Abstract
Background
Even with significant advances in surgical techniques and treatment, salvage chemotherapy remains the major treatment strategy for patients with unresectable or metastatic gastric cancer (GC). Practical and technical advances have simplified safe and convenient use of supplemental home parenteral nutrition (HPN). We aimed to clarify the role of HPN in patients with incurable GC undergoing salvage chemotherapy.
Methods
We enrolled 25 patients with GC with a nutritional risk index (NRI) of ≦ 97.5 undergoing HPN. Their nutritional status, laboratory data, and quality of life (QoL) were analyzed using the Research and Treatment of Cancer quality of life questionnaire-C30 before and after HPN administration at 0.5, 1, 2, and 3 months. We enrolled 25 patients with an NRI of > 97.5 not undergoing HPN as the control group.
Results
Total protein (P = 0.008), prealbumin (P < 0.001), and total cholesterol (P = 0.023) levels improved significantly after 0.5 months of HPN administration. The study group also demonstrated a marked improvement in nitrogen balance (P = 0.004) and prealbumin levels (P < 0.012) after 1 month. Gains in body weight after 1 month and body mass index after 2 months of HPN administration remained comparable with those of the control group. Global QoL scores were maintained and comparable with those of the control group.
Conclusions
Supplemental HPN therapy for malnourished patients with unresectable or metastatic GC undergoing salvage chemotherapy is feasible and revealed marked improvement in nutritional status. Early HPN intervention should be considered an important part of palliative treatment for advanced GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Taiwan, gastric cancer (GC) is the sixth and fifth most common cancer in men and women, respectively, accounting for 3.8% of malignancies and 5.0% of malignancy-related deaths in 2013 [1]. Although significant advances have been made in surgical techniques and anticancer treatments, metastatic GC in a significant number of patients and locally advanced stage III GC in a proportion of patients remain unresectable. Therefore, salvage chemotherapy is a treatment option for palliative purposes.
Patients with unresectable or metastatic GC frequently experience malnutrition, which may be secondary to immune response and systemic inflammation, adverse events of chemotherapy, psychological factors, and gastrointestinal (GI) malfunction or bowel obstruction due to carcinomatosis. Furthermore, malnutrition, especially in the context of skeletal muscle wasting (sarcopenia), has been associated with an increased risk of developing intolerance to chemotherapy. The dosage of anticancer agents can cause increased toxicity, for which measures taken will potentially lead to dose reduction or treatment interruption, impaired quality of life (QoL), reduced performance status, and shortened survival time [2,3,4]. Malnutrition with poor or inadequate enteral nutrition (including oral nutrition supplements) in patients who lack adequate GI access or function indicates a need for parenteral nutrition (PN) [5]. PN can supplement daily nutritional needs that are unmet by enteral nutrition in patients with unresectable or metastatic GC and facilitate support or restoration of nutritional status. In patients with a terminal condition, PN may also prevent death from starvation and dehydration, for example, in patients with carcinomatosis with bowel obstruction.
Practical and technical advances have simplified the safe and convenient use of home parenteral nutrition (HPN). HPN can shorten in-hospital stays and increase the proportion of care in outpatient settings. In addition, HPN is suitable for the long-term care of patients. Therefore, it is a part of palliative care for patients with cancer and carries the potential to increase their survival and improve their QoL [6, 7].
Methods
The protocol was approved by the local ethics committees (KMUHIRB-2013-02-71) and was performed in accordance with the guidelines contained in the Declaration of Helsinki of 1975, as revised in 1996. Written informed consent was obtained from patients before any study activities were performed. The study was registered at www.clinicaltrials.gov (NCT03121807) under the trial name: Home Parenteral Nutrition for Malnourished Unresectable Stage IV Gastric Cancer (date of registration: 4 January 2017).
Patient eligibility
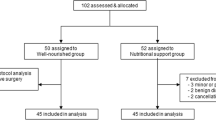
In this prospective observational study, patients (aged ≥ 20 years) with unresectable locally advanced stage III or metastatic (stage IV) GC who underwent salvage chemotherapy from October 2014 to January 2019 were screened for eligibility. A total of 25 patients with malnourishment and a nutritional risk index (NRI) of ≦ 97.5 comprised the study group, who received HPN. Exclusion criteria included the following laboratory results: absolute neutrophil count < 1.5 × 103/μL, platelet count < 60 × 103/μL, hemoglobin < 8.0 g/dL, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) > 3.0 × upper limit of normal (ULN) or AST and ALT > 5.0 × ULN in patients with liver metastasis, total bilirubin > 2.0 × ULN, and creatinine > 2.0 × ULN or calculated creatinine clearance < 60 mL/min. Additional exclusion criteria included the following: heart failure (New York Heart Association functional class > III) or stroke history; known diabetic ketoacidosis within 7 days before initiation of the study; body mass index (BMI) > 30 kg/m2; drug abuse or chronic alcoholism; other life-threatening diseases; recent emergent surgery; ongoing infection; pregnancy or lactation; history of immunosuppressive therapy or recent immunological diseases; and participation in another clinical study with an investigational drug or an investigational medical device within 1 month of the initiation of the study.
For ethical reasons, we could not reject administering supplemental HPN to malnourished patients. Therefore, we enrolled 25 well-nourished patients with unresectable GC who also underwent salvage chemotherapy for comparison. No supplemental HPN was provided for these patients.
Intervention
For the study group, the PN regimen included a total caloric supplement of 910–1800 kcal/day, consisting of 33–60, 120–240, and 30–60 g/day of amino acids, glucose, and lipids, respectively, according to patients’ calorie-protein needs, which were estimated by dieticians of the nutrition therapy team based on oral intake or tubal feeding. The adequacy of supplemental HPN was assessed and the amount of supplemental HPN was adjusted every 2 weeks in accordance with the nitrogen balance of each patient. In addition, the regimen included electrolytes, microelements, and vitamins, dosed according to the nutritional status of the patient. PN was infused daily in an infusion time range between 18 and 24 h depending on the amount of infusion.
Nutritional, laboratory, and QoL measurements
NRI, body weight, and BMI measurements as well as blood tests were performed before HPN administration and at 0.5, 1, 2, and 3 months after HPN administration. Assessments included nitrogen balance, protein (albumin, prealbumin, total protein, and transferrin), lipids (triglycerides, cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL)), hematology (leukocyte count and hemoglobin), general chemistries (blood urea nitrogen (BUN) and creatinine), and liver function tests (total bilirubin, AST, and ALT). Nitrogen balance was calculated as daily nitrogen intake (including enteral nutrition and PN) minus daily nitrogen loss (24-hour urine urea nitrogen, urine nonurea nitrogen, and fecal and skin loss, which were assumed to be 4 g/day). QoL was assessed using the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ)-C30 according to the same schedule.
Sample size
According to our preliminary results, nitrogen balance was − 1.92 g/day (SD, 4.48 g/day) for patients with NRI ≦ 97.5, with a correlation of 0.687 after HPN administration for 1 month. Using a two-sided sample size calculation before and after the study to detect a 2-g/day or greater difference to make nitrogen balance positive, the required sample size of the study group to obtain 80% power at alpha = 0.05 was 25. For comparison, we enrolled 25 patients with NRI > 97.5 as the control group, in which no HPN was administered, and the corresponding parameters were collected for comparison.
Statistical analysis
The results between groups were compared using the Mann–Whitney U test or Kruskal–Wallis test for categorical unpaired data. The Wilcoxon rank-sum test or Friedman test was used to analyze paired data. Fisher’s exact test was used to compare dichotomous variables. Pearson’s chi-square test was used to analyze nominal variables. McNemar’s test was used to analyze paired categorical data. The means were compared using a two-sample test, analysis of variance (ANOVA), or linear regression, as appropriate. However, for all aforementioned inferential analysis methods, the center effect was not considered when comparing treatments. Therefore, ANOVA incorporating the center effect and Cochran–Mantel–Haenszel test stratified by the center effect were applied to replace the two-sample t test and Fisher’s exact test. For efficacy analyses and some of the safety analyses (including laboratory data and vital sign data), analysis of covariance was applied when comparing treatment modes, with their respective baselines as covariates. Baseline data were defined as the data obtained before the first administration of treatment before surgery. This approach was based on the potential effect of baseline data on the endpoints. Endpoints were defined as the net change in post-treatment data from baseline data. Statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, IL, USA). Results with P < 0.05 were considered statistically significant.
Results
Clinical characteristics at baseline and outcomes
In the study group, NRI improved and was higher than 97.5 in 8 out of 22 patients at 0.5 months, 3 out of 19 patients at 1 month, 6 out of 12 patients at 2 months, and 3 out of 7 patients at 3 months. Seven patients survived longer than 3 months, four patients survived longer than 4 months, and the remaining two survived longer than 5 months, including one patient whose NRI was higher than 97.5 at each time point after 0.5 month. Furthermore, median overall survival was significantly shorter in the study group than in the control group (2 vs. 19 months, 95% confidence interval (CI), 0.6–3.4 vs. 1.6–36.4, P < 0.001, HR = 4.39, 95% CI, 2.0–9.6, Fig. 1). The median HPN administration period was 135 days (range, 16–569 days). The study and control groups before HPN administration are compared in Table 1. Patients with malnourishment in the study group exhibited significantly lower serum levels of albumin (P < 0.001), prealbumin (P < 0.001), cholesterol (P = 0.009), HDL (P = 0.049), LDL (P = 0.037), hemoglobin (P = 0.022), and Na (P = 0.003) compared with those in the control group.
Differences in parameters after HPN intervention in the study group
Table 2 shows the differences in the parameters of patients in the study group after HPN intervention. Net change in body weight started to be positive at 1 month and BMI at 2 months although these changes were nonsignificant. In terms of lab assessments, albumin did not change significantly; however, prealbumin, total protein, triglycerides, cholesterol, LDL, and transferrin levels increased throughout the study period. Prealbumin increased significantly at 0.5 month (P < 0.001) and 1 month (P = 0.012); total protein (P = 0.008), cholesterol (P = 0.023), and LDL (P = 0.024) increased significantly at 0.5 month; and triglycerides increased significantly at 2 months (P = 0.018). Positive changes in nitrogen balance began at 1 month, and the difference was significant (P = 0.004). Total bilirubin was elevated significantly at 0.5 month (P = 0.030) although the level was generally less than 2.0 mg/dL. AST and ALT were elevated after HPN administration, but the levels remained less than ULN.
Differences between the study group after HPN intervention and control group
Table 3 demonstrates differences in parameters between the study and control groups at different observation times. NRI in the study group improved especially at 2 and 3 months but was still worse than that in the control group (P = 0.004 and 0.010, respectively). Body weight, BMI, prealbumin, cholesterol, HDL, and LDL of the study group were comparable with those of the control group after 1 month (P = 0.849), 2 months (P = 0.077), 1 month (P = 0.051), 0.5 month (P = 0.352), 2 months (P = 0.129), and 0.5 month (P = 0.749), respectively. Nitrogen balance was significantly better in the study group than in the control group at 1 month (P = 0.011) and 3 months (P = 0.023).
QoL at baseline
Differences in scale scores of EROTC QLQ-C30 between the study group before treatment and control group are summarized in Table 4. At baseline, compared with those in the control group, the scores of physical functioning (PF) (P < 0.032), fatigue (FA) (P = 0.013), and appetite loss (AP) (P = 0.004) were significantly worse in the study group, but the global QoL scores did not differ (P = 0.087) between the two groups before HPN treatment.
Differences in QoL scores after HPN intervention in the study group
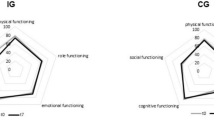
Changes in EORTC scores after treatment initiation are shown in Table 5. For PF, the score decreased significantly at 0.5 month (P = 0.016), 1 month (P = 0.048), and 3 months (P < 0.001). The score for role functioning (RF) decreased significantly at 0.5 month (P < 0.001), 1 month (P = 0.001), 2 months (P = 0.022), and 3 months (P = 0.006) after HPN treatment. Both emotional functioning (EF) and social functioning (SF) scores declined significantly only at 3 months (P = 0.042 and 0.033, respectively). The scores of FA (P = 0.010) and financial difficulties (FI) (P = 0.048) increased significantly at 3 months, whereas dyspnea (DY) scores increased significantly at 2 months (P = 0.028). No significant change in global QoL scores was observed during the study period.
Differences in QoL scores between the study group after HPN intervention and the control group
Compared with the control group at different time points after HPN intervention, the scores of PF, RF, and SF were significantly impaired in the study group throughout the study period, except for the SF scores at 2 months (P = 0.091). The FA score was significantly worse in the study group than in the control group throughout the study period (P = 0.001, 0.011, 0.007, and 0.002 at 0.5, 1, 2, and 3 months, respectively). Compared with those of the control group, the dyspnea scores of the study group were significantly worse at 2 months (P = 0.016) and 3 months (P = 0.023), and AP score was significantly worse at 3 months (P = 0.035, Table 6).
Discussion
Malnutrition is a condition frequently encountered in patients with cancer, especially in those with GC. Symptoms caused by GC itself can include anorexia, nausea, vomiting, fatigue, pain, gastric outlet obstruction, and impaired food intake. Moreover, the toxicities of anticancer drugs, including mucositis, constipation, and diarrhea, can exacerbate these symptoms and further reduce oral feeding. This is a vicious circle and, with a long-term lack of enteral stimulation by food or tube feeding, malnutrition results in small intestine mucosal atrophy, reduction of mucosal cell proliferation, and corruption of intestinal mucosal barrier function [8]. In addition, the immune response to cancer and inflammatory cytokines released by cancer cells results in systemic inflammation [9, 10], which together with the cancer itself, increases metabolism and energy and protein needs in turn. Essentially, psychological stress, depression, and decreased physical activity because of (or leading to) reduced performance status can also influence food intake. When nutrient intake fails to meet protein and energy needs in the context of elevated metabolic rate, depletion of body reserves results. Decline in skeletal muscle mass, function, and quality, critical for patients with cancer, eventually result in sarcopenia. Sarcopenia not only further limits physical activity, but also adversely impacts the risk of toxicity of anticancer drugs [11,12,13] and is associated with shorter survival, as demonstrated by rapidly accumulating evidence [14,15,16,17,18]. These findings are consistent with the results of the current study, in which malnourished patients had short median overall survival; this demonstrated that malnutrition may reflect not only disease severity but also disease prognosis. NRI is a nutritional screening tool composed of the two variables of body weight loss and serum albumin level that is simple and easily practiced, especially for outpatient visits. However, even with its lower sensitivity and specificity compared with that of the patient-generated subjective global assessment (PG-SGA), both are predictive of outcomes [19].
PN is a solution for patients whose enteral nutrition does not satisfy their daily nutrition requirements. For patients with unresectable or metastatic GC, HPN should be considered because inadequate food intake and anticancer therapy may last for a protracted time. HPN reduces hospital stay and financial costs and provides additional time to these patients to be cared for in their familiar home environments. For appropriately selected patients with advanced or terminal cancer in the context of a nonfunctioning gut or malignant bowel obstruction, HPN, as part of a palliative treatment, can also prevent death from starvation and dehydration. In the appropriate setting, such carcinomatosis and small bowel obstruction, can offer the potential to prolong survival and improve QoL.
In the present study, the gains in body weight after 1 month and in BMI after 2 months following HPN administration in the study group remained comparable with those in the control group. Prealbumin, cholesterol, and LDL which were significantly lower in the study group before HPN administration, increased significantly at 0.5 month and were comparable with those in the control group after treatment. Although NRI improved, the difference was not significant. However, negative nitrogen balance at baseline notably became positive after 1 month in the study group. Eventually, nitrogen balance was significantly higher in the study group than in the control group at 3 months. Improvement in nutritional status was not reflected by the albumin level in the 3-month observation time. This is consistent with the albumin half-life of 21 days, which implies that 100 days are required to reach a new steady level [20]. Consistently, low levels of hemoglobin in the study group corresponded with more serious GC, which directly resulted in bleeding and consequent chronic anemia, as well as serious malnutrition. Although total bilirubin, AST, and BUN were elevated slightly in the study group, the values remained less than ULN. Long-term HPN was notably not associated with any deterioration in liver or kidney functions.
Before HPN was introduced, the baseline score of PF was significantly lower, and FA and AP scores were significantly more severe in the study group than in the control group. Appetite increased after 0.5 month of HPN administration, whereas FA did not change. The PF and RF scores were impaired as the disease progressed. The EF and SF scores declined significantly at 3 months and symptoms of FA and DY deteriorated at the end of their life. The FI scores in the study group were comparable with those in the control group. This may partially result from fewer in-hospital costs and partially because the Taiwan national health insurance system covers almost all treatment expense for patients with cancer. Deterioration of EORTC symptom scales in the study group is mainly related to progression of disease at the end of life, whereas metabolic and infectious complications are the most commonly encountered side effects of HPN administration. Despite the adverse changes mentioned, the global QoL score was maintained and was comparable with that of the patients in the control group.
HPN administration in patients with incurable cancer and life expectancy shorter than 2–3 months has been debated [21, 22]. However, the World Health Organization (WHO) defined palliative care as “an approach that improves the quality of life of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment, and treatment of pain and other problems—physical, psychosocial, and spiritual” [23]. Moreover, PN has become an integral part of palliative care for patients with cancer [6]. Recent studies have demonstrated significant improvements in global the QoL and nutritional status of patients with cancer after 4 weeks of HPN administration [24,25,26]. Cotogni et al. also reported that global QoL, PF, RF, EF, FA, and AP exhibited a significant favorable trend in patients with advanced cancer receiving oncological treatment with a median survival of 4.7 months. These results were consistent with those of a study conducted by Vashi et al. [27], which demonstrated that HPN administration was positively associated with improved QoL and nutritional status in patients with advanced cancer with compromised enteral intake and malnutrition. Furthermore, the greatest benefit was observed in patients with at least 3 months of HPN administration; however, significant improvement was also observed in those receiving HPN for 1 or 2 months. Although it is not demonstrated in the current study, Chermesh et al. concluded that HPN administration prevents death from starvation in patients with incurable cancer without oral intake and prolongs survival [28]. Moreover, HPN administration does not deteriorate QoL among caregivers [29]. Therefore, HPN administration meets the WHO definition and can be a critical, integral part of palliative care.
This study had several limitations. First, this was an observational prospective study with a small number of participants, and it may not have sufficient power to draw definite conclusions. However, performing a randomized trial is challenging because it involves including patients with malnourishment in the control group, which is unacceptable for ethical reasons. Second, the appropriateness of offering HPN to patients who are not expected to live longer than 2–3 months can be debated. However, recent evidence has demonstrated positive results of HPN in certain patients with terminal cancer. As such, HPN meets the WHO definition of palliative care.
Conclusion
Supplemental HPN administration had a positive impact on nutritional status and QoL in patients with malnourishment and incurable GC shortly after the initiation of treatment, despite being a small-sample study with limitations. Supplemental HPN was well tolerated, with no liver or kidney damage and can be reasonably included as an integral part of palliative care for appropriately chosen patients with terminal cancer with malnutrition. Early HPN intervention is imperative for malnourished patients with unresectable or metastatic GC undergoing salvage chemotherapy.
Availability of data and materials
The data and materials analyzed in the current study are available from the corresponding author on reasonable requests.
References
Ministry of Health and Culture (2019) http://www.mohw.gov.tw
Andreyev HJ, Norman AR, Oates J, Cunningham D (1998) Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 34(4):503–509
Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, Smith IE, O'Brien ME (2004) Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 90(10):1905–1911. https://doi.org/10.1038/sj.bjc.6601781
Massicotte MH, Borget I, Broutin S, Baracos VE, Leboulleux S, Baudin E, Paci A, Deroussent A, Schlumberger M, Antoun S (2013) Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. J Clin Endocrinol Metab 98(6):2401–2408. https://doi.org/10.1210/jc.2013-1115
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hutterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Muhlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC (2017) ESPEN guidelines on nutrition in cancer patients. Clin Nutr 36(1):11–48. https://doi.org/10.1016/j.clnu.2016.07.015
Druml C, Ballmer PE, Druml W, Oehmichen F, Shenkin A, Singer P, Soeters P, Weimann A, Bischoff SC (2016) ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr 35(3):545–556. https://doi.org/10.1016/j.clnu.2016.02.006
Bozzetti F (2019) The role of parenteral nutrition in patients with malignant bowel obstruction. Support Care Cancer. https://doi.org/10.1007/s00520-019-04948-1
Altmann GG (1972) Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat 133(4):391–400. https://doi.org/10.1002/aja.1001330403
Roxburgh CS, McMillan DC (2014) Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer 110(6):1409–1412. https://doi.org/10.1038/bjc.2014.90
Dantzer R (2004) Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 500(1-3):399–411. https://doi.org/10.1016/j.ejphar.2004.07.040
Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, Butts CA, Scarfe AG, Sawyer MB (2007) Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res 13(11):3264–3268. https://doi.org/10.1158/1078-0432.CCR-06-3067
Palmela C, Velho S, Agostinho L, Branco F, Santos M, Santos MP, Oliveira MH, Strecht J, Maio R, Cravo M, Baracos VE (2017) Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J Gastric Cancer 17(1):74–87. https://doi.org/10.5230/jgc.2017.17.e8
Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, Choi SK, Muss HB (2017) Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res 23(14):3537–3543. https://doi.org/10.1158/1078-0432.CCR-16-2266
Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC (2019) The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle 10(1):111–122. https://doi.org/10.1002/jcsm.12357
Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, Ma LL, Yu Z, Shen X (2016) Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine 95(13):e3164. https://doi.org/10.1097/MD.0000000000003164
da Silva JR Jr, Wiegert EVM, Oliveira L, Calixto-Lima L (2019) Different methods for diagnosis of sarcopenia and its association with nutritional status and survival in patients with advanced cancer in palliative care. Nutrition 60:48–52. https://doi.org/10.1016/j.nut.2018.09.003
Mintziras I, Miligkos M, Wachter S, Manoharan J, Maurer E, Bartsch DK (2018) Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: Systematic review and meta-analysis. Int J Surg 59:19–26. https://doi.org/10.1016/j.ijsu.2018.09.014
Fattouh M, Chang GY, Ow TJ, Shifteh K, Rosenblatt G, Patel VM, Smith RV, Prystowsky MB, Schlecht NF (2019) Association between pretreatment obesity, sarcopenia, and survival in patients with head and neck cancer. Head Neck 41(3):707–714. https://doi.org/10.1002/hed.25420
Faramarzi E, Mahdavi R, Mohammad-Zadeh M, Nasirimotlagh B (2013) Validation of nutritional risk index method against patient-generated subjective global assessment in screening malnutrition in colorectal cancer patients. Chin J Cancer Res 25(5):544–548. https://doi.org/10.3978/j.issn.1000-9604.2013.10.04
Geisler JP, Linnemeier GC, Thomas AJ, Manahan KJ (2007) Nutritional assessment using prealbumin as an objective criterion to determine whom should not undergo primary radical cytoreductive surgery for ovarian cancer. Gynecol Oncol 106(1):128–131. https://doi.org/10.1016/j.ygyno.2007.03.008
Bozzetti F, Cozzaglio L, Biganzoli E, Chiavenna G, De Cicco M, Donati D, Gilli G, Percolla S, Pironi L (2002) Quality of life and length of survival in advanced cancer patients on home parenteral nutrition. Clin Nutr 21(4):281–288
Bozzetti F, Arends J, Lundholm K, Micklewright A, Zurcher G, Muscaritoli M, Espen (2009) ESPEN guidelines on parenteral nutrition: non-surgical oncology. Clin Nutr 28(4):445–454. https://doi.org/10.1016/j.clnu.2009.04.011
WHO (2007) https://www.who.int/cancer/publications/cancer_control_palliative/en/
Culine S, Chambrier C, Tadmouri A, Senesse P, Seys P, Radji A, Rotarski M, Balian A, Dufour P (2014) Home parenteral nutrition improves quality of life and nutritional status in patients with cancer: a French observational multicentre study. Support Care Cancer 22(7):1867–1874. https://doi.org/10.1007/s00520-014-2164-9
Senesse P, Tadmouri A, Culine S, Dufour PR, Seys P, Radji A, Rotarski M, Balian A, Chambrier C (2015) A prospective observational study assessing home parenteral nutrition in patients with gastrointestinal cancer: benefits for quality of life. J Pain Symptom Manag 49(2):183–191 e182. https://doi.org/10.1016/j.jpainsymman.2014.05.016
Cotogni P, De Carli L, Passera R, Amerio ML, Agnello E, Fadda M, Ossola M, Monge T, De Francesco A, Bozzetti F (2017) Longitudinal study of quality of life in advanced cancer patients on home parenteral nutrition. Cancer Med 6(7):1799–1806. https://doi.org/10.1002/cam4.1111
Vashi PG, Dahlk S, Popiel B, Lammersfeld CA, Ireton-Jones C, Gupta D (2014) A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 14:593. https://doi.org/10.1186/1471-2407-14-593
Chermesh I, Mashiach T, Amit A, Haim N, Papier I, Efergan R, Lachter J, Eliakim R (2011) Home parenteral nutrition (HTPN) for incurable patients with cancer with gastrointestinal obstruction: do the benefits outweigh the risks? Med Oncol 28(1):83–88. https://doi.org/10.1007/s12032-010-9426-2
Santarpia L, Bozzetti F (2018) Acute impact of home parenteral nutrition in patients with late-stage cancer on family caregivers: preliminary data. Support Care Cancer 26(2):667–671. https://doi.org/10.1007/s00520-017-3884-4
Funding
This work was supported by grants from the Ministry of Science and Technology (MOST108-2321-B-037-001, MOST107-2321-B-037-003, MOST107-2314-B-037-116, MOST107-2314-B-037-022-MY2, and MOST107-2314-B-037-023-MY2) and the Ministry of Health and Welfare (MOHW107-TDU-B-212-123006, MOHW107-TDU-B-212-114026B, MOHW108-TDU-B-212-133006, and MOHW109-TDU-B-212-134026), which were funded by the health and welfare surcharge of tobacco products. Further funding was obtained from Kaohsiung Medical University Hospital (KMUH108-8R34, KMUH108-8R35, KMUH108-8M33, KMUH108-8M35, KMUH108-8M36, KMUHS10801, KMUHSA10804, KMUHS10807, and KMUH-DK109005~3), the Center for Cancer Research (KMU-TC108A04), and the Cohort Research Center Grant (KMU-TC108B07). In addition, this study was supported by the Grant of Taiwan Precision Medicine Initiative, Academia Sinica, Taiwan, ROC.
Author information
Authors and Affiliations
Contributions
C.J. Ma conducted the treatment, interpreted the final results, and drafted the manuscript. C.W. Huang and Y.S. Yeh collected data. H.L. Tsai, W.C. Su, and T.K. Chang participated in data analysis. L.C. Sun and Y.L. Shih assisted in treatment. F.J. Yu and D.C. Wu assisted in data interpretation. J.Y. Wang was responsible for study design and coordination. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
The protocol was approved by the local ethics committees (KMUHIRB-2013-02-71) and was performed in accordance with Declaration of Helsinki of 1975, as revised in 1996.
Consent to participate
Written informed consent was obtained from patients before any study activities were performed.
Consent for publish
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, CJ., Huang, CW., Yeh, YS. et al. Supplemental home parenteral nutrition improved nutrition status with comparable quality of life in malnourished unresectable/metastatic gastric cancer receiving salvage chemotherapy. Support Care Cancer 29, 1977–1988 (2021). https://doi.org/10.1007/s00520-020-05687-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05687-4