Abstract
Purpose
The cuff pressure of a tracheal tube may increase during robot-assisted laparoscopic surgery for prostatectomy (RALP), which requires pneumoperitoneum in a steep head-down position, but there have been no studies which confirmed this.
Methods
In study 1, we studied how frequently the cuff pressure significantly increased during anesthesia for the RALP. In study 2, we studied if the SmartCuff (Smiths Medical Japan, Tokyo) automatic cuff pressure controller would minimize the changes in the intracuff pressure. With approval of the study by the research ethics committee (approved number: 20115), we measured the cuff pressures in anesthetized patients undergoing RALP and in those undergoing gynecological laparotomy (as a reference cohort), with and without the use of the SmartCuff.
Results
In 21 patients undergoing RALP, a clinically meaningful increase (5 cmH2O or greater) was observed in all the 21 patients (P = 0.00; 95% CI for difference: 86–100%), whereas in 23 patients undergoing gynecological laparotomy, a clinically meaningful decrease (5 cmH2O or greater) was observed in 21 of 23 patients (91%, P < 0.0001; 95% CI for difference: 72–99%). With the use of the SmartCuff, there was no significant increase in the incidence of a clinically meaningful change in the intracuff pressure in either cohort.
Conclusion
The cuff pressure of a tracheal tube would frequently increase markedly in patients undergoing RALP, whereas it would frequently decrease markedly in patients undergoing gynecological laparotomy. The SmartCuff may inhibit the changes in the cuff pressure during anesthesia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Robot-assisted laparoscopic surgery has increasingly been used as a minimally invasive procedure, and its indications have been expanding. Prostatectomy is one such an indication, as the robot-assisted laparoscopic prostatectomy (RALP) has some potential advantages over conventional laparotomy, including a shorter operative time, less intraoperative blood loss, less postoperative pain, a lower risk of erection dysfunction, and a shorter hospital stay.
One major possible problem associated with anesthesia for RALP is that the procedure may require a high intraperitoneal pressure (15–20 mmHg (or 20–27 cmH2O)) [1] in a steep head-down position (25–30°), which may increase the intra-cranial, intra-ocular pressures, and the airway pressure. The steep head-down position may also cause stagnation of blood flow in the upper parts of the body, leading to edema of the airway mucosa due to impaired cervical lymphatic circulation. Therefore, pneumoperitoneum, in addition to steep head-down position, may increase the cuff pressure of a tracheal tube. Nevertheless, at the time of carrying out this study and analyzing the results, there had been no reports investigating the degree of change in the cuff pressure of a tracheal tube during anesthesia for the RALP. Therefore, the main purpose of this study was to assess how frequently the intracuff pressure markedly increases during anesthesia for the RALP.
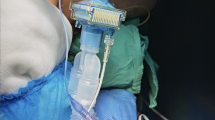
The SmartCuff (Smiths Medical Japan, Tokyo), an automatic cuff pressure controller, has become commercially available in 2018 (Fig. 1). After this device was introduced into clinical practice at our hospital, we carried out another observational study, with the main aim to assess if the use of the SmartCuff would prevent the changes in the cuff pressure during anesthesia.
Methods
The research ethics committee of Dokkyo Medical University Saitama Medica Center approved the study to be reported (approved number: 20115; approved date: 25th December, 2020). The ethics committee judged that no written informed consent from each patient would be required, as this was a series of observational cohort studies. Nevertheless, with the instruction made by the research ethics committee, we notified the public, information concerning the research, including the purpose of utilization of information in the research, and opportunities to refuse that the research was commenced or continued on the research subject had been ensured for the research subjects, and other factors, at least for 6 months. We started to analyze the obtained data 12 months after the notification to the public.
Study 1: changes in the intracuff pressure during anesthesia
RALP cases
At our hospital, more than 90% of prostatectomy have been performed by RALP, using the da Vinci Surgical System (Intuitive surgical Inc, Sunnyvale, USA) or the hinotori™ Surgical Robot System (Medicaroid Corporation, Kobe, Japan).
We studied 21 patients who underwent the RALP, during July 2019 to January 2020. We excluded patients when at least one of the following was present: emergency surgery, morbidly obese (body mass index (BMI) > 35), cranial nerve system disease, cervical spine or cervical cord disease, pathological deformity of the airway, history of difficult airway management or tracheostomy, at a high risk of pulmonary aspiration, untreated coagulation abnormalities, or active infections.
In the operating room, a blood pressure cuff, an electrocardiogram, and a pulse oximeter were applied, and an intravenous cannula was inserted either at the back of the hand or the wrist. The patient’s head was placed on a pillow (6–7 cm in height). After preoxygenation of the patient with 100% oxygen through a facemask for more than 3 min, anesthesia was induced with intravenous propofol (1.5–2 mg.kg−1), fentanyl (2–4 μg.kg−1), and neuromuscular blockade was produced with rocuronium (0.6 mg.kg−1). Adequacy of neuromuscular blockade was confirmed with a neuromuscular blockade monitor (TOF watch®, Merck & Co., Kenilworth, NJ, USA), and the trachea was intubated using a McGrath® Mac (Covidien, Tokyo, Japan) videolaryngoscope, using a standardized method reported previously [2], so that the effect of intubation factor would be minimum between patients. A tracheal tube of 8.0-mm ID, with a taper-guard cuff (Covidien, Tokyo, Japan), was used. If the trachea could not be intubated at the first attempt, the patient was excluded from the data analysis. Adequate depth of a tracheal tube in the trachea was determined by each anesthesiologist by auscultation of the chest, with the depth mark of the tube approximately 21–23 cm at the gap between the upper and lower teeth. With this positioning, the cuff of the tracheal tube would be located at the lower segment of the cervical trachea, 3–4 cm beyond the glottis.
The lungs were ventilated with a pressure control mode, with a setting of the upper limit of inspiratory pressure 25 cmH2O and the positive end-expiratory pressure of 5 cmH2O, and the ratio of inspiratory and expiratory times (I: E ratio) of 1:2. Respiratory rate was adjusted to keep the end-tidal carbon dioxide concentration to be 35–45 mmHg. A gastric tube was inserted and gastric contents, if any, were removed by suction. If semi-solid materials were identified in the stomach, the patient was excluded from the study (because of increased risk of pulmonary aspiration). The gastric tube was left in place until the end of anesthesia for drainage.
Anesthesia was maintained either with 1.5–2.2% sevoflurane in oxygen or with target-controlled intravenous propofol infusion (with the target blood concentration of 2.3–2.8 μg.ml−1), and analgesia was provided by intermittent injection of 0.05–0.1 mg fentanyl and continuous infusion of remifentanil 0.2–0.5 μg.kg−1.min−1. Rocuronium was injected intermittently during anesthesia to maintain the train of four (TOF) to be the maximum of 1 (this was a part of clinical protocol for anesthesia, agreed between the Departments of Urology and Anesthesiology, to prevent straining (“buckling”) during RALP).
At the beginning of surgery, the cuff pressure of a tracheal tube was adjusted to be 25 cmH2O, using the VBM Cuff Control Inflator (Smiths Medical, Tokyo, Japan). We adjusted to this pressure, because the American Thoracic Society guidelines recommend that the cuff pressure to be maintained > 20 cmH2O, to prevent ventilator-associated pneumonia [3], and that blood flow in the tracheal mucosa may decrease when the cuff pressure exceeds 30 cmH2O [3].
Intracuff pressures were measured using the VBM Cuff Control Inflator, during pneumoperitoneum (with the intra-abdominal pressure of 15 mmHg) and after head-down (of 30-degrees): it was measured at the start of pneumoperitoneum (0 s), every 10 s for the initial 1 min, then every minute until 5 min, and thereafter at 10 min and 15 min, after the start of pneumoperitoneum. The patient was turned to the head-down position (with the intra-abdominal pressure reduced to 10 mmHg), the cuff pressure was measured every minute for the initial 5 min, 10 min, 15 min, and 30 min later. Because the cuff pressure was higher during inspirations than during expirations, we decided to record the cuff pressures during inspirations. If the cuff pressure increased higher than 45 cmH2O, the cuff pressure was re-adjusted to 25 cmH2O, and observation was terminated.
Gynecological cases
It was not known how the intracuff pressure, if any, would change during anesthesia (without the use of nitrous oxide), in patients undergoing conventional “typical” operation that would not directly affect the intracuff pressure. Therefore, we decided to study the change in the intracuff pressure in patients undergoing conventional “typical” operation, as a reference cohort. We considered that gynecological (open) laparotomy would be suitable, as the operation site is remote from the respiratory organs, and operation is usually performed with the patient in the supine position.
We studied 23 patients undergoing gynecological laparotomy, with the patient in the supine position, during July 2019 to January 2020. We excluded patients when at least one of the following was present: emergency surgery, morbidly obese (body mass index (BMI) > 35), pregnant, cranial nerve system disease, cervical spine or cervical cord disease, pathological deformity of the airway, history of difficult airway management or tracheostomy, at a high risk of pulmonary aspiration, untreated coagulation abnormalities, or active infections.
Anesthesia was induced and the trachea intubated with the same procedures as in for patients who underwent the RALP. A tracheal tube of 7.0-mm ID, with a taper-guard cuff (Covidien, Tokyo, Japan), was used. Adequate depth of a tracheal tube in the trachea was determined by each anesthesiologist by auscultation of the chest, with the depth mark of the tube approximately 19–21 cm at the gap between the upper and lower teeth. With this positioning, the cuff of the tracheal tube would be located at the lower segment of the cervical trachea, 3 to 4 cm beyond the glottis.
The cuff pressure was adjusted to 25 cmH2O, when surgery started, and the cuff pressure was measured every 10 s for the initial 1 min, then every minute until 5 min, and every 5 min thereafter until 30 min, after the start of surgery. If the cuff pressure decreased less than 10 cmH2O, or gas leakage was detected around the cuff, the cuff pressure was re-adjusted to 25 cmH2O, and observation was terminated.
Study 2: efficacy of the SmartCuff
After the SmartCuff was introduced into clinical practice at our hospital in summer 2019, we investigated the efficacy of the SmartCuff in 28 patients who underwent RALP, and in 23 patients who underwent gynecological laparotomy (as a reference cohort), during January 2020 to October, 2020. The SmartCuff is a handy automated cuff controller, which can directly connect to the pilot balloon valve of the tracheal tube, continuously measure the cuff pressure, and automatically adjust the cuff pressure to a set pressure.
The anesthesia method and procedure were the same as in study 1. At the beginning of surgery, the SmartCuff was connected to a tracheal tube, and the cuff pressure was adjusted to be 25 cmH2O. The cuff pressures were recorded at the timings as defined in the study 1. The presence or the absence of postoperative respiratory complications was assessed as in study 1.
In all the four cohorts, we visited each patient approximately 24 h after operation, and asked if the patient had respiratory complications (sore throat, hoarseness, and dysphagia).
Statistical analysis
In both Studies 1 and 2, the primary endpoint was the incidence of a clinically meaningful change in the cuff pressure during anesthesia. McNemar test was used, in each cohort, to assess if the incidence of a clinically meaningful change of the cuff pressure (5 cmH2O or greater), or even a greater change (10 cmH2O or greater) was increased.
As the secondary outcome measures, paired t test was used to compare the intracuff pressure between 0 and 5 min after the start of measurements, and between the maximum (or the minimum) pressure and the pressure at 0 min of the start of measurements, in each cohort. 95% confidence intervals for difference were calculated for the incidence of a clinically meaningful change in the cuff pressure, and for the change in the cuff pressure. Fisher’s exact test was used to compare the incidence of a clinically meaningful change of the cuff pressure (5 cmH2O or greater), and to compare the incidence of postoperative complications, with and without the use of the SmartCuff.
We considered that the increase in the cuff pressure of 5 cmH2O would be clinically meaningful, and that its incidence in one third of patients during anesthesia (i.e., 33%) would be clinically meaningful. Manual calculations using Altman’s nomogram [4, 5] as well as G*Power 3 [6] have indicated that twenty patients would be required to detect this difference in each cohort, with a power of 0.8, and P = 0.05. A P value of less than 0.05 was considered significant for the primary outcome measure. Regarding hypothesis tests related to the secondary outcome measures, we principally regarded the results as subsidiary information. Nevertheless, we judged that there was a significant difference, when P < 0.001.
Results
Characteristics of patients undergoing the robot-assisted laparoscopic prostatectomy (RALP) and of patients undergoing gynecological laparotomy, and operation and anesthesia time are shown in Tables 1 and 2.
Study 1: changes in the intracuff pressure during anesthesia
RALP cases
In 21 patients who underwent the RALP, the intracuff pressure of a tracheal tube immediately started to increase during insufflation of gas to the peritoneal cavity, and continued to be high during the head-down position (Fig. 2). The mean cuff pressure significantly increased to 33 (standard deviation (SD): 2.9) cmH2O, 5 min after the start of pneumoperitoneum (P < 0.0001, mean (95% CI) difference from the start: 7.6 (6.3–9.0) cmH2O). The mean maximum cuff pressure was 35.0 (SD: 3.4) cmH2O, with the range of 30–43 cmH2O, and the increase was significant (P < 0.0001, mean (95% CI) difference: 10.0 (8.4–11.5) cmH2O). Roughly 15 min after the head-down positioning, the cuff pressure started to decrease toward 25 cmH2O.
A clinically meaningful increase (5 cmH2O or greater) in the cuff pressure was observed in all the 21 patients (100%), and this incidence was significant (P = 0.00; 95% CI 86–100%). The increase of 10 cmH2O or greater (that means 35 cmH2O or higher) was observed in 9 of 21 patients (43%) (P = 0.008; 95% CI 22–66%).
Gynecological cases
In 23 patients undergoing gynecological laparotomy, the cuff pressure started to decrease from 25 cmH2O to the mean pressure of 15 cmH2O, on average 30 min after the start of surgery (Fig. 2). The mean cuff pressure was 23 (SD: 2.0) cmH2O, 5 min after the start of surgery, and the decrease was significant (P < 0.0001, mean (95% CI) difference: 2.3 (1.4–3.1) cmH2O). The mean minimum cuff pressure was 16.4 (SD: 3.8) cmH2O, with the range of 10–22 cmH2O, and the decrease was significant (P < 0.0001, mean (95% CI) difference: 8.6 (6.9–10.1) cmH2O). In one patient, gas leakage around the cuff occurred, 25 min after the start of surgery, when the cuff pressure was 13 cmH2O. The cuff was inflated to prevent gas leakage, and the measurements were terminated in this case.
A clinically meaningful decrease (5 cmH2O or greater) in the cuff pressure was observed in 21 of 23 patients (91%), and this incidence was significant (P < 0.0001; 95% CI 72–99%). The decrease of 10 cmH2O or greater (that means 15 cmH2O or lower) was observed in 8 of 23 patients (35%) (P = 0.008; 95% CI 16–57%).
Study 2: efficacy of the SmartCuff
RALP cases
When the SmartCuff was used in 28 patients undergoing RALP, the cuff pressure mildly increased during anesthesia (Fig. 3). The mean maximum cuff pressure was 28.0 (SD: 0.9) cmH2O, with the range of 26–30 cmH2O, and the increase was significant (P < 0.0001, mean (95% CI) difference: 3.0 (2.7–3.4) cmH2O).
A clinically meaningful increase (5 cmH2O or greater) in the cuff pressure was observed in 1 of 28 patients (4%), and this incidence was not significant (P = 1.0; 95%CI for difference: 0–18%).
Gynecological cases
When the SmartCuff was used in 23 patients undergoing gynecological laparotomy, the cuff pressure did not change significantly and remained constant, during anesthesia (Fig. 3).
Comparisons with and without the use of the SmartCuff
In patients undergoing the RALP, the incidence of a clinically meaningful increase was significantly lower with the use of the SmartCuff (0 of 28 patients (0%)) than without (21 of 21 patients (100%)) (P < 0.0001; 95% CI for difference: 86–100%).
In patients undergoing gynecological laparotomy, the incidence of a clinically meaningful decrease was significantly lower with the use of the SmartCuff (0 of 23 patients (0%)) than without (21 of 23 patients (91%)) (P < 0.0001; 95% CI for difference: 77–100%).
The incidences of postoperative sore throat, dysphagia, or hoarseness, 24 h after surgery are shown in Table 3.
Discussion
In study 1, we have found that in patients undergoing RALP, the cuff pressure of a tracheal tube markedly increased immediately after the start of pneumoperitoneum, and remained high after head-down. In contrast, in patients undergoing gynecological laparotomy, the cuff pressure markedly decreased over time. In study 2, we have found that, when the SmartCuff was used, the changes in the cuff pressure were not clinically meaningful, both in patients undergoing RALP and in patients undergoing gynecological laparotomy.
After completion of data analysis, one article [7] has been published to report the intracuff changes in patients who were undergoing robot-assisted laparoscopic pelvic surgeries. The study has shown that the cuff pressure increased after pneumoperitoneum, and remained high after head-down. Our results are consistent with the results of this study [7].
In the tracheal mucosa, the arterial pressure is about 40 cmH2O, and the capillary pressure about 25 cmH2O [8]. If the cuff pressure exceeds the capillary pressure, ischemia, congestion, edema or necrosis of the tracheal mucosa and the cartilage may occur [8]. One study using bronchoscopic observation of the tracheal lumen has shown that, at the cuff pressure of 25 cmH2O, both tracheal mucosa and the blood vessels appeared normal, but at 30 cmH2O, some areas of the mucosa were less pink, and at 40 cmH2O, the mucosa became very pale [8]. The increased cuff pressure may also cause complications such as postoperative sore throat, dysphagia, and hoarseness [9]. On the other hand, if the cuff pressure become too low, the incidence of gas leakage around the cuff or of pulmonary aspiration is increased, leading to insufficient ventilation or ventilator-associated pneumonia [10, 11], and thus the American Thoracic Society guidelines recommend that the cuff pressure to be maintained > 20 cm H2O [3]. Because of these reasons, the cuff pressure around 25 cmH2O has been considered to be optimal.
The cuff pressure may be influenced during anesthesia by several factors, such as patient’s body position, intraperitoneal insufflation of gas for laparoscopy, the use of nitrous oxide, and the degree of neuromuscular blockade [12,13,14,15]. In addition, numerous physical factors can affect the intracuff pressure, e.g., elasticity, the diameter, and the shape of the cuff; the change in the volume of the air, and in the temperature, in the cuff; the outer diameter of a tracheal tube and the internal diameter of the airway lumen; the shape and the smoothness of the airway lumen (e.g., corrugated cartilaginous portion and membranous portion); and relative sizes of the diameter of the cuff and diameter of the airway lumen.
Our study design cannot elucidate the mechanisms for the change in the cuff pressure. A few possible mechanisms have been reported for the changes in the cuff pressure during general anesthesia [16, 17], but these are not confirmed by studies, and thus speculative. One plausible explanation for the increase in the cuff pressure in patients undergoing RALP is that intraperitoneal insufflation would raise the diaphragm and would compress the airway structures, such as the trachea, so that the gap between a tracheal tube and the inner surface of the trachea may be narrowed. Steep head-down position would also compress the airway structures and would increase the external pressure to the trachea, by increasing blood flows in the upper part of the body and by increasing the weight incurring to the neck and thorax. These changes might have led to the increase in the cuff pressure.
In contrast, one of plausible reasons for the decrease in patients undergoing gynecological laparotomy may relate to the cuff design. Currently, a tracheal tube with a low-pressure, high-volume cuff is mainly used, because compared with a high-pressure, low-volume cuff, a low-pressure, high-volume cuff is less likely to produce an excessive pressure to the tracheal membrane. As the diameter of a low-pressure, high-volume cuff is designed to be longer than the estimated internal diameter of the tracheal lumen, the cuff can seal the gap between the tracheal tube and tracheal lumen, before the cuff is inflated maximally. Therefore, when the cuff is inflated with an appropriate volume of air in the trachea (for example, with the minimum volume of air to prevent air leakage around the cuff), the cuff would not be inflated evenly, producing wrinkling of the cuff. The air would distribute over time more evenly in the cuff, so that the area of the cuff attaching to the tracheal lumen would decrease, leading to the decrease in the cuff pressure. The same might have occurred in patients undergoing the RALP, in whom the cuff pressure appeared to decrease after the initial increase by the other factors described above.
The cuff pressure of a tracheal tube is conventionally measured intermittently using a cuff control inflator, and can be adjusted periodically. However, since the cuff pressure may frequently fluctuate due to body movements, repositioning, and surgical procedures, it may be difficult for a conventional device to maintain a constant cuff pressure.
Several types of automated cuff controllers have been reported [18,19,20,21], but there may be considerable differences in the efficacy between the devices in maintaining the cuff pressure [19, 20], and in preventing gas leakage around the cuff [21]. In our study, the SmartCuff could maintain the intracuff pressure during anesthesia in all the patients, and no gas leakage was observed in any patients, indicating that the SmartCuff would effectively inhibit both increase and decrease in the cuff pressure. Another advantage of the SmartCuff includes that the device is handy, battery powered, and it does not make any noticeable noise, so that it may be useful for a sedated patient whose trachea is intubated.
When the SmartCuff was used, the intracuff pressure was constantly 25 cmH2O in patients who underwent gynecological laparotomy, whereas it was approximately 28 cmH2O in patients who underwent RALP. The reasons for this difference are not clear, but one difference we noticed was that, in RALP cases, the cuff pressure fluctuated between 28 and 25 cmH2O during inspirations and expirations. The SmartCuff adjusts the cuff pressure to a set pressure, using a measured pressure which was measured at a longer proportion of time. As the I:E ratio was 1: 2, it is likely that the SmartCuff adjusted the pressure to be 25 cmH2O during expirations, resulting to the cuff pressure increasing to the mean of 28 cmH2O during inspirations.
Limitations of the study include that this was not a randomized controlled study, so that there may be confounding factors when direct comparison are made between the cohorts. Because of this, we regarded these comparisons as the secondary outcome measures. Nevertheless, we considered it reasonable to regard that there were significant differences, if P < 0.001. Another limitation of the study is that we did not formally compare between the cohorts for the incidence of postoperative complications, because this was not a randomized controlled study, and because a formal power analysis was carried out for this secondary outcome measure (the fact which indicates that the number of patients studied would be too small to compare for this outcome measure).
Conclusion
In conclusion, we have shown that the cuff pressure of a tracheal tube would frequently increase markedly in patients undergoing RALP, whereas it would frequently decrease markedly in patients undergoing gynecological laparotomy. The SmartCuff may inhibit the changes in the cuff pressure during anesthesia.
Data availability
Data are available from the authors.
References
Valdivieso RF, Hueber PA, Zorn KC. Robot assisted radical prostectomy: how I do it. Part I. patient preparation and positioning. Can J Urol. 2013;20:6957–61.
Tsunoda N, Asai T. A double-curved tube for McGrath®MAC videolaryngoscope-guided tracheal intubation. Br J Anaesth. 2022;128:e14–6.
Guidelines for the management of adults with hospital-acquired, ventilator-associated. And healthcare-associated pneumonia. Am J Resp Crit Care Med. 2005;171:388–416.
Asai T. Statistics notes-28: Sample size determination-2 (in Japanese with English abstract). Masui (Jpn J Anesthesiol). 2022;71:350–7.
Altman DG. Clinical traial. In: Altman DG, editor. Practical statistics for medical research. London: Chapman & Hall; 1991. p. 440–76.
Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Gupta P, Tandon S, Dhar M, Agarwal A, Pathak S, Prabakaran P. A prospective observational study on changes in endo-tracheal tube cuff pressure and its correlation with airway pressures during various stages of robotic pelvic surgeries. J Anaesthesiol Clin Pharmacol. 2022;38:270–4.
Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow. Endoscopic study of effects of four large volume cuffs. Br Med J. 1984;288:965–8.
El-Boghdadly K, Bailey CR, Wiles MD. Postoperative sore throat: a systematic review. Anaesthesia. 2016;71:706–17.
Asai T, Shingu K. Leakage of fluid around high-volume, low-pressure cuffs. A comparison of four tracheal tubes. Anaesthesia. 2001;56:39–42.
Rello J, Soñora R, Jubert P, Artigas A, Rué M, Vallés J. Pneumonia in intubated patients: role of respiratory airway care. Am J Resp Crit Care Med. 1996;154:111–5.
Sole ML, Penoyer DA, Su X, Jimenez E, Kalita SJ, Poalillo E, Byers JF, Bennett M, Ludy JE. Assessment of endotracheal cuff pressure by continuous monitoring: a pilot study. Am J Crit Care. 2009;18:133–43.
Yildirim ZB, Uzunkoy A, Cigdem A, Ganidagli S, Ozgonul A. Changes in cuff pressure of endotracheal tube during laparoscopic and open abdominal surgery. Surg Endosc. 2012;26:398–401.
Combes X, Schauvliege F, Peyrouset O, Motamed C, Kirov K, Dhonneur G, Duvaldestin P. Intracuff pressure and tracheal morbidity: influence of filling with saline during nitrous oxide anesthesia. Anesthesiology. 2001;95:1120–4.
Sole ML, Su X, Talbert S, Penoyer DA, Kalita S, Jimenez E, Ludy JE, Bennett M. Evaluation of an intervention to maintain endotracheal tube cuff pressure within therapeutic range. Am J Crit Care. 2011;20:109–17.
Lobato EB, Paige GB, Brown MM, Bennett B, Davis JD. Pneumoperitoneum as a risk factor for endobronchial intubation during laparoscopic gynecologic surgery. Anesth Analg. 1998;86:301–3.
Wu CY, Yeh YC, Wang MC, Lai CH, Fan SZ. Changes in endotracheal tube cuff pressure during laparoscopic surgery in head-up or head-down position. BMC Anesthesiol. 2014;14:75.
Willis BA, Latto IP, Dyson A. Tracheal tube cuff pressure. Clinical use of the Cardiff Cuff Controller. Anaesthesia. 1988;43:312–4.
Valencia M, Ferrer M, Farre R, Navajas D, Badia JR, Nicolas JM, Torres A. Automatic control of tracheal tube cuff pressure in ventilated patients in semirecumbent position: a randomized trial. Crit Care Med. 2007;35:1543–9.
Kunitz O, Jansen R, Ohnsorge E, Haaf-vonBelow S, Schulz-Stübner S, Rossaint R. Cuffdruckmessung und Regelung im Erwachsenenalter [Cuff pressure monitoring and regulation in adults]. Anaesthesist. 2004;53:334–40 (German).
Weiss M, Doell C, Koepfer N, Madjdpour C, Woitzek K, Bernet V. Rapid pressure compensation by automated cuff pressure controllers worsens sealing in tracheal tubes. Br J Anaesth. 2009;102:273–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tsunoda, N., Asai, T. & Okuda, Y. Tracheal tube cuff pressure during anesthesia for robotic-assisted laparoscopic prostatectomy and the efficacy of an automatic cuff pressure controller (SmartCuff): observational studies of 1-sample paired data. J Anesth 37, 234–241 (2023). https://doi.org/10.1007/s00540-022-03151-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-022-03151-7