water bodies, whereas specimen BPI 911245 was collected on a dead hanging vine stem and therefore directly exposed to desiccation, wind and solar radiation among other environmental factors. Similarly, Wu and Zhuang (2005) commented on the wide range of conidial variation in their collections from China.
In culture, sporulation was abundant in our isolates and many conidia were morphologically similar to those encountered on natural substrate with visible pores in the distosepta (Fig. 6c). However, many aberrant conidia were also produced. They displayed abnormal conidial features such as partial or complete lack of pigmentation, long, slender, flexuous or sinuous subcylindrical conidia, swollen apical cells or aseptate to 1–3-euseptate apical appendages with swollen cells, and missing or inconspicuous, non-darkened or thickened pores or the obconical basal cells missing. Curiously, aberrant Y-shaped-conidia with more or less pentagonal or triangular central cells and two divergent arms were observed (Fig. 6d). They often end in subhyaline apical appendages with swollen cells at the tips and septal pores present along the arms or sometimes missing. Abnormalities also extended to hyaline or subhyaline, terminal or intercalary conidiophores reduced to a single conidiogenous cell (Fig. 6e) often with verrucose ornamented walls, and bearing typically pigmented, brown, or unusually non-pigmented, aberrant conidia. The presence of macronematous conidiophores on natural substrate in contrast with their relative absence in culture further confirms the phenotypic plasticity of L. brachypus under different conditions. This feature, together with the presence of conidia having both disto- and eusepta, further supports the lack of taxonomic relevance of these characters in the phylogeny and classification of ellisembia-like fungi. Phenotypic plasticity in general is suspected to have an important role in colonization of new environments and geographical range shifts (Bonamour et al. 2019) and it may help to explain the widespread distribution of L. brachypus. The fungus is quite common in the United States where it has been recorded in several states besides the type locality in West Virginia (MycoPortal 2023). It has been reported also in Asia, Africa, Europe, Australia, and New Zealand (Ellis 1958; Kirk 1985; Révay 1988; Wu and Zhuang 2005) and it is probably the most widespread member of this clade. Strains represented by molecular data in GenBank originated only from Asia (China and Thailand). Our North American collections provide strains and sequence data from the geographical area of the type specimen, originally described as Helminthosporium brachypus Ellis & Everh. on wood in the state of West Virginia, southeastern USA, more than a century ago (Millspaugh and Nuttall 1896). Furthermore, the present specimens help to document its presence for the first time in subtropical Texas.
Phylogenetically, our strains from North America did not show significant molecular differences compared with the Asian ones, at least in the ITS and LSU markers, and only the TEF1 showed some apparent intra and interspecific variation. However, Yang et al. (2023) recently described Ellisembia aquirostrata, transferred here to Lomaantha, which is morphologically identical to L. brachypus except for its different cultural features and phylogenetic distinctiveness. This could be a first example of cryptic diversity hidden within this widespread fungus and reflects the need to use molecular data in the future for proper delimitation of L. brachypus s. str. from morphologically similar taxa. Other species in the literature such as E. minibrachypus Subram., E. pruni (Jian Ma & X.G. Zhang) Santa Izabel, A.C. Cruz & Gusmão or E. phoebes (Ch.K. Shi & X.G. Zhang) Santa Izabel, A.C. Cruz & Gusmão (Subramanian 1994; Ma and Zhang 2007; Shi and Zhang 2007; Santa Izabel et al. 2013) are seemingly conspecific with L. brachypus based on morphological features.
The unusual strain E. folliculata MAFF 240276
Surprisingly, a strain named E. folliculata MAFF 240276 with only an available ITS sequence clustered with Chaetosphaeria guttulata Z.L. Luo, K.D. Hyde & H.Y. Su MFLU 18–1617 outside the clade of ellisembia-like chaetosphariaceous fungi (Fig. 7). The specimen source of this strain was originally collected on dead culms of Sasa senanensis Rehder (Poaceae) in Japan (Shirouzu and Harada 2008). Based on its description, it agrees well in conidial dimensions (45–70 × 10–11 μm) and number of distosepta (6–11) with L. folliculata. In the drawing Fig. 3 p. 128, cell lumina are reduced and some distosepta are darkened and thickened with pores depicted for some of them. Conidial morphology, however, somewhat differ from the usual cylindrical shape of this species. Figure 3 shows subcylindrical, fusiform to ellipsoidal and narrowly obclavate conidia, although these variations have been previously documented for other specimens of L. folliculata (Sinclair et al. 1990). On the other hand, C. guttulata (Luo et al. 2019) is an atypical anamorphic member of the family having a unique conidiogenesis among Chaetosphaeriaceae (Wu and Diao 2022). It consists of non-phialidic, polyblastic, sympodially extending conidiogenous cells bearing numerous darkened and protuberant conidiogenous loci and hyaline, ovoid or fusiform, 3-septate conidia with a darkened scar at the slightly fimbrillate base. The fungus was described from a single specimen collected on submerged decaying wood in a freshwater stream in China. Morphologically, it resembles species of Minimelanolocus R.F. Castañeda & Heredia in Chaetothyriales (Eurotiomycetes) which are often collected from freshwater habitats (Liu et al. 2015; Dong et al. 2018; Wan et al. 2021). This may suggest contamination during isolation in pure culture and a mismatched phylogenetic placement. More collections and data are needed to confirm the status of C. guttulata and strain MAFF 240276, and whether other ellisembia-like fungi unrelated to the Lomaantha lineage may be present in Chaetosphaeriaceae.
Best scoring RAxML phylogenetic tree inferred from a concatenated ITS-LSU dataset showing the expanded Lomaantha and related genera in Chaetosphaeriaceae (Sordariomycetes). The Lomaantha clade is highlighted in a red colored box and the Texas strains of L. brachypus are in bold. The clade containing the strain Ellisembia folliculata MAFF 240276 is highlighted in a blue colored box. Bootstrap support values ≥ 70% are shown at the nodes and Bayesian posterior probabilities ≥ 0.95 are indicated by thickened branches
In conclusion, the taxonomy of ellisembia-like fungi is still in a state of flux. Phylogenetic analyses of molecular data are helping nowadays to clarify relationships among Ellisembia and related taxa, but there is still a long way before a more natural, less schematic classification of these fungi can be achieved. Special emphasis should be made on the recollection, isolation and sequencing of the many rare or poorly collected ellisembia-like taxa in order to systematically achieve this goal in the near future.
Data availability
All specimens are deposited in herbaria BPI or PRC, and strains in culture collections CBS or CCF; all sequence data are available in GenBank (https://www.ncbi.nlm.nih.gov) under the accession numbers given in Table 1; sequence alignments are available in figshare (https://figshare.com/) and new combinations were registered in MycoBank (https://www.mycobank.org/).
References
Afshari N, Gomes de Farias AR, Bhunjun CS, Phukhamsakda C, Lumyong S, Hyde KD (2023) Distoseptispora dipterocarpi sp. nov. (Distoseptisporaceae), a lignicolous fungus on decaying wood of Dipterocarpus in Thailand. Curr Res Environ Appl Mycol 13(1):68–78. https://doi.org/10.5943/cream/13/1/5
Arambarri AM, Cabello M, Mengascini A (1989) Estudio sistemático de los hyphomycetes del Río Santiago III. (Buenos Aires, Argentina). Bol Soc Argent Bot 26(1–2):1–6
Bao DF, Hyde KD, McKenzie EHC, Jeewon R, Su HY, Nalumpang S, Luo ZL (2021) Biodiversity of lignicolous freshwater hyphomycetes from China and Thailand and description of sixteen species. J Fungi 7:669(1–42). https://doi.org/10.3390/jof7080669
Bonamour S, Chevin LM, Charmantier A, Teplitsky C (2019) Phenotypic plasticity in response to climate change: the importance of cue variation. Phil Trans R Soc B 374:20180178. https://doi.org/10.1098/rstb.2018.0178
Clark LG, Triplett JK (2023) Arundinaria Michx. flora of North America http://floranorthamerica.org/Arundinaria. Accessed 8 June 2023
Crous PW, Wingfield MJ, Burgess TI et al (2018) Fungal planet description sheets: 716–784. Persoonia 40:240–393. https://doi.org/10.3767/persoonia.2018.40.10
Crous PW, Osieck ER, Jurjević Ž et al (2021) Fungal planet description sheets: 1284–1382. Persoonia 47:178–374. https://doi.org/10.3767/persoonia.2021.47.06
Delgado G, Miller AN, Piepenbring M (2018) South Florida microfungi: Castanedospora, a new genus to accommodate Sporidesmium pachyanthicola (Capnodiales, Ascomycota). Cryptog Mycol 39:109–127. https://doi.org/10.7872/crym/v39.iss1.2018.109
Delgado G, Miller AN, Hashimoto A, Iida T, Ohkuma M, Okada G (2022) A phylogenetic assessment of Endocalyx (Cainiaceae, Xylariales) with E. grossus comb. et stat. nov. Mycol Prog 21:221–242. https://doi.org/10.1007/s11557-021-01759-9
Dong W, Hyde KD, Bhat DJ, Zhang H (2018) Introducing Aculeata aquatica gen. et sp. nov., Minimelanolocus thailandensis sp. nov. and Thysanorea aquatica sp. nov. (Herpotrichiellaceae, Chaetothyriales) from freshwater in northern Thailand. Mycol Prog 17:617–629. https://doi.org/10.1007/s11557-018-1389-2
Ellis MB (1958) Clasterosporium and some allied Dematiaceae-Phragmosporae. I. Mycol Pap 70:1–89
Fuckel KWGL (1874) Symbolae mycologicae. Zweiter Nachtrag Jahrb Nassau Ver Naturkd 27–28:1–99
Hennebert GL (2017) Glischroderma Fuckel. Mycotaxon 132(4):745–757. https://doi.org/10.5248/132.745
Hernandez A, Sutton B (1997) Imimyces and Linkosia, two new genera segregated from Sporidesmium sensu lato, and redescription of Polydesmus. Mycol Res 101(2):201–209. https://doi.org/10.1017/S0953756296002419
Holubová-Jechová V (1987) Studies on hyphomycetes from Cuba V. Six new species of dematiaceous hyphomycetes from Havana Province. Česká Mykol 41(1):29–36
Hopple JS Jr, Vilgalys R (1994) Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 86(1):96–107. https://doi.org/10.1080/00275514.1994.12026378
Hughes SJ, Illman WI (1974a) Sporidesmium brachypus. Fungi Canadenses No. 57. National Mycological Herbarium, Biosystematics Research Institute, Ottawa
Hughes SJ, Illman WI (1974b) Sporidesmiium foliculatum. Fungi Canadenses No. 60. National Mycological Herbarium, Biosystematics Research Institute, Ottawa
Hyde KD, Tennakoon DS, Jeewon R et al (2019) Fungal diversity notes 1036–1150: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 96:1–242
Katoh K, Standley DM (2013) MAFFT Multiple Sequence Alignment Software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 19;20(4):1160–1166. https://doi.org/10.1093/bib/bbx108
Kirk PM (1981) New or interesting microfungi I. Dematiaceous hyphomycetes from Devon. Trans Br Mycol Soc 76(1):71–87. https://doi.org/10.1016/S0007-1536(81)80010-1
Kirk PM (1985) New or interesting microfungi. XIV Dematiaceous Hyphomycetes from Mt Kenya. Mycotaxon 23:305–352
Koukol O, Delgado G (2021) Why morphology matters: the negative consequences of hasty descriptions of putative novelties in asexual ascomycetes. IMA Fungus 12:26. https://doi.org/10.1186/s43008-021-00073-z
Kuo CH, Goh TK (2019) Ellisembia acicularia sp. nov. and four new records of freshwater hyphomycetes from streams in the Alishan area, Chiayi County, Taiwan. Nova Hedwig 108(1–2):185–196. https://doi.org/10.1127/nova_hedwigia/2018/0493
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808. https://doi.org/10.1093/oxfordjournals.molbev.a026092
Liu XY, Udayanga D, Luo ZL, Chen LJ, Zhou DQ, Su HY, Hyde KD (2015) Backbone tree for Chaetothyriales with four new species of Minimelanolocus from aquatic habitats. Fungal Biol 119:1046–1062. https://doi.org/10.1016/j.funbio.2015.08.005
Luo ZL, Hyde KD, Liu JK, Bhat DJ, Bao DF, Li WL, Su HY (2018) Lignicolous freshwater fungi from China II: Novel Distoseptispora (Distoseptisporaceae) species from northwestern Yunnan province and a suggested unified method for studying lignicolous freshwater fungi. Mycosphere 9(3):444–461. https://doi.org/10.5943/mycosphere/9/3/2
Luo ZL, Hyde KD, Liu JK et al (2019) Freshwater Sordariomycetes. Fungal Divers 99:451–660. https://doi.org/10.1007/s13225-019-00438-1
Ma J, Zhang XG (2007) Taxonomic studies of Sporidesmium from China. Mycotaxon 99:367–371
Ma J, Wang Y, O’Neill NR, Zhang XG (2011) A revision of the genus Lomaantha, with the description of a new species. Mycologia 103(2):407–410. https://doi.org/10.3852/10-176
Ma J, Xu ZH, Zhang K, Zhang XG, Qiu L (2020) Three new species of Ellisembia from Jiangxi Province, eastern China. Mycosystema 39(10):1846–1853. https://doi.org/10.13346/j.mycosystema.200140
Maciá-Vicente JG, Bai B, Qi R, Ploch S, Breider F, Thines M (2022) Nutrient availability does not affect community assembly in root-associated fungi but determines fungal effects on plant growth. mSystems. 7(3):0030422. https://doi.org/10.1128/msystems.00304-22
Magyar D, Shoemaker RA, Bobvos J, Crous PW, Groenewald JZ (2011) Pyrigemmula, a novel hyphomycete genus on grapevine and tree bark. Mycol Prog 10:307–314. https://doi.org/10.1007/s11557-010-0703-4
Matsushima T (1975) Icones microfungorum a Matsushima lectorum. Published by the author, Kobe
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, pp 1–8
Millspaugh CF, Nuttall LW (1896) Flora of West Virginia. Publ Field Mus Nat Hist Bot ser 1(2):1–276
Morgan-Jones G (1977) Notes on hyphomycetes. XXI. Sporidesmium carrii sp. nov. Mycotaxon 6(2):356–358
MycoPortal (2023) http://mycoportal.org/portal/index.php. Accessed 15 August 2023
Pereira A, Pontes HM, Hernandez A, Santana J (2022) Fungi associated with Bactris gasipaes Kunth (Arecaceae) in Brazil: checklist and new records of Didymostilbe capsici, Ellisembia antillana and Aculeata aquatica (anamorphic Ascomycota). Nova Hedwig 114(1–2):197–220. https://doi.org/10.1127/nova_hedwigia/2022/0679
Qiao M, Du X, Bian ZH, Peng J, Yu ZF (2017) Ellisembia pseudokaradkensis sp. nov. from Hainan, China. Mycotaxon 132(4):813–817
Qiao M, Guo JS, Tian WG, Yu ZF (2018) Ellisembia hainanensis sp. nov. from Hainan, China. Mycotaxon 133(1):97–101
Réblová M, Nekvindová J (2023) New genera and species with chloridium-like morphotype in the Chaetosphaeriales and Vermiculariopsiellales. Stud Mycol 106:199–258. https://doi.org/10.3114/sim.2023.106.04
Réblová M, Winka K (2000) Phylogeny of Chaetosphaeria and its anamorphs based on morphological and molecular data. Mycologia 92:939–954. https://doi.org/10.2307/3761589
Réblová M, Winka K (2001) Generic concepts and correlations in ascomycetes based on molecular and morphological data: Lecythothecium duriligni gen. et sp. nov. with a Sporidesmium anamorph, and Ascolacicola austriaca sp. nov. Mycologia 93:478–493. https://doi.org/10.2307/3761734
Réblová M, Nekvindová J, Fournier J, Miller AN (2020) Delimitation, new species and teleomorph-anamorph relationships in Codinaea, Dendrophoma, Paragaeumannomyces and Striatosphaeria (Chaetosphaeriaceae). MycoKeys 74:17–74. https://doi.org/10.3897/mycokeys.74.57824
Réblová M, Nekvindová J, Hernández M (2021a) Reflections on Menisporopsis, Multiguttulispora and Tainosphaeria using molecular and morphological data. J Fungi 7:438(1–36). https://doi.org/10.3390/jof7060438
Réblová M, Nekvindová J, Miller AN (2021b) Phylogeny and taxonomy of Catenularia and similar fungi with catenate conidia. MycoKeys 81:1–44. https://doi.org/10.3897/mycokeys.81.67785
Réblová M, Hernández M, Sklenář F, Nekvindová J, Réblová K, Kolařík M (2022) Consolidation of Chloridium: new classification into eight sections with 37 species and reinstatement of the genera Gongromeriza and Psilobotrys. Stud Mycol 103:87–212. https://doi.org/10.3114/sim.2022.103.04
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1 alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98. https://doi.org/10.3852/mycologia.97.1.84
Révay A (1988) Dematiaceous hyphomycetes inhabiting forest debris in Hungary III. Stud Bot Hung 20:95–100
Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16(6):276–277. https://doi.org/10.1016/s0168-9525(00)02024-2
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Santa Izabel TS, Cruz ACR, Gusmão LFP (2013) Conidial fungi from the semi-arid Caatinga biome of Brazil. Ellisembiopsis gen. nov., new variety of Sporidesmiella and some notes of Sporidesmium complex. Mycosphere 4(2):156–163. https://doi.org/10.5943/mycosphere/4/2/1
Seifert KA, Morgan-Jones G, Gams W, Kendrick B (2011) The genera of hyphomycetes. CBS KNAW Fungal Biodiversity Center, Utrecht
Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD (2006) Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycol Res 110:916–928. https://doi.org/10.1016/j.mycres.2006.06.004
Shi CK, Zhang XG (2007) Taxonomic studies of Sporidesmium from Guangxi, China. Mycotaxon 99:359–366
Shirouzu T, Harada Y (2008) Lignicolous dematiaceous hyphomycetes in Japan: five new records for Japanese mycoflora, and proposals of a new name, Helminthosporium magnisporum, and a new combination, Solicorynespora foveolata. Mycoscience 49:126–131. https://doi.org/10.1007/S10267-007-0399-8
Shoemaker RA, Hambleton S (2001) “Helminthosporium” asterinum, Polydesmus elegans, Imimyces, and allies. Can J Bot 79(5):592–599. https://doi.org/10.1139/b01-038
Sinclair RC, Eicker A, Morgan-Jones G (1990) Dematiaceous hyphomycetes from South Africa. I. Some phragmosporous, holoblastic and tretic species. S African J Bot 56:507–513. https://doi.org/10.1016/S0254-6299(16)31016-X
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Su HY, Hyde KD, Maharachchikumbura SSN et al (2016) The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province, China. Fungal Divers 80:375–409. https://doi.org/10.1007/s13225-016-0362-0
Subramanian CV (1954) Three new hyphomycetes. J Indian Bot Soc 33:28–35
Subramanian CV (1992) A reassessment of Sporidesmium (Hyphomycetes) and some related taxa. Proc Indian Acad Sci (plant Sci) B58:179–190
Subramanian CV (1994) Hyphomycetes from South East Asia - novelties from Singapore and Malaysia. Kavaka 22(23):52–76
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Turland NJ, Wiersema JH, Barrie FR et al (eds.) (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum vegetabile 159. Koeltz botanical books, Glashütten. https://doi.org/10.12705/Code.2018
Wan YL, Bao DF, Luo ZL, Bhat DJ, Xu YX, Su HY, Hao YE (2021) Two new species of Minimelanolocus (Herpotrichiellaceae, Chaetothyriales) from submerged wood in Yunnan, China. Phytotaxa 480(1):45–56. https://doi.org/10.11646/phytotaxa.480.1.4
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wu WP, Diao Y (2022) Anamorphic chaetosphaeriaceous fungi from China. Fungal Divers 116:1–546. https://doi.org/10.1007/s13225-022-00509-w
Wu WP, Zhuang W (2005) Sporidesmium, Endophragmiella and related genera from China. Fungal Diversity Press, Hong Kong
Xia JW, Wang JY, Yang CL, Li Z, Zhang XG (2016) Ellisembia henanensis sp. nov. and two new hyphomycete records from central China. Mycotaxon 131(3):597–603. https://doi.org/10.5248/131.597
Xia JW, Li HH, Zhang XG (2017) Two new species of hyphomycetes from southern China. Mycosystema 36(11):1483–1486. https://doi.org/10.13346/j.mycosystema.170140
Yan XY, Huang JE, Song HY, Gao Y, Hu HJ, Zhai ZJ, Yan JQ, Huo GH, Hu DM (2023) A new species of Dictyochaeta (Sordariomycetes, Chaetosphaeriales, Chaetosphaeriaceae) from freshwater habitats in China. Biodivers Data J 11:e97439. https://doi.org/10.3897/BDJ.11.e97439
Yang J, Maharachchikumbura SSN, Liu JK, Hyde KD, Jones EBG, Al-Sadi AM, Liu ZY (2018) Pseudostanjehughesia aquitropica gen. et sp. nov. and Sporidesmium sensu lato species from freshwater habitats. Mycol Prog 17:591–616. https://doi.org/10.1007/s11557-017-1339-4
Yang J, Liu LL, Jones EBG, Li WL, Hyde KD, Liu ZY (2021) Morphological variety in Distoseptispora and introduction of six novel species. J Fungi 7:945. https://doi.org/10.3390/jof7110945
Yang J, Liu LL, Jones EBG et al (2023) Freshwater fungi from karst landscapes in China and Thailand. Fungal Divers 119:1–212. https://doi.org/10.1007/s13225-023-00514-7
Zhang K, Guo W, Heredia G, Delgado JP, Ma J, Castañeda RF (2020) Anasporidesmiella gen. nov. for an atypical Sporidesmiella species and for A. manifesta sp. nov. Mycotaxon 135:719–727. https://doi.org/10.5248/135.719
Zhang H, Zhu R, Qing Y, Yang H, Li C, Wang G, Zhang D, Ning P (2022a) Polyphasic identification of Distoseptispora with six new species from freshwater. J Fungi 8:1063. https://doi.org/10.3390/jof8101063
Zhang JY, Ma J, Xiao YP, Boonmee S, Kang JC, Lu YZ (2022b) Morphological and phylogenetic analyses reveal five new species in Chaetosphaeriaceae. J Fungi 8:643. https://doi.org/10.3390/jof8060643
Zheng H, Wan Y, Li J, Castañeda RF, Yu Z (2020) Phialolunulospora vermispora (Chaetosphaeriaceae, Sordariomycetes), a novel asexual genus and species from freshwater in southern China. MycoKeys 76:17–30. https://doi.org/10.3897/mycokeys.76.57410
Zheng H, Li J, Guo JS, Qiao M, Yu ZF (2021) Anacraspedodidymum submersum sp. nov. (Chaetosphaeriaceae, Chaetosphaeriales), a new species of freshwater hyphomycetes from southwest China. Int J Syst Evol Microbiol 71(2):1–8. https://doi.org/10.1099/ijsem.0.004650
Acknowledgements
We gratefully acknowledge Uwe Braun (Martin-Luther-Universität, Germany) for kindly notifying us about the need to revise the typification of S. coronatum, and Hans-Josef Schroers (Section Editor of Mycological Progress) and Karol Marhold (Slovak Academy of Sciences, Slovakia) for suggesting and confirming the proper epitypification strategy to follow. Hermine Lotz-Winter and Ralph Mangelsdorff (Goethe Universität Frankfurt, Germany) are thanked for their advice that greatly helped to recollect E. coronata in its type locality and the identification of its host plant. We thank also the following curators, Michelle Price and Juan Carlos Zamora (G), Curtis J. Hansen (AUA), Lee Davies (K), Markéta Šandová (PRM), and Donat Magyar (National Public Health Center, Hungary) for the loan of herbarium specimens in their care, and Anita Tiller and Kari Hernandez (MERCA) for hosting loans to G.D. at their institution.
Funding
This project was partially financed by the Institutional Support for Science and Research of the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: G.D. and O.K. Field work and morphological and cultural studies: G.D., M.P., and O.K. SEM microscopy: W.C. Molecular work: O.K. and J.M.V. Phylogenetic analyses: G.D. and O.K. Writing and reviewing the paper: G.D. and O.K. Editing the draft: G.D., O.K., J.M.V., M.P., and W.C. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
All authors have approved and agreed with the submission of the final manuscript.
Competing interests
The authors declare no competing interest.
Additional information
Section Editor: Hans-Josef Schroers
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Delgado, G., Koukol, O., Maciá-Vicente, J.G. et al. Redefining Ellisembia sensu stricto with a reassessment of related taxa in Sordariomycetes. Mycol Progress 23, 32 (2024). https://doi.org/10.1007/s11557-024-01967-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-024-01967-z