Abstract
Purpose
Preoperative malnutrition is associated with poor postoperative outcomes in patients with pancreatic cancer. This study evaluated the effectiveness of current practice in nutritional support for patients with pancreatic cancer.
Methods
Observational multicenter HPB network study conducted at the Isala Clinics Zwolle, Medical Spectrum Twente, Medical Center Leeuwarden, and University Medical Center Groningen between October 2021 and May 2023. Patients with a suspected pancreatic malignancy scheduled for surgery were screened for malnutrition using the Patient-Generated Subjective Global Assessment (PG-SGA) questionnaire and referred to a dedicated dietician for nutritional support comprising pancreatic enzyme replacement therapy, dietary advice, and nutritional supplements to achieve adequate caloric and protein intake. At baseline, 1 day preoperatively, and 3 months postoperatively, the nutritional status and muscle thickness were evaluated.
Results
The study included 30 patients, of whom 12 (40%) classified as malnourished (PG-SGA ≥ 4) at baseline. Compared to well-nourished patients, malnourished patients were younger, were predominantly female, and had a higher body mass index, despite having lost more body weight in the past 6 months. All malnourished patients and 78% of the well-nourished patients received nutritional support. Consequently, a preoperative increase in caloric and protein intake and body weight were observed. Postoperatively, despite a further increase in caloric intake, a considerable decrease in protein intake, body weight, and muscle thickness was observed.
Conclusion
Malnutrition is prevalent in patients undergoing pancreatic surgery. Nutritional support by a dedicated dietician is effective in enhancing patients’ preoperative nutritional status. However, postoperative monitoring of adequate nutritional intake in patients could be improved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is an aggressive malignancy, and surgical resection remains the most important treatment modality to provide patients with the best chance of long-term survival [1]. However, pancreatic surgery is highly invasive, and patients’ postoperative recovery is often impeded by (severe) postoperative complications [2]. Currently, greater attention is paid to the preoperative optimization of patient-related modifiable risk factors, a practice known as prehabilitation, with the aim of enhancing postoperative outcomes in patients. Multiple patient-related risk factors are identified as valid indicators for poor postoperative outcomes and are deemed modifiable in the preoperative phase in patients undergoing major abdominal surgery [3, 4]. One significant preoperative risk factor in patients with pancreatic cancer is the development and progression of malnutrition, a complex condition characterized by increased tumor metabolism, inadequate nutrient intake, and malabsorption [5]. Severe malnutrition leads to cachexia, a metabolic syndrome characterized by the pathological loss of skeletal muscle mass and adipose tissue [6]. Ultimately, malnutrition contributes to poor surgical and oncological outcomes due to a reduced physical reserve in patients which is necessary to withstand the physical demands of surgery [7,8,9,10,11].
Malnutrition is frequently observed in patients with cancer, with incidence rates ranging from 50 to 80% being reported [7, 12,13,14]. The multifactorial etiology of malnutrition in patients with pancreatic cancer encompasses various factors, including tumor-related factors, pancreatic endocrine and exocrine insufficiency, disease-related symptoms, and treatment-related side effects [5, 15, 16]. In particular, the endocrine and exocrine function of the pancreas can be affected in patients with pancreatic cancer. Endocrine pancreatic insufficiency can lead to type 3c diabetes mellitus (DM), also known as pancreatogenic DM, and contributes to maldigestion and malabsorption of nutrients [5]. However, exocrine pancreatic insufficiency, in particular, contributes to the malabsorption of essential nutrients due to the inadequate secretion of digestive enzymes into the small intestine [5]. Treatment for exocrine pancreatic insufficiency typically involves pancreatic enzyme replacement therapy (PERT), which includes taking digestive enzymes with meals to aid in digestion and absorption [9, 17]. Additionally, dietary modifications and, if necessary, oral nutritional supplements or tube feeding may be recommended in order to address caloric and protein deficiencies [9]. Notably, only a few years ago, Latenstein et al. found that nearly half of the malnourished patients with pancreatic cancer did not receive preoperative nutritional support [18].
In the current study, we sought to determine the effectiveness of current practice concerning preoperative nutritional support in (malnourished) patients with pancreatic cancer undergoing surgery in a regional hepato-pancreato-biliary (HPB) network.
Material and methods
Study design
This observational multicenter study was conducted between October 2021 and May 2023 in a regional HPB network consisting of four hospitals: Isala Clinics Zwolle, Medical Spectrum Twente, Medical Center Leeuwarden, and University Medical Center Groningen (UMCG). The aim of this study was to investigate the current practice concerning nutritional support in patients undergoing pancreatic surgery. Hereto, in all consecutive patients over 18 years old with a suspected pancreatic malignancy who were scheduled for an elective pancreatoduodenectomy and had provided informed consent, it was recorded whether nutritional support was provided perioperatively and what this support consisted of. Patients requiring neoadjuvant chemotherapy were excluded. All included patients were screened for malnutrition using the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) questionnaire and were classified as either malnourished (score ≥ 4) or well nourished (score < 4) [19]. Additionally, malnourished patients were compared to well-nourished patients and the impact of perioperatively provided nutritional support was evaluated. This study was approved by the Medical Ethics Committee of the UMCG (Netherlands research register number 202000299), and written consent was obtained from all patients prior to inclusion. The study was performed in accordance with the ethical standards as stated in the 1964 Declaration of Helsinki and its later amendments. Lastly, STROBE guidelines were adhered to as applicable for this study [20].
Perioperative nutritional support: current practice
Whether patients were preoperatively referred to a dedicated dietician for nutritional support was at the discretion of the consulting surgeon. In all participating hospitals, a dedicated dietician conducted a comprehensive nutritional assessment for patients who were referred for nutritional support to determine their specific dietary requirements [21]. Based on this assessment, the dietician gave patients dietary advice to achieve adequate caloric and protein intake and provided them with a prescription for nutritional supplements, tube feeding, or parenteral nutrition if necessary. Furthermore, the dietician initiated PERT if patients had symptoms of exocrine pancreatic insufficiency. Lastly, the dietician continuously evaluated the nutritional treatment plan, with active and regular follow-up of the patient.
Study objectives
The primary objective of this study was to assess the perioperative referral rates for nutritional support among patients suspected of pancreatic cancer and scheduled to undergo pancreatic surgery. Also, it was recorded what the nutritional support consisted of. Additionally, the study aimed to evaluate the efficacy of optimizing the nutritional status, particularly in malnourished patients, during the relatively brief preoperative period.
Study assessments
To evaluate the effects of nutritional support on patients’ nutritional status, nutritional intake, muscle thickness, and functional capacity in both the preoperative and postoperative periods, the below mentioned study assessments were performed at inclusion within a week of the patients’ visit to the surgical outpatient department (T0), 1 day prior to surgery (T1), and 3 months after surgery (T2).
Nutritional status and support
To assess their nutritional status, patients were asked to fill out the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) questionnaire [19]. The questionnaire is patient-led and evaluates alterations in body weight, dietary intake, gastrointestinal symptoms, and functional capacity during the past month. The questionnaire has been found to effectively identify malnutrition in patients with cancer [22]. Subsequently, during each study visit, body mass index (BMI) was calculated. Additionally, the total number of patients preoperatively referred to a dedicated dietician was recorded. Regarding referred patients, the total number of preoperative consultations (either face-to-face or by telephone) was registered. Furthermore, the number of days between the first consultation with the dietician and the day of surgery was listed. Patients’ utilization of oral nutritional supplements, tube feeding, or parenteral nutrition to complement their dietary intake was documented. Lastly, whether and when patients started PERT was recorded.
Nutritional intake
Patients were asked to keep a nutritional diary and to record their dietary intake for three consecutive days prior to each study visit. Based on these nutritional diaries, energy intake and protein intake were calculated using an online nutrition calculator (Mijn Eetmeter, Stichting Voedingscentrum Nederland, Rotterdam, the Netherlands). Energy intake was expressed in kilocalories (kcal) per kilogram (kg) of body weight per day, whereas protein intake was expressed in grams (g) of protein per kilogram of body weight per day.
Muscle thickness
Previous research indicated that combined muscle thickness measurement performed by point-of-care ultrasound (POCUS) is a valid indicator for skeletal muscle status [23]. Therefore, muscle thickness of the m. biceps brachii, m. rectus femoris, m. vastus intermedius, and m. rectus abdominis was measured in patients by three researchers (R.N.M.H., A.G.W., and D.K.) using POCUS (Philips FUS6882 Lumify L12-4, Koninklijke Philips N.V., the Netherlands) according to a previously published protocol [24]. The average of these measurements was used for the final analysis. An example of the acquired images is provided in Fig. 1.
Functional capacity
Patients’ functional capacity was assessed by performing the five times sit to stand test (FTSST). The FTSST is a validated test used to assess functional independence in patients; it includes the assessment of lower limb strength, balance control, and mobility [25]. Moreover, as a proxy for muscle strength, patients’ handgrip strength was assessed using a handheld dynamometer (Jamar FAB12- 0604 + , JLW Instruments, Chicago, IL, USA) [26].
Patient characteristics and postoperative surgical outcomes
Baseline characteristics (i.e., age, sex, American Society of Anesthesiology (ASA) score, smoking status, and relevant comorbidities), intraoperative variables (i.e., operation time and estimated blood loss), and 30-day postoperative outcomes (i.e., length of hospital stay, complications graded according to the Clavien-Dindo classification [27], length of intensive care unit (ICU) or medium care unit (MCU) stay, ICU and MCU readmission rate, unplanned hospital readmission, and 30-day mortality rate) were collected and analyzed.
Statistical analysis
In light of the observational and explorative design of this study, no formal statistical tests were executed. For both patient characteristics and study-specific outcomes between malnourished patients and well-nourished patients, descriptive statistics were applied. For all continuous variables, the median and interquartile range are provided to convey information about the distribution of variables among the study cohort. Categorical data are presented as numbers and percentages. Paired line graphs were used to represent the distribution of daily energy intake, daily protein intake, body weight, and muscle thickness measurements during each study visit for malnourished and well-nourished patients. Additionally, the daily energy intake and protein intake of individual malnourished patients were represented in parallel plots. The R software version 4.2.2. (R Foundation for Statistical Computing, Vienna, Austria) was used for descriptive analyses, and GraphPad Prism version 10.0.2 (GraphPad Software, Boston, Massachusetts, USA) was used to represent the data.
Results
Study cohort
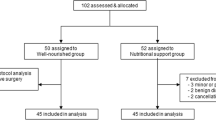
A total of 30 patients were included in this study. In Table 1, the baseline characteristics of the included patients are provided. Twelve patients (40%) were classified as malnourished, and 18 (60%) were classified as well-nourished, based on preoperative PG-SGA SF scores. Malnourished patients were overall younger (60.5 years versus 70 years, respectively), were predominantly female (75% versus 44%, respectively), and had a higher BMI (26.0 kg/m2 versus 23.5 kg/m2, respectively), despite having lost more body weight in the past 6 months, compared to well-nourished patients.
Perioperative nutritional support and its effect on nutritional intake and body weight
In contrast to the 14 (78%) well-nourished patients, all 12 malnourished patients were referred to a dedicated dietician for perioperative nutritional support. The median time between the first consultation with the dietician and the day of surgery was 22 (14.5–38) days versus 25 (16–31) days for the malnourished and well-nourished patient cohorts, respectively. For patients in the malnourished group, an average of 1.8 follow-up consultations with the dietician were held preoperatively, whereas for patients in the well-nourished group, 1.4 follow-up consultations were held. The type of perioperative nutritional support prescribed to patients in both groups is provided in Table 2. Malnourished patients often required preoperative oral nutritional supplements to increase their caloric and protein intake. Furthermore, they regularly required preoperative PERT (75%) to correct for exocrine pancreatic insufficiency. Additionally, PERT was postoperatively prescribed to nearly all patients in the study cohort.
As a result of the dietary advice and prescribed nutritional supplements, malnourished patients were able to increase their daily total energy intake from 19.7 (14.9–25.7) kcal/kg body weight/day at T0 to 24.3 (20.1–35.7) kcal/kg body weight/day at T1 and their daily total protein intake from 1.07 (0.67–1.39) g/kg body weight/day at T0 to 1.22 (1.19–1.59) g/kg body weight/day at T1. Although malnourished patients increased their daily total postoperative energy intake even more to 27.6 (18.7–32.9) kcal/kg body weight/day at T2, their daily total protein intake decreased to 1.08 (0.78–1.64) g/kg body weight/day at T2. Preoperatively, the median body weight increased from 72.4 (64.5–93.1) kg to 74.0 (65.3–89.8) kg for malnourished patients. However, postoperatively, the median body weight decreased to 71.5 (59.6–82.4) kg. For well-nourished patients, the daily total energy intake increased from 26.3 (19.9–29.7) kcal/kg body weight/day at T0 to 26.8 (20.2–32.2) at T1 and decreased to 25.0 (20.3–30.9) kcal/kg body weight/day at T2. Their daily total protein intake increased from 1.22 (1.07–1.39) g/kg body weight/day at T0 to 1.28 (0.97–1.60) g/kg body weight/day at T1 and decreased to 1.08 (0.72–1.33) g/kg body weight/day at T2. For the median body weight in well-nourished patients, a constant decline was observed from 78.4 (70.4–82.9) kg at T0 to 77.8 (71.4–83.0) kg at T1 and 73.7 (67.6–78.6) kg at T2. The dynamic changes in nutritional intake and body weight are graphically represented in Fig. 2.
Additionally, in the supplementals (Fig. S1), the individual changes in daily total energy and protein intake in malnourished patients are represented.
Perioperative functional capacity and muscle thickness
An overview of the dynamic changes in perioperative functional capacity is provided in Table 3. Overall, the median handgrip strength increased slightly or remained relatively stable preoperatively but decreased postoperatively in both groups. Regarding the FTSST, no clinically relevant changes were observed in either group.
In Fig. 3, the dynamic changes in muscle thickness of the four muscle groups are graphically represented. The median muscle thickness decreased postoperatively for all muscle groups in both patient cohorts. However, postoperatively, the decrease in median muscle thickness was most prominent in patients who were preoperatively classified as well nourished.
Surgical details
Finally, no noteworthy differences in surgical details were observed (Table 4). Postoperatively, the median length of stay was shorter for malnourished patients (9.5 days versus 12 days for well-nourished patients), and there were no unplanned readmissions for malnourished patients compared to 4 (22%) unplanned readmissions for well-nourished patients. Pancreatic adenocarcinoma was more frequently diagnosed in malnourished patients (75% versus 28% in well-nourished patients) and, consequently, they were more frequently treated with adjuvant chemotherapy (58% versus 11% in well-nourished patients).
Discussion
In this prospective observational multicenter study within a regional HPB network, we observed a high incidence of preoperative malnutrition in patients suspected of pancreatic cancer undergoing pancreatic surgery. The majority of malnourished patients were diagnosed with pancreatic adenocarcinoma. All malnourished patients and the majority of well-nourished patients were referred to a dedicated dietician for perioperative nutritional support. Furthermore, PERT and oral nutritional supplements were frequently prescribed to address exocrine pancreatic insufficiency and nutritional deficiencies, respectively. Notably, the implementation of the PACAP-1 trial in the Netherlands contributed to the current practice of referring numerous patients preoperatively to a dietician and prescribing PERT [28]. Nutritional support had a clear effect, with a preoperative increase in caloric and protein intake and body weight, and functional capacity remaining stable. However, despite a further increase in caloric intake, protein intake and body weight decreased substantially postoperatively. Moreover, across the entire study cohort, a considerable decline in muscle thickness was observed for the repeated measures of all four muscle groups.
The high incidence of malnutrition reported in this study aligns with previous research indicating that patients suspected of pancreatic cancer undergoing pancreatic surgery have an increased risk of preoperative malnutrition [7, 12,13,14]. If left untreated, malnutrition is associated with unfavorable surgical and oncological outcomes [11, 29, 30]. Therefore, the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines on nutrition in patients with cancer state that nutritional support must aim to mitigate metabolic derangements by optimizing nutritional intake to match the total energy expenditure of 25–30 kcal/kg/day and protein requirements of 1.2–1.5 g/kg/day in patients with cancer [31]. In this study, nutritional support improved preoperative energy intake in malnourished patients from 19.7 to 24.3 kcal/kg body weight/day and protein intake from 1.07 to 1.22 g/kg body weight/day. Well-nourished patients were also able to achieve adequate preoperative nutritional intake, which remained relatively stable (energy intake ranging from 26.3 to 26.8 kcal/kg body weight/day and protein intake ranging from 1.22 to 1.28 g/kg body weight/day). By comparison, the majority of previous studies investigating nutritional support in patients undergoing pancreatic surgery have solely focused on in-hospital postoperative nutritional intake [32, 33]. However, in a similar study, Min Park et al. reported that malnourished patients undergoing pancreatic surgery were provided with preoperative nutritional support and consequently achieved a high median caloric intake of 32.1 kcal/kg body weigh/day and a protein intake of 1.30 g/kg body weight/day [34]. Nonetheless, they commenced preoperative nutritional support only 1 week before surgery, casting doubt on its clinical relevance. Preferably, the time between the initiation of nutritional support and the surgery should be at least 14 days [35]. The latest ESPEN guidelines on nutrition in surgical patients highlight the importance of the timely commencement of preoperative nutritional support in malnourished patients and the delay of surgery, if necessary, to optimize nutritional status [36].
Adequate nutritional intake remains equally important in the postoperative phase to promote postoperative functional recovery. In this study, the postoperative total energy intake remained adequate, with an intake of 27.6 kcal/kg/day and 25.0 kcal/kg/day in the malnourished and well-nourished patient groups, respectively. However, in both groups, the total protein intake decreased to 1.08 g/kg/day, which might be due to fewer patients receiving oral nutritional supplements postoperatively. Maintaining an adequate nutritional status during adjuvant chemotherapy is crucial, because malnutrition has been linked to diminished tolerance to chemotherapy, increased treatment toxicity, lower treatment adherence, and the necessity for dose adjustments [37,38,39]. Conversely, the adverse effects of chemotherapy can also lead to inadequate nutrient absorption and intake and subsequent malnutrition [40]. The latter might also have influenced the results of this study, because more than half of the patients in the malnourished group received adjuvant chemotherapy during the T2 study visit.
In both study groups, a substantial postoperative decrease in body weight and muscle thickness was observed. The role of surgery-induced trauma in triggering the catabolic response, leading to a loss of muscle mass in the postoperative period, is widely recognized [41]. However, another explanation could be the decrease in postoperative protein intake. Insufficient nutritional intake in combination with insufficient physical activity was previously identified as the leading risk factor for postoperative loss of muscle mass [42]. Importantly, inadequate postoperative nutritional intake was previously associated with a decreased 1-year survival in patients undergoing cancer surgery [42].
The administration of chemotherapy is linked to muscle wasting, and the fact that several patients in this study received adjuvant chemotherapy might have adversely affected postoperative muscle thickness [43]. This once more highlights the importance of adequate postoperative nutritional support. Notably, the decrease in body weight and muscle thickness was most prominent in patients who were preoperatively classified as well-nourished. Previously, Hogenbirk et al. observed a similar phenomenon when investigating the occurrence of surgery-related muscle loss and its association with in-hospital nutritional intake in the first postoperative week in patients undergoing pancreatic surgery [44]. The authors suggested that this could be attributed to the malnourished patients’ inability to further lose muscle mass postoperatively. Additionally, having received extensive nutritional advice and support compared to well-nourished patients, malnourished patients might be more aware of the importance of sufficient nutritional intake following surgery.
The strengths of the present study include the implementation of a comprehensive and multifaceted approach to adequately assess the perioperative nutritional status of patients. Additionally, the multicenter design of this study provides a realistic overview of the management of malnutrition in patients suspected of pancreatic cancer undergoing surgery. Highlighting the significance of equal treatment for malnutrition within a regional HPB network is crucial, as practice variation is undesirable and may lead to suboptimal treatment of malnutrition. Nevertheless, a few limitations must be addressed. Firstly, the study’s explorative design with a small sample size increased the risk of a selection bias. Secondly, due to factors such as the severity of disease symptoms and preexisting conditions, considerable baseline variability was observed among included patients in nutritional intake, body weight, functional capacity, and muscle thickness. This variability might have resulted in regression to the mean, distorting true values, for example, in patients with particularly low muscle thickness. In future research, solely focusing on malnourished patients might be worthwhile. Finally, because this study had an observational design, the results could not be compared to a valid control group with malnourished patients who did not receive perioperative nutritional support. However, this study has provided novel insights into the effect of a systematic approach to nutritional support by a dedicated dietician on patients diagnosed with resectable pancreatic cancer.
In conclusion, preoperative nutritional support by a dedicated dietician improves the nutritional status of both malnourished and well-nourished patients. Therefore, we recommend, based on the findings of this study, that all patients undergoing pancreatic surgery be referred to a dedicated dietitian for a full nutritional assessment and subsequent preoperative nutritional support. Nevertheless, we simultaneously suggest that greater attention should be directed toward postoperative monitoring of adequate nutritional intake in patients.
Data availability
The datasets generated or analyzed in the present study are not publicly available because the data are linked to a vulnerable patient population. However, these data are available upon reasonable request from the corresponding author (a.g.wijma@umcg.nl).
References
Park W, Chawla A, O’Reilly EM (2021) Pancreatic cancer: a review. JAMA 326:851–862
Jasmijn Smits F, Verweij ME, Daamen LA et al (2022) Impact of complications after pancreatoduodenectomy on mortality, organ failure, hospital stay, and readmission: analysis of a nationwide audit. Ann Surg 275:E222–E228
Gillis C, Ljungqvist O, Carli F (2022) Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br J Anaesth 128:434–448
van Wijk L, van der Snee L, Buis CI et al (2021) A prospective cohort study evaluating screening and assessment of six modifiable risk factors in HPB cancer patients and compliance to recommended prehabilitation interventions. Perioper Med (Lond). 10. https://doi.org/10.1186/S13741-020-00175-Z
Gilliland TM, Villafane-Ferriol N, Shah KP et al (2017) Nutritional and metabolic derangements in pancreatic cancer and pancreatic resection. Nutrients 9. https://doi.org/10.3390/NU9030243
Ni J, Zhang L (2020) Cancer cachexia: definition, staging, and emerging treatments. Cancer Manag Res 12:5597–5605
La Torre M, Ziparo V, Nigri G et al (2013) Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol 107:702–708
Kim E, Kang JS, Han Y et al (2018) Influence of preoperative nutritional status on clinical outcomes after pancreatoduodenectomy. HPB (Oxford) 20:1051–1061
Gianotti L, Besselink MG, Sandini M et al (2018) Nutritional support and therapy in pancreatic surgery: a position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery 164:1035–1048
Ratnayake CB, Loveday BP, Shrikhande SV et al (2018) Impact of preoperative sarcopenia on postoperative outcomes following pancreatic resection: a systematic review and meta-analysis. Pancreatology 18:996–1004
Pierobon ES, Moletta L, Zampieri S et al (2021) The prognostic value of low muscle mass in pancreatic cancer patients: a systematic review and meta-analysis. J Clin Med 10. https://doi.org/10.3390/JCM10143033
Ryan AM, Power DG, Daly L et al (2016) Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc 75:199–211
Bossi P, Delrio P, Mascheroni A et al (2021) The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients 13. https://doi.org/10.3390/NU13061980
Menozzi R, Valoriani F, Ballarin R et al (2023) Impact of nutritional status on postoperative outcomes in cancer patients following elective pancreatic surgery. Nutrients 15. https://doi.org/10.3390/NU15081958
Vujasinovic M, Valente R, Del Chiaro M et al (2017) Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients 9. https://doi.org/10.3390/NU9030183
Poulia KA, Sarantis P, Antoniadou D et al (2020) Pancreatic cancer and cachexia-metabolic mechanisms and novel insights. Nutrients 12. https://doi.org/10.3390/NU12061543
Pezzilli R, Caccialanza R, Capurso G et al (2020) Pancreatic enzyme replacement therapy in pancreatic cancer. Cancers (Basel) 12. https://doi.org/10.3390/CANCERS12020275
Latenstein AEJ, Dijksterhuis WPM, Mackay TM et al (2020) Cachexia, dietetic consultation, and survival in patients with pancreatic and periampullary cancer: a multicenter cohort study. Cancer Med 9:9385–9395
De Groot LM, Lee G, Ackerie A et al (2020) Malnutrition screening and assessment in the cancer care ambulatory setting: mortality predictability and validity of the Patient-Generated Subjective Global Assessment short form (PG-SGA SF) and the GLIM criteria. Nutrients 12:1–13
Vandenbroucke JP, Von Elm E, Altman DG et al (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 4:1628–1654
Reber E, Gomes F, Vasiloglou MF et al (2019) Nutritional risk screening and assessment. J Clin Med 8. https://doi.org/10.3390/jcm8071065
Bauer J, Capra S, Ferguson M (2002) Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 56:779–785
Hogenbirk RNM, Viddeleer AR, Hentzen JEKR et al (2022) Thickness of biceps and quadriceps femoris muscle measured using point-of-care ultrasound as a representation of total skeletal muscle mass. J Clin Med 11. https://doi.org/10.3390/JCM11226606
Hentzen JEKR, Van Wijk L, Buis CI et al (2019) Impact and risk factors for clinically relevant surgery-related muscle loss in patients after major abdominal cancer surgery: study protocol for a prospective observational cohort study (MUSCLE POWER). Int J Clin Trials 6:138
Muñoz-Bermejo L, Adsuar JC, Mendoza-Muñoz M et al (2021) Test-retest reliability of five times sit to stand test (FTSST) in adults: a systematic review and meta-analysis. Biology (Basel) 10. https://doi.org/10.3390/BIOLOGY10060510
Amasene M, Besga A, Medrano M et al (2021) Nutritional status and physical performance using handgrip and SPPB tests in hospitalized older adults. Clin Nutr 40:5547–5555
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
MacKay TM, Smits FJ, Latenstein AEJ et al (2020) Impact of nationwide enhanced implementation of best practices in pancreatic cancer care (PACAP-1): a multicenter stepped-wedge cluster randomized controlled trial. Trials 21. https://doi.org/10.1186/S13063-020-4180-Z
Chan MY, Chok KSH (2019) Sarcopenia in pancreatic cancer - effects on surgical outcomes and chemotherapy. World J Gastrointest Oncol 11:527–537
Bundred J, Kamarajah SK, Roberts KJ (2019) Body composition assessment and sarcopenia in patients with pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford) 21:1603–1612
Arends J, Bachmann P, Baracos V et al (2017) ESPEN guidelines on nutrition in cancer patients. Clin Nutr 36:11–48
Bozzetti F, Mariani L (2014) Perioperative nutritional support of patients undergoing pancreatic surgery in the age of ERAS. Nutrition 30:1267–1271
Afaneh C, Gerszberg D, Slattery E et al (2015) Pancreatic cancer surgery and nutrition management: a review of the current literature. Hepatobiliary Surg Nutr 4:59–71
Park HM, Kang YH, Lee DE et al (2022) Effect of preoperative nutritional support in malnourished patients with pancreatobiliary cancer: a quasi-experimental study. BMC Nutr 8. https://doi.org/10.1186/S40795-022-00555-2
Martínez-Ortega AJ, Piñar-Gutiérrez A, Serrano-Aguayo P et al (2022) Perioperative nutritional support: a review of current literature. Nutrients 14. https://doi.org/10.3390/NU14081601
Weimann A, Braga M, Carli F et al (2021) ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr 40:4745–4761
Arrieta O, De la Torre-Vallejo M, López-Macías D et al (2015) Nutritional Status, body surface, and low lean body mass/body mass index are related to dose reduction and severe gastrointestinal toxicity induced by afatinib in patients with non-small cell lung cancer. Oncologist 20:967–974
Bozzetti F (2017) Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 28:2107–2118
Klute KA, Brouwer J, Jhawer M et al (2016) Chemotherapy dose intensity predicted by baseline nutrition assessment in gastrointestinal malignancies: a multicentre analysis. Eur J Cancer 63:189–200
de van der Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, Baracos VE (2018) Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol 29(5):1141–1153
Gillis C, Carli F (2015) Promoting perioperative metabolic and nutritional care. Anesthesiology 123:1455–1472
Hogenbirk RNM, van der Plas WY, Hentzen JEKR et al (2023) Postoperative muscle loss, protein intake, physical activity and outcome associations. Br J Surg 110:183–192
Aversa Z, Costelli P, Muscaritoli M (2017) Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol 9:369–382
Hogenbirk RNM, Hentzen JEKR, van der Plas WY et al (2023) Surgery-related muscle loss after pancreatic resection and its association with postoperative nutritional intake. Cancers (Basel) 15. https://doi.org/10.3390/CANCERS15030969
Acknowledgements
The authors greatly appreciate the support and dedication of the patients who volunteered to participate in the study. Furthermore, they thank the staff of the surgical departments of the participating hospitals for their collaboration and assistance.
Funding
This study was supported by a grant from the Vrienden Integrale Oncologische Zorg (VIOZ).
Author information
Authors and Affiliations
Contributions
Conceptualization, R.N.M.H., S.B., and J.M.K.; Methodology, A.G.W., R.N.M.H., S.B., and J.M.K. Validation, A.G.W., R.N.M.H., S.B., and J.M.K. Formal Analysis, A.G.W., S.B., and J.M.K. Investigation, A.G.W., R.N.M.H., D.A.K., and H.D. Writing – Review & Editing, A.G.W., R.N.M.H., H.D., D.A.K., E.S.J.B., M.T.D.V., S.B., M.S.L.L., V.B.N., E.M.M., F.J.H.H., M.W.N., and J.M.K. Original Draft Preparation, A.G.W.; Visualization, A.G.W.; Supervision, S.B. and J.M.K. Funding Acquisition, S.B. and J.M.K.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Medical Ethics Committee of the UMCG in the Netherlands (Netherlands research registration number 202000299). This study was performed in accordance with the ethical standards set by the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Competing interests
The authors declare no competing interests.
Disclaimer
The study protocol has not undergone any peer review by the funding body, nor will they play a role in the analysis and interpretation of future results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
520_2024_8683_MOESM1_ESM.tif
Supplementary file1 (TIF 3650 KB) Figure S1. Individual malnourished patients’ daily total energy and protein intake during each study visit.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wijma, A.G., Hogenbirk, R.N.M., Driessens, H. et al. Nutritional support in pancreatic cancer patients and its effect on nutritional status: an observational regional HPB network study investigating current practice. Support Care Cancer 32, 487 (2024). https://doi.org/10.1007/s00520-024-08683-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08683-0